Abstract
Background
Although highly heterogeneous among countries, the incidence rates of low birth weight (LBW), preterm birth (PTB), and small for gestational age (SGA) have been increasing globally over the past two decades. To better understand the cause of these secular trends, this study aimed to investigate the effects of age, period, and birth cohort on LBW, PTB, and SGA rates in Shanghai.
Methods
Data from 2,958,695 singleton live births at 24–41 gestational weeks between 2004 and 2020 were obtained for this study. Age-period-cohort models based on Poisson regression were used to evaluate the independent effects of maternal age, delivery period, and maternal birth cohort on the trends in LBW, PTB, and SGA.
Results
The overall prevalence rates of LBW, PTB, and SGA were 2.9%, 4.7%, and 9.3%, respectively, and significant changes were observed (average annual change: + 10.7‰, + 9.1‰, -11.9‰) from 2004 to 2020. Cohort effect increased steadily, from 1960 (risk ratio [RR] = 0.71, 95% confidence interval [CI]: 0.65–0.78) to 1993 (RR = 0.97, 95% CI: 0.94–1.01) for LBW and from 1960 (RR = 0.69, 95% CI: 0.64–0.75) to 2004 (RR = 1.02, 95% CI: 0.94–1.12) for PTB. A strong cohort effect was found with the highest risk of SGA (RR = 1.82, 95% CI: 1.72–1.93) in 1960 and the lowest risk (RR = 0.57, 95% CI: 0.54–0.61) in 2004, compared with the reference cohort of 1985. There was a “U-shaped” maternal age effect on LBW and PTB and a weak period effect on the three birth outcomes.
Conclusions
Our findings suggested a significant independent effect of age, period, and birth cohort on the three birth outcomes. The increasing rates of LBW and PTB motivated us to focus on young and advanced pregnant women. Meanwhile, the prevalence of SGA decreased steadily, illustrating the need for further research on the mechanisms underlying these trends.
Similar content being viewed by others
Background
Adverse birth outcomes (ABOs), including low birth weight (LBW), preterm birth (PTB), small for gestational age (SGA), and stillbirths and miscarriage, were the leading causes of neonatal mortality and morbidity in young children [1,2,3]. Numerous studies reported ABOs as a significant global public health problem over the past two decades [1,2,3]. It was estimated that 12 million PTB and 32 million SGA babies were born in sub-Saharan African and South Asian countries, accounting for most of global ABOs [2, 4]. PTB rates were reported to be approximately 5% in Europe and 18% in Africa [4]. Kaforau et al. also estimated the mean prevalence rates of LBW and PTB in 11 countries in the Pacific region to be 12% and 13%, respectively [5]. South Asia had the highest rate of SGA, where 40% were categorised as SGA [6]. In China, the estimated prevalence rates of LBW, PTB, and SGA were 7.2%, 6.1%, and 12.3%, respectively [2, 7, 8]. These findings demonstrated that ABOs mainly occur in low- and middle-income countries and that ABO incidence rates were highly heterogeneous worldwide [4, 6].
LBW, referring to a birth weight < 2500 g, was a valuable marker of immaturity at delivery [1]. PTB, mostly defined as birth before 37 completed weeks of gestation, commonly led to neonatal mortality and morbidity [9]. SGA means the birth weight falls below a gestational age and sex-specific cut-off point, which was commonly the lowest 10th centile or 2 standard deviations (SDs) below the average [10, 11]. Therefore, SGA can be considered a retrospective indicator of intrauterine growth restriction [3]. Infants born with ABOs were at an increased risk of respiratory distress syndrome, stunting, mental retardation, and early childhood mortality [6].
Since the Reform and Opening up in the 1990s, Chinese people have undergone a dramatic economic and nutritional transition [12]. Along with changes in sociodemographic and individual characteristics of pregnant women, such as shifting to an urban lifestyle (residence in urban areas, sedentary behaviour and increased mental pressure), older age of delivery (≥ 35 years) and a higher level of maternal education, the epidemiology of ABOs has also changed [13, 14]. These changes may contribute to the future burden of chronic diseases, given the potential risks of fetal growth restriction. Previous studies have established how maternal age has a “U-shaped” effect on ABOs [15, 16]. Although studies have examined secular trends in PTB and SGA in China, these analyses used either age or period as an additional factor [8, 14]. Age-period-cohort (APC) analysis was a classic model used to demonstrate trends in health outcomes because it can simultaneously examine the effect of maternal age (age effect, defined as variations caused by physiological changes and social status changes) [17], delivery year (period effect, representing a set of social events and environmental factors such as medical technology and public health policies before outcomes), and maternal birth year (cohort effect, reflecting individual experience and exposure factors during their lifetime) [18]. To better understand the cause of the secular trends, this study aimed to clarify the effects of maternal age, period of delivery, and maternal birth cohort on LBW, PTB, and SGA in Shanghai.
