Abstract
Background
Improving the understanding of non-clinical factors that lead to the increasing caesarean section (CS) rates in many low- and middle-income countries is currently necessary to meet the challenge of implementing effective interventions in hospitals to reverse the trend. The objective of this study was to study the influence of organizational factors on the CS use in Argentina, Vietnam, Thailand and Burkina Faso.
Methods
A cross-sectional hospital-based postpartum survey was conducted in 32 hospitals (8 per country). We selected women with no potential medical need for CS among a random sample of women who delivered at each of the participating facilities during the data collection period. We used multilevel multivariable logistic regression to analyse the association between CS use and organizational factors, adjusted on women’s characteristics.
Results
A total of 2,092 low-risk women who had given birth in the participating hospitals were included. The overall CS rate was 24.1%, including 4.9% of pre-labour CS and 19.3% of intra-partum CS. Pre-labour CS was significantly associated with a 24-hour anaesthetist dedicated to the delivery ward (ORa = 3.70 [1.41; 9.72]) and with the possibility to have an individual room during labour and delivery (ORa = 0.28 [0.09; 0.87]). Intra-partum CS was significantly associated with a higher bed occupancy level (ORa = 1.45 [1.09; 1.93]): intrapartum CS rate would increase of 6.3% points if the average number of births per delivery bed per day increased by 10%.
Conclusion
Our results suggest that organisational norms and convenience associated with inadequate use of favourable resources, as well as the lack of privacy favouring women’s preference for CS, and the excessive workload of healthcare providers drive the CS overuse in these hospitals. It is also crucial to enhance human and physical resources in delivery rooms and the organisation of intrapartum care to improve the birth experience and the working environment for those providing care.
Trial registration
The QUALI-DEC trial is registered on the Current Controlled Trials website (https://www.isrctn.com/) under the number ISRCTN67214403.
Similar content being viewed by others
Background
While the global caesarean section (CS) rate has significantly increased in recent decades (from 7% in 1990 to 21% in 2018) and some regions of Asia or South America present CS rates above 40% and 50% [1, 2], recent trends and projections show that CS rates will continue to rise unequally in the absence of global effective intervention to revert the trend [1]. Between 1990 and 2018, the largest increases in CS rates occurred in Latin America and Asia [1, 2]. Although the increase in CS rates is partly due to an increasing number of institutional deliveries [3], there is a significant increase in the use of CS in health care facilities [3, 4]. Indeed, previous studies on hospital-based CS rates using the Robson classification showed increased rates over time (from 2004 to 2011), and particularly in women in groups 1 to 4 of Robson’s classification which are considered to be low-risk women compared with the other groups [5]. This trend has raised global concern among healthcare providers and governments since there is no evidence of benefit from performing a CS without medical indication [4, 6,7,8].
Medical indications alone are thus unlikely to explain this phenomenon and the contribution of non-clinical factors in CS use should also be investigated [3, 9, 10]. Factors related to women, healthcare providers or health systems have been proposed to interact and contribute to the increasing use of CS [11]. Women-related factors documented in low- and middle-income countries (LMICs) include socio-economic status or age. Women of higher socio-economic status have a higher probability to give birth by CS compared to poorer women [3, 12,13,14,15]. Advanced maternal age also increases the probability of CS [13, 16]. Some antenatal factors such attending antenatal care (ANC) visits in a private facility or a high number of antenatal care (ANC) visits also may influence the mode of birth and the decision for CS [13, 14, 17].
Factors related to healthcare providers have been also identified as influencing the CS use [11, 18,19,20,21]. Previous studies have shown that fear of litigation or reputational damage, financial incentives or lack of cooperation and trust between healthcare providers may influence decision-making for CS [18, 22]. However, it appears that physicians often perceive these factors to be associated with the characteristics of the health care systems in which they practice, rather than with their own beliefs or characteristics [18].
Organizational factors associated with CS use have been also investigated [11]. In France, low staffing levels for obstetricians and midwives are associated with higher CS rates [23]. In the United States, hospitals with high profits on CS procedures are more likely to perform CSs [24]. In LMICs, giving birth in the private sector increases the likelihood of having CS in many countries [13, 14, 17, 25,26,27]. A hospital’s high level of care (ability to provide comprehensive obstetric care) is associated with an increased risk of CS use in Bangladesh [28]. Implementation of clinical practice guidelines with mandatory second opinion or combined with opinion lead, audit and feedback reduced CS rates in some middle-income countries [11]. As organizational factors are potentially suitable for change within the facility, improving understanding of these drivers and how they interact can help envision and develop strategies that support the reduction of unnecessary CS in LMICs.
The QUALI-DEC project
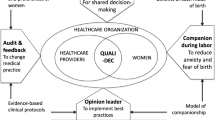
In response to the growing global importance of non-clinical factors in the rise of CS, a consortium of researchers started the QUALI-DEC project: “Appropriate use of CS through QUALIty DECision-making by women and providers” to implement four non-clinical interventions to reduce unnecessary CS in LMICs [29]: (i) opinion leaders to improve evidence-based clinical practices; (ii) audits and feedback to help healthcare providers identify unnecessary CS; (iii) implementation of companionship during labour and childbirth to support parturient women; (iv) a Decision-Analysis Tool (DAT) to help women make an informed decision on delivery mode. The research project is implemented in 32 health facilities in four countries: Burkina Faso, Argentina, Viet Nam, and Thailand. Despite overall high CS rates in the participating hospitals, there is still some variability in the observed rates. In order to tailor the implementations of these interventions to the local context, it is important to understand the determinants of the mode of birth in the study settings.
With this background, the objective of this study was to assess the influence of organizational factors on the use of CS in a low-risk population before starting the intervention phase of QUALI-DEC project, with the ultimate aim of better tailoring the components of the intervention in each country.
Materials and methods
Study design
This study is an ancillary study of the overall QUALI-DEC project, a hybrid efficacy-implementation type III multisite trial registered on the Current Controlled Trials website (number ISRCTN67214403) [29]. The effectiveness of the intervention will be assessed using an interrupted time series analysis and a comparative before-and-after cross-sectional study design. This ancillary study used data from the baseline (pre-intervention) cross-sectional survey. This survey was conducted among a representative sample of postpartum women before discharge from the hospital and will be replicated at the end of the intervention period. The baseline survey took place in all the 32 hospitals participating in the QUALI-DEC project in Burkina Faso, Argentina, Thailand and Vietnam (8 per country). The selection of participating hospitals in each country for the QUALI-DEC project was made purposively for their high CS rates by national or local health authorities in discussion with the research consortium and intended to reflect the diversity of health facilities in each country, regarding their mode of organization.
Participants and sample size
Women who had delivered a live-born child after 22 weeks’ gestation age (26 weeks in Burkina Faso) were eligible to participate in the baseline cross-sectional survey. Women who had a vital health problem in the post-partum period and those who had given birth to a stillborn child, a neonatal death or a newborn with a malformation were not eligible. Abortions or miscarriages were not considered as deliveries and women who delivered at home or in another health facility (postnatal transfer) were excluded from the survey. The sample size estimate was not calculated specifically for this study but for the effectiveness-implementation research (Quali-Dec). The calculation was based on the expected before-after difference in women’s satisfaction scores, i.e. 470 women per country [29]. The minimum number of women to approach during recruitment was 564 women in each country (71 women per hospital), assuming a 10% non-response rate and 10% ineligible women.
For this study, we selected women who were, a priori, at lower risk of CS: single, full-term pregnancy with cephalic presentation and no previous CS. Women who gave preterm birth (< 37 weeks’ gestation), those with a previous CS, multiple pregnancies or non-cephalic presentations were all excluded. Women with pre-labor emergency CS were also excluded because this type of CS was usually performed for high risk women (e.g. eclampsia, cord prolapse, …).
Inclusion of participants and data collection
Prior to the cross-sectional survey, a baseline formative research [30] was first conducted in 2020 as part of the QUALI-DEC project. This formative research collected the relevant institutional and organisational data in each hospital to adapt the intervention to the context. This data was collected by each local principal investigator using a data collection form. Data were then extracted by the principal data manager and the final database was controlled by each country principal investigator.
The design of the cross-sectional survey was based on the World Health Organization (WHO) Global Health Survey of Maternal and Perinatal Health [31]. In each country, data collection took place daily in all hospitals until the required number of participants was reached, but still continuing for two full weeks if the sample size was reached earlier. To achieve this goal, we estimated that 5–6 post-partum women had to be interviewed each day. For hospitals with a high volume of activity, a random sampling of women were used to reach 10 selected women per day, among those who had given birth the previous day. We assumed that 4–5 women would refuse to participate or would be ineligible, which would leave us with 5–6 recruitments and interviews per day. The survey period depended on the number of births in each hospital and ranged from 14 days to 46 days between December 2020 and June 2022.
A data collector assigned all randomly selected women an identification number and assessed their eligibility using a screening form. If the eligibility criteria were met, a social scientist approached the post-partum women and offered them to participate during their hospital stay. A consent form was completed when the women agreed to participate and the social scientist interviewed the participants in face-to-face using a tablet for data collection form. The questionnaire was developed based on a literature review and validated by the experts comprising the QUALI-DEC project team. A pilot survey was conducted to test the questionnaire, which was modified where necessary. The information collected was organized in seven modules: women’s characteristics, antenatal care and preference for mode of birth; birth outcomes; women’s knowledge about modes of birth; labour companionship; women’s birth experience and satisfaction; gender dimensions and social equity; wealth characteristics and out-of-pocket expenses. Information on medical history, pregnancy, labour and delivery were simultaneously extracted for all included women by a clinical data collector from medical records using a standard data collection form. Data was entered in duplicate by two local data abstractors into an electronic system specifically designed with validation checks (REDCap®). Final consistency checks were carried out by the principal data manager, with regular support from the country-level data managers.
Outcomes and explanatory variables
We defined two primary outcomes: (i) pre-labour CS; and (ii) intrapartum CS. The first outcome refers to women who had a planned CS performed before labour as compared to women who attempted a vaginal delivery but ended up with either a vaginal delivery or an emergency intra-partum CS. The second outcome refers to women with a trial of labour but who had an emergency intrapartum CS as compared to women with natural or instrumental vaginal delivery. Furthermore, the rate of instrumental deliveries was very low (1.5% of births) in the 32 hospitals surveyed.
A pre-defined list (Supplementary information Table S1), representative of the main indications for CS in the four participating countries, was used to collect indications for CS, as declared by healthcare providers and recorded in medical records. The various indications for CS were not mutually exclusive (i.e. several indications could be recorded for the same CS).
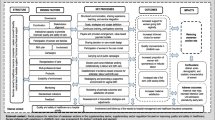
We grouped the explanatory variables into women’s characteristics or organizational factors (Fig. 1). The women’s characteristics fall into three categories, starting with the variables that we assumed had a proximal effect on outcomes: (i) birth-related factors; (ii) pregnancy-related factors; and (iii) socio-demographic characteristics. Birth-related factors included the type of labour onset (spontaneous or induced labour), the birthweight (low, normal or high) and pre-existing medical or obstetrical complication that may represent a potential medical need for CS. This last variable is a composite variable assessed at the time of birth, defined as currently having or having had at least one condition from a list of complications extracted from medical records (hypertension and complications; prelabour rupture of membranes; suspected intrauterine growth restriction; diabetes type I/II/gestational; cardiac or renal disease; chronic respiratory conditions; HIV; cholestasis; vaginal bleeding; genital ulcer disease; condyloma accuminata). Pregnancy-related factors included parity, whether or not the woman attended ANC visits in another private facility (outside the hospital where the woman delivered). Sociodemographic factors comprised the place of residency (urban or rural), marital status, maternal age at delivery, maternal level of education, maternal occupation and wealth index. The wealth index was a context-specific composite index, developed through variable selection and component analysis carried out in collaboration with local investigators.
The organizational factors, which we have assumed to have a distal effect on outcomes, included the status of the maternity unit where the woman delivered (academic or not, reference level, totally public or private practice for all/some doctors, functioning ultrasound machine in the delivery ward or not, anaesthetist fully dedicated to the delivery ward or shared with other services, individual delivery room or not), the workload of healthcare providers and the intrapartum care capacity. Workload was modelled as the ratio of the average number of birth per day (annual number of births divided by 365) to the total number of providers (obstetrician or nurse/midwives) working in the maternity unit. The intrapartum capacity was assessed based on the ratio of the average number of births per day (annual number of births divided by 365) to the total number of beds in admission ward or the delivery ward. These organizational factors were selected based of the literature review [13, 14, 16, 18, 23, 32, 33].
Finally, the country was considered as a separate variable, insofar as it captures unmeasured factors, such as those linked to healthcare systems, medical practices or culture of care.
Data analysis
All analyses were performed using the statistical analysis software Stata/SE® 17. We first described the characteristics of participants using frequencies and percentages for qualitative variables and means and standard deviations (SD) for quantitative variables. Univariate regressions were performed to analyse the crude association between explanatory variables and both primary outcomes.
Multivariable logistic regression models were used to analyse the determinants of having a pre-labour or intrapartum CS. To account for the clustering of outcomes by hospital, a mixed-effects logistic regression models with random intercept were used to present adjusted odds ratios (ORa) and confidence intervals.
A woman’s variable was selected if it was significantly associated to the outcome in bivariate analysis (p-value < 0.2). Women’s variables were progressively introduced into the model in the following sequence: (i) birth-related factors; (ii) pregnancy-related factors; (iii) sociodemographic factors. At each step, we selected the variables that provided the most parsimonious model according to the likelihood ratio using a forward stepwise procedure (with a selection of variables having a p-value < 0.1). The country of residence was forced into the model. Organizational factors were also forced into the model including maternal characteristics in two steps: one model including all variables related to the status of the maternity unit and another model including variables related to workload and care capacity but excluding variables on maternity status. We tested the interactions between organizational factors and parity because the impact of organization on the use of CS might be different in women with previous experience of birth or not [32]. We also tested this type of interaction regarding women’s country of residence because organization of care was quite different in the four participating countries. We measured the sensitivity of the outcomes to changes in continuous variables (i.e. elasticity). In this way, we used Stata’s margins command, which estimates the response margins of the outcome for specified values of covariates. The addition of the eyex option allows us to estimate the change in the probability of having a CS caused by a percentage variation (10%) in workload and care capacity.
Results
A total of 5,840 eligible women gave birth in the 32 hospitals during the data collection period, and 3,127 were randomly selected and provided consent (Supplementary information Figure S1). As per the analysis plan, we further excluded 978 women because of their high risk of undergoing CS and 57 women because they had an emergency pre-labour CS. We then analysed 2,092 women with a priori low risk for CS.
Five hundred five participants (24.1%) delivered by CS: 102 women (4.9%) with pre-labour CS and 403 women (19.3%) with intrapartum CS. Pre-labour CS rates by hospital ranged from 0 to 2.6% in Burkina Faso; from 1.9 to 8.8% in Argentina; from 1.2 to 17.9% in Thailand; and from 0 to 15.7% in Vietnam. Intrapartum CS rates by hospital ranged from 8.9 to 26.8% in Burkina Faso; from 7.5 to 19.2% in Argentina; from 8.4 to 26.3% in Thailand; and from 4 to 60% in Vietnam (Fig. 2).
In more than 30% of pre-labour CSs, the maternal request was one of the indications (Fig. 3). The most common indications for intrapartum CS were fetal distress (30%), cephalopelvic disproportion (26%) and dystocia/failure to progress (17.6%) (Fig. 4).
Women’s characteristics and organizational factors are presented in Tables 1 and 2, respectively. Most hospitals were teaching facilities with functioning ultrasound devices in the delivery ward. In contrast, few of them had a 24-hour anaesthetist dedicated to the maternity unit and offered women the possibility of giving birth in an individual delivery room.
Most women’s and institutional characteristics were significantly associated to both outcomes in bivariate analysis with p-value less than 0.2 (Table 3). Regarding women’s characteristics, only marital status was excluded from the multivariable models for pre-labour CS. Marital status and maternal age were excluded from the models for intrapartum CS.
Taking into account women’s characteristics, regression model 2 shows that pre-labour CS was significantly associated with a 24-hour anaesthetist dedicated to the delivery ward (ORa = 3.70 [1.41; 9.72], p < 0.01) and with the possibility to have an individual room during labour and delivery (ORa = 0.28 [0.09; 0.87], p = 0.03) (Table 4). Workload and care capacity indicators (model 3) were not significantly associated with the use of pre-labour CS.
Model 6 shows that intra-partum CS was significantly associated with the average number of births per delivery bed per day: ORa = 1.45 [1.09; 1.93], p < 0.01 (Table 5). The other organizational factors had no effect on intra-partum CS rates.
Based on the elasticity estimation, intrapartum CS rate would increase of 6.3% points if the average number of births per delivery bed per day increased by 10% (elasticity = 0.63 [0.16; 1.09], p < 0.01). This result is illustrated in Fig. 5 where we plotted the aggregate intrapartum CS rate by hospital, calculated from the regression model (model 6) as a function of the number of births per delivery bed per day in the corresponding hospital. Interaction tests do not show any different impact of organizational factors on CS rates between countries, and between nulliparous and multiparous women.
Discussion
This multi-site, multi-country study shows that CS use among low-risk women varied not only between countries, but also between hospitals within the same country. This variability is partly explained by individual and organizational factors. The availability of an anaesthetist fully dedicated to the maternity unit and the lack of individual delivery rooms in the hospital increased the use of pre-labour CS, while intra-partum CS increased when delivery bed occupancy increased as well.
Our analysis shows that CS rates were particularly high in those women who can be considered to be “lower risk” women from the perspective that they had a single, full-term pregnancy with cephalic presentation and no previous CS. These results are consistent with previous studies in LMICs that have shown that the population of low-risk women has high CS rates and is the main contributors to overall CS rates in these countries [3, 5, 34, 35].
In our study, there was one pre-labour CS for every four intra-partum CS. Fetal distress, cephalopelvic disproportion and failure to progress were the main indications for intra-partum CS. These finding are consistent with the results of previous studies in similar context [13, 36,37,38]. Most pre-labour CS were performed in Argentina and Thailand, countries where women’s preference for a pre-labour CS seems to be marked, revealed by women and accepted by obstetricians [39,40,41]. This is correlated with our findings which show that crude odds ratios of intrapartum CS are usually equal or smaller than those of pre-labour CS and that more than 30% of pre-labour CS were performed on maternal request in our sample. Accepting maternal request for CS without medical indication has been reported to be a way for obstetricians to avoid the potential litigation they fear and may be partly responsible for the high pre-labour CS rate in low-risk women [18, 22]. This practice might be reinforced by women’s preference for CS due to fear of pain during vaginal delivery but also to their perception of CS as a safe procedure [42,43,44].
In this study, women who gave birth in a participating hospital with a dedicated 24-hour anaesthetist in the maternity ward had an increased risk of pre-labour CS than in a hospital with an anaesthetist who shares his activity with other services. Similar results were found in Senegal and Mali [16]. Obviously, the full availability of an anaesthetist makes it easy to plan a CS, whether on medical indication or on maternal request. Although the presence of a dedicated anaesthetist is highly desirable to ensure that women who require a CS do not have difficulty accessing it, there is also a risk that hospitals perform excessive pre-labour CS routinely if no intervention is put in place to ensure quality of care and to reduce medically inappropriate surgery [11].
Our findings highlight the need to maintain a favourable environment in the maternity unit in order to improve quality of care and decrease unnecessary CS. Women who gave birth in a hospital with individual delivery rooms were less likely to give birth by pre-labour CS than women who gave birth in hospitals with only shared delivery rooms. Lack of privacy in shared delivery rooms is associated with a negative birth experience and women’s preference for pre-labour CS [43, 45]. The possibility to have an individual room reassures women of their privacy during delivery, usually allows a companion to be present by their side and thus might encourage them to attempt a vaginal birth. Additionally, we showed of positive relationship between intrapartum CS rates and delivery bed occupancy as a proxy of care capacity. This finding is not in line with that found in China [46], but is in accordance with two studies conducted in France [23, 32]. In this country, authors have shown that staffing levels and high number of births by delivery room also affect the use of caesarean section. Overcrowded maternity units is perceived by providers as a barrier to the quality of intrapartum care and may influence providers’ decision to perform a CS during labour when one could have waited longer [18]. Women who have had a poor experience during childbirth, relating to the conditions in shared rooms, can also request intrapartum CS for their next pregnancy [42].
This multi-site and multi-country design is a strength of this study that allows for the generalisation of results in similar contexts. Moreover, we carried out a comprehensive data collection on potential determinants of CS rates [31]. Our results show well known individual risk factors for pre-labour or intrapartum CS in LMICs: complications during pregnancy [16, 23, 47, 48], high birth weight [16, 23, 49, 50], maternal age [13, 16, 50,51,52], education [13, 50], employment [53].
Our study might have some limitations. Our sample of hospitals is not representative of all hospitals in the four participating countries. Our results only apply to hospitals with moderate to high caesarean section rates in in Burkina Faso, Argentina, Thailand and Vietnam. Moreover, organisational data such as bed occupancy levels or workload for healthcare providers were annual averages collected in 2020. These indicators may change over time and may not reflect the situation at the time of the post-partum survey. However, due to ongoing collaboration with researchers in countries who did not report significant changes, we believe that these variations, if they occurred, were minimal. In addition, our decision of not including institutional and organisational variables in the same statistical model (due to the collinearity), may have biased the elasticity measure due to a lack of adjustment for hospital characteristics.
Our results do not allow us to know whether women’s level of wealth is a determining factor in accessing individual rooms, but it is likely that the poorest women have less access to this type of room as compared with the richest. Anyway, wealth index was not significantly associated with pre-labour CS in our analysis which suggests that this “individual room” effect entails other components than the wealth index. Only two private hospitals were included (in Vietnam), compared to 30 public hospitals with or without private ward. Since several studies have shown that there is an association between private hospitals and CS use in LMICs [13, 14, 18, 25, 33, 50, 51], we may have underestimated CS rates among low-risk women in the participating countries and the effect of this private practice could not be identified. Nevertheless, our results show that women who attended ANC in another private facility have higher pre-labour CS rates than other women. Financial incentives, perceived better time management or obstetrician compliance with performing a CS on maternal request in the private sector may explain this association [13, 14, 18, 25, 33]. Finally, factors related to the healthcare providers, quality of care and compliance with medical guidelines were not taken into account in this study [18].
The results of this study have practical implications for policy makers, hospitals managers and healthcare providers to reduce unnecessary CSs, as evidenced by the high CS rates among low-risk women in the 32 participating hospitals. Firstly, in contexts where the availability of an anaesthetist fully dedicated to the maternity unit facilitates overuse of CS, efforts must be made to improve decision making of pre-labour CS [11]. To this end, non clinical interventions such as mandatory second-opinion, opinion leaders and peer-review of CS indications are recommended to reduce unnecessary CS [11, 54].
Secondly, to encourage women to attempt vaginal delivery when appropriate, hospitals should make effort to reassure women and offer them as much privacy as possible. Individual delivery room is optimal, but using curtains in shared delivery rooms could be an option [11]. More privacy will facilitate companionship during labour and delivery. This approach helps women reduce their anxiety, communicate with healthcare providers and deliver vaginally [55,56,57].
Regarding limited care capacity in referral hospital with high delivery rates, efforts should be made to adapt the number of delivery beds to the expected number of women in labour. Our results suggest that hospitals with between 1 and 2 births/bed/24 h is associated with lower intra-partum CS rates as compared with 3 births/bed/24 h. To this end, health policy should focus on the quality of care in level 1 or 2 hospitals and encourage women with low obstetrical risk to give birth in these facilities rather than in referral hospitals. Women could benefit from giving birth in smaller hospitals, possibly closer to their home, with the same quality of care as in large hospitals.
Conclusion
Lack of privacy, easy access to surgery and limited intrapartum care capacity increase the use of CS in low risk women of participating hospitals. In this context it is crucial to improve the conditions and environment of the delivery room and the organisation of intrapartum care for a better birth experience. It is also important to implement non clinical interventions targeting health care professionals to improve decision-making regarding the mode of birth.
Availability of data and materials
The datasets generated and/or analysed during the current study will be available on Zenodo (https://www.zenodo.org/) under the community “QUALI-DEC - Appropriate use of Caesarean section through QUALIty DECision-making by women and providers (847567)”. The database anonymisation process is underway and run by the principal data manager of the project. All datasets relating to the Quali-Dec baseline survey will be available by the end of the project (end 2024). Zenodo is a general-purpose open-access repository developed under the European OpenAIRE program and operated by the European Organization for Nuclear Research (CERN). Zenodo will allow the deposition of datasets, reports, and any other digital artifacts related to research. For each repository, a persistent DOI will be created to easily cite the stored items. The metadata of each record will be indexed and searchable directly in Zenodo’s search engine immediately after publishing.
Abbreviations
- CS:
-
Caesarean section
- LMICs:
-
Low- and middle-income countries
- ANC:
-
Antenatal care
- DAT:
-
Decision analysis tool
- WHO:
-
World Health Organization
- REDCap:
-
Research electronic data capture
- OR:
-
Odds ratio
- SD:
-
Standard deviation
- CI:
-
Confidence interval
- ORa:
-
Adjusted odds ratio
References
Betran AP, Ye J, Moller AB, Souza JP, Zhang J. Trends and projections of caesarean section rates: global and regional estimates. BMJ Glob Health. 2021;6(6):e005671.
Betrán AP, Ye J, Moller AB, Zhang J, Gülmezoglu AM, Torloni MR. The increasing trend in caesarean section rates: global, regional and national estimates: 1990–2014. PLoS One. 2016;11(2). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4743929/. Cited 2021 Jun 3.
Boerma T, Ronsmans C, Melesse DY, Barros AJD, Barros FC, Juan L, et al. Global epidemiology of use of and disparities in caesarean sections. Lancet. 2018;392(10155):1341–8.
Department of Reproductive Health and Research, World Health Organization. WHO statement on caesarean section rates. 2015. Available from: http://apps.who.int/iris/bitstream/handle/10665/161442/WHO_RHR_15.02_eng.pdf;jsessionid=C5F0C940EFD7594686FE1A9529E123E6?sequence=1. Cited 2021 Nov 23.
Vogel JP, Betrán AP, Vindevoghel N, Souza JP, Torloni MR, Zhang J, et al. Use of the Robson classification to assess caesarean section trends in 21 countries: a secondary analysis of two WHO multicountry surveys. Lancet Glob Health. 2015;3(5):e260-270.
Ye J, Zhang J, Mikolajczyk R, Torloni MR, Gülmezoglu AM, Betran AP. Association between rates of caesarean section and maternal and neonatal mortality in the 21st century: a worldwide population-based ecological study with longitudinal data. BJOG. 2016;123(5):745–53.
Betran AP, Torloni MR, Zhang J, Ye J, Mikolajczyk R, Deneux-Tharaux C, et al. What is the optimal rate of caesarean section at population level? A systematic review of ecologic studies. Reprod Health. 2015;12:57.
Ye J, Betrán AP, Guerrero Vela M, Souza JP, Zhang J. Searching for the optimal rate of medically necessary cesarean delivery. Birth. 2014;41(3):237–44.
O’Leary C, De Klerk N, Keogh J, Pennell C, De Groot J, York L, et al. Trends in mode of delivery during 1984–2003: can they be explained by pregnancy and delivery complications? BJOG. 2007;114(7):855–64.
Bell JS, Campbell DM, Graham WJ, Penney GC, Ryan M, Hall MH. Do obstetric complications explain high caesarean section rates among women over 30? A retrospective analysis. BMJ. 2001;322(7291):894–5.
Betrán AP, Temmerman M, Kingdon C, Mohiddin A, Opiyo N, Torloni MR, et al. Interventions to reduce unnecessary caesarean sections in healthy women and babies. Lancet. 2018;392(10155):1358–68.
Boatin AA, Schlotheuber A, Betran AP, Moller AB, Barros AJD, Boerma T, et al. Within country inequalities in caesarean section rates: observational study of 72 low and middle income countries. BMJ. 2018;360:k55.
Begum T, Rahman A, Nababan H, Hoque DME, Khan AF, Ali T, et al. Indications and determinants of caesarean section delivery: evidence from a population-based study in Matlab, Bangladesh. PLoS One. 2017;12(11):e0188074.
Mia MN, Islam MZ, Chowdhury MR, Razzaque A, Chin B, Rahman MS. SSM Popul Health. 2019;8:100415.
Tenaw Z, Kassa ZY, Kassahun G, Ayenew A. Maternal Preference, mode of delivery and associated factors among women who gave birth at public and private hospitals in Hawassa City, Southern Ethiopia. Ann Glob Health. 2019;85(1):115.
Briand V, Dumont A, Abrahamowicz M, Traore M, Watier L, Fournier P. Individual and institutional determinants of caesarean section in referral hospitals in Senegal and Mali: a cross-sectional epidemiological survey. BMC Pregnancy Childbirth. 2012;12(1):1–8.
Vieira GO, Fernandes LG, de Oliveira NF, Silva LR, de Vieira T. Factors associated with cesarean delivery in public and private hospitals in a city of northeastern Brazil: a cross-sectional study. BMC Pregnancy Childbirth. 2015;15:132.
Panda S, Begley C, Daly D. Clinicians’ views of factors influencing decision-making for caesarean section: a systematic review and metasynthesis of qualitative, quantitative and mixed methods studies. PLoS One. 2018;13(7):e0200941.
Panda S, Begley C, Daly D. Clinicians’ views of factors influencing decision-making for CS for first-time mothers-a qualitative descriptive study. PLoS One. 2022;17(12):e0279403.
Long Q, Kingdon C, Yang F, Renecle MD, Jahanfar S, Bohren MA, et al. Prevalence of and reasons for women’s, family members’, and health professionals’ preferences for cesarean section in China: a mixed-methods systematic review. PLoS Med. 2018;15(10):e1002672.
Takegata M, Smith C, Nguyen HAT, Thi HH, Thi Minh TN, Day LT, et al. Reasons for increased caesarean section rate in Vietnam: a qualitative study among Vietnamese mothers and health care professionals. Healthcare. 2020;8(1):41.
Elaraby S, Altieri E, Downe S, Erdman J, Mannava S, Moncrieff G, et al. Behavioural factors associated with fear of litigation as a driver for the increased use of caesarean sections: a scoping review. BMJ Open. 2023;13(4):e070454.
Zbiri S, Rozenberg P, Goffinet F, Milcent C. Cesarean delivery rate and staffing levels of the maternity unit. PLoS One. 2018;13(11):e0207379.
Sakai-Bizmark R, Ross MG, Estevez D, Bedel LEM, Marr EH, Tsugawa Y. Evaluation of hospital cesarean delivery-related profits and rates in the United States. JAMA Netw Open. 2021;4(3):e212235.
Neuman M, Alcock G, Azad K, Kuddus A, Osrin D, More NS, et al. Prevalence and determinants of caesarean section in private and public health facilities in underserved South Asian communities: cross-sectional analysis of data from Bangladesh, India and Nepal. BMJ Open. 2014;4(12):e005982.
Bhatia M, Banerjee K, Dixit P, Dwivedi LK. Assessment of variation in cesarean delivery rates between public and private health facilities in India from 2005 to 2016. JAMA Netw Open. 2020;3(8):e2015022.
Phadungkiatwattana P, Tongsakul N. Analyzing the impact of private service on the cesarean section rate in public hospital Thailand. Arch Gynecol Obstet. 2011;284(6):1375–9.
han MN, Islam MM, Akter S. Spatial distribution of caesarean deliveries and their determinants in Bangladesh: evidence from linked data of population and health facility survey. Lancet Reg Health Southeast Asia. 2023;0(0). Available from: https://www.thelancet.com/journals/lansea/article/PIIS2772-3682(23)00013-6/fulltext. Cited 2023 Jul 4.
Dumont A, Betrán AP, Kaboré C, de Loenzien M, Lumbiganon P, Bohren MA, et al. Implementation and evaluation of nonclinical interventions for appropriate use of cesarean section in low- and middle-income countries: protocol for a multisite hybrid effectiveness-implementation type III trial. Implement Sci. 2020;15(1):72.
Bohren MA, Opiyo N, Kingdon C, Downe S, Betrán AP. Optimising the use of caesarean section: a generic formative research protocol for implementation preparation. Reprod Health. 2019;16(1):170.
Shah A, Faundes A, Machoki M, Bataglia V, Amokrane F, Donner A, et al. Methodological considerations in implementing the WHO global survey for monitoring maternal and perinatal health. Bull World Health Organ. 2008;86(2):126–31.
Duvillier C, Rousseau A, Bouyer C, Goffinet F, Rozenberg P. [Impact of organizational factors on the cesarean delivery occurrence in a low-risk population]. Gynecol Obstet Fertil Senol. 2018;46(10–11):706–12.
Lin HC, Xirasagar S. Institutional factors in cesarean delivery rates: policy and research implications. Obstet Gynecol. 2004;103(1):128–36.
Betrán AP, Gulmezoglu AM, Robson M, Merialdi M, Souza JP, Wojdyla D, et al. WHO global survey on maternal and perinatal health in Latin America: classifying caesarean sections. Reprod Health. 2009;6:18.
Schantz C, Ravit M, Traoré AB, Aboubakar M, Goyet S, de Loenzien M, et al. Why are caesarean section rates so high in facilities in Mali and Benin? Sex Reprod Healthc. 2018;16:10–4.
Dorji T, Dorji P, Gyamtsho S, Tamang ST, Wangden T, Wangmo S, et al. Rates and indications of caesarean section deliveries in Bhutan 2015–2019: a national review. BMC Pregnancy Childbirth. 2021;21(1):698.
Ehtisham S, Akhtar Hashmi H. Determinants of caesarean section in a tertiary hospital. J Pak Med Assoc. 2014;64(10):1175–8.
Maskey S, Bajracharya M, Bhandari S. Prevalence of cesarean section and its indications in a tertiary care hospital. JNMA J Nepal Med Assoc. 2019;57(216):70–3.
Perrotta C, Romero M, Sguassero Y, Ingram C, Righetti N, Gialdini C, et al. Women’s mode of birth preferences and preparedness of hospitals to support vaginal birth in the public health sector in Argentina. Repro Female Child Health. 2022;1:111–21.
Perrotta C, Romero M, Sguassero Y, Straw C, Gialdini C, Righetti N, et al. Caesarean birth in public maternities in Argentina: a formative research study on the views of obstetricians, midwives and trainees. BMJ Open. 2022;12(1):e053419.
Nuampa S, Ratinthorn A, Lumbiganon P, Rungreangkulkij S, Rujiraprasert N, Buaboon N, et al. “Because it eases my childbirth plan”: a qualitative study on factors contributing to preferences for caesarean section in Thailand. BMC Pregnancy Childbirth. 2023;23(1):280.
Coates D, Thirukumar P, Spear V, Brown G, Henry A. What are women’s mode of birth preferences and why? A systematic scoping review. Women Birth. 2020;33(4):323–33.
Colomar M, Opiyo N, Kingdon C, Long Q, Nion S, Bohren MA, et al. Do women prefer caesarean sections? A qualitative evidence synthesis of their views and experiences. PLoS One. 2021;16(5):1–23.
Jenabi E, Khazaei S, Bashirian S, Aghababaei S, Matinnia N. Reasons for elective cesarean section on maternal request: a systematic review. J Matern Fetal Neonatal Med. 2020;33(22):3867–72.
Valizadeh F, Heshmat F, Mohammadi S, Motaghi Z. Affecting factors of parturient women’s privacy preservation in the maternity ward: a qualitative study. J Family Reprod Health. 2021;15(3):186–95.
Hong X. Factors related to the high cesarean section rate and their effects on the “price transparency policy” in Beijing, China. Tohoku J Exp Med. 2007;212(3):283–98.
Shah A, Fawole B, M’imunya JM, Amokrane F, Nafiou I, Wolomby JJ, et al. Cesarean delivery outcomes from the WHO global survey on maternal and perinatal health in Africa. Int J Gynaecol Obstet. 2009;107(3):191–7.
Banchani E, Tenkorang EY. Risk factors for caesarean sections in Ghana: evidence from the Ghana maternal health survey. J Biosoc Sci. 2022;54(1):21–38.
Yaya S, Uthman OA, Amouzou A, Bishwajit G. Disparities in caesarean section prevalence and determinants across sub-saharan Africa countries. Glob Health Res Policy. 2018;3(1):1–9.
Verma V, Vishwakarma RK, Nath DC, Khan HTA, Prakash R, Abid O. Prevalence and determinants of caesarean section in South and South-East Asian women. PLoS One. 2020;15(3):e0229906.
Leone T, Padmadas SS, Matthews Z. Community factors affecting rising caesarean section rates in developing countries: an analysis of six countries. Soc Sci Med. 2008;67(8):1236–46.
Kim SY, Park JY, Bak SE, Jang YR, Wie JH, Ko HS, et al. Effect of maternal age on emergency cesarean section. J Matern Fetal Neonatal Med. 2020;33(23):3969–76.
Taye MG, Nega F, Belay MH, Kibret S, Fentie Y, Addis WD, et al. Prevalence and factors associated with caesarean section in a comprehensive specialized hospital of Ethiopia: a cross-sectional study; 2020. Ann Med Surg. 2021;67:102520.
Chen I, Opiyo N, Tavender E, Mortazhejri S, Rader T, Petkovic J, et al. Non-clinical interventions for reducing unnecessary caesarean section. Cochrane Database Syst Rev. 2018;2018(9):CD005528.
World Health Organization. Companion of choice during labour and childbirth for improved quality of care. 2020.
Hodnett ED, Gates S, Hofmeyr GJ, Sakala C. Continuous support for women during childbirth. Cochrane Database Syst Rev. 2013;7:CD003766.
Bohren MA, Berger BO, Munthe-Kaas H, Tunçalp Ö. Perceptions and experiences of labour companionship: a qualitative evidence synthesis. Cochrane Database Syst Rev. 2019;(3). Available from: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD012449.pub2/full. Cited 2023 Jun 12.
Acknowledgements
We thank all women who participated to the survey and healthcare providers who facilitated the data collection in participating hospitals.
Clinical Trial Registry: The QUALI-DEC trial is registered on the Current Controlled Trials website (https://www.isrctn.com/) under the number ISRCTN67214403.
Funding
The QUALI-DEC project is co-funded by the European Union’s Horizon 2020 research and innovation program under grant agreement No. 847567 and by the UNDP-UNFPA-UNICEF-WHO-World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), a co-sponsored program executed by the World Health Organization (WHO) in the Department of Sexual and Reproductive Health and Research (SRH). The contents of this article are solely the responsibility of the authors and do not reflect the views of the EU, UNDP, UNFPA, UNICEF, WHO, or the World Bank or their respective institutions. The first author (C.E.) received salary support from the Ecole Doctorale Pierre Louis de Santé Publique (Sorbonne Université - Université Paris Cité) as part of a thesis funding.
Author information
Authors and Affiliations
Consortia
Contributions
CE designed the study, analysed the data, interpreted the results and wrote the first draft of the article; APB coordinated the survey, participated in the design of the study and the interpretation of the results, and contributed to the writing of the article; CK, PL, GC, CG and MQNH contributed to the data collection; AD designed the study, participated to the interpretation of the results, and contributed to the writing of the article. All authors provided feedback and made revisions to the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
All methods have been approved by the appropriate ethics committee and were carried out in accordance with relevant guidelines and regulations. We received authorisation from the Department of Reproductive Health of the Ministry of Health in Vietnam, and the eight participating hospitals ethically approved the research. We obtained ethical clearance for the study from the local and institutional review boards from the Centro Rosarino de Estudios Perinatales of Rosario, Argentina (Record Notice No. 1/20), Khon Kaen University in Thailand, the Ethics Committee for Health Research of Burkina Faso (Decision No. 2020-3-038), the Research Project Review Panel (RP2) in the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (WHO study No. A66006), and the French Research Institute for Sustainable Development (coordinator). For all individual interviews, formal written informed consent was obtained from participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary information Table S1.
Pre-defined list used to collect indication for CS (Quali-Dec post-partum survey).
Additional file 2: Supplementary Figure S1.
Flow-chart representing the recruitment of participants into the baseline Quali-Dec survey.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Etcheverry, C., Betrán, A.P., de Loenzien, M. et al. How does hospital organisation influence the use of caesarean sections in low- and middle-income countries? A cross-sectional survey in Argentina, Burkina Faso, Thailand and Vietnam for the QUALI-DEC project. BMC Pregnancy Childbirth 24, 67 (2024). https://doi.org/10.1186/s12884-024-06257-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12884-024-06257-w