Methods
Study population
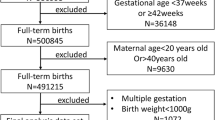
Birth data from the year 2004 to 2020 was collected from the birth registry system of the Shanghai Municipal Centre for Disease Control and Prevention (SCDC), which was established in 2003 and covers all hospitals with authorized delivery services in Shanghai. After excluding twin or multiple births (n = 81,120, 2.62%); those with missing sex, parity, or maternal education data (n = 754, 0.03%); gestational age < 24+0 weeks or > 41+6 weeks (n = 39,277, 1.27%); outliers with ≥ 3 SDs from the gestational age; and sex-specific mean birthweight (n = 18,253, 0.59%), a total of 2,958,695 singleton live births were included in the data analysis. The flow of the study population selection is shown in Supplementary Fig. 1 [see Additional file].
Definition of LBW, PTB, and SGA
The main ABOs investigated in this study included LBW, PTB, and SGA. According to previous studies, LBW was defined as a birth weight of less than 2500 g [5]. PTB was defined as delivery before the 37 completed weeks of gestation (or 259 days) [9], and SGA was defined as a birth weight < 10th centile for gestational age, gender-specific reference [10]. The reference of birth weight percentiles created by Mikolajczyk was adopted in this study [19], which could be adapted to the local population conveniently, without losing the predictive ability of ABOs. After identifying the mean birth weight and SD at 40 weeks, we obtained the birth weight percentiles according to the assumption of normal distribution for gestational age between 24 and 41 weeks. We excluded births at very early or late gestational ages based on the birth weight percentiles used for SGA, as described above. The birth weight percentiles are shown in Supplementary Table 1 [see Additional file].
Statistical analysis
Maternal and neonatal characteristics, including maternal age, educational attainment, gravidity, parity, birth weight, gestational age, and incidence rates of LBW, PTB, and SGA, were analysed over a 5-year period. The age-standardised rate (ASR) was calculated by direct standardisation of the entire study population.
We used APC models to evaluate the net effects of maternal age, delivery period, and maternal birth cohort on the trends in LBW, PTB, and SGA based on the Poisson log-linear regression model. To resolve collinearity among age, period, and cohort (C = P-A), the method proposed by Carstensen was used [20]. Because of the small proportion of younger and older pregnant women (aged < 15, 0.07‰ and > 44, 0.62‰), maternal age < 15 was recoded as 15, and maternal age > 44 was recoded as 44. For visualization of trends, study populations were then categorised into 5-year age groups (15–19, 20–24, 25–29, 30–34, 35–39, and 40–44) and 5-year calendar period groups (2004–2008, 2009–2013, 2014–2018, and 2019–2020) according to their maternal age and date of delivery, respectively, and the birth cohort was computed by subtracting maternal age from the period.
Five sub-models were derived from APC modelling, including age, age–drift, age–cohort, age–period, and APC models. Overall linear trends, interpreted as estimated average annual changes, were extracted from the ‘drift’ variable in age-drift models. The model goodness-of-fit was evaluated based on residual deviance statistics. We examined the significance of pairwise comparisons of the sub-models using χ2 tests. Stratified APC models based on parity were also performed. All statistical tests were two-sided and P-values < 0.05 were considered significant, using the APC-fit function in the Epi package in R (version 4.1.0) [21].
Results
The maternal and neonatal characteristics of the study population according to the delivery period were presented in Table 1. We included 295,8695 singleton live births, of which 52.9% were males and 47.1% were females. Maternal age at childbearing increased significantly, with a mean age of 26.9 years in 2004, which increased to 30.1 years in 2020. The percentage of highly educated mothers increased over time, whereas the proportion of multiparous mothers increased from a quarter to more than a third. However, there was no clear trend of change in birth weight during this period, although a very small decrease was observed.
In the years from 2004 to 2020, the overall prevalence rates of LBW, PTB, and SGA were 2.9%, 4.7%, and 9.3%, respectively,. After standardisation, significant changes were observed in the trends of LBW, PTB, and SGA. The ASR for LBW increased from 2.8% in 2004 to 3.4% in 2020 with an average annual increase of 10.7‰ (95% confidence interval [CI], 9.2‰-12.2‰) and the ASR for PTB increased from 4.7% to 5.3% (annual increase: 9.1‰, 95% CI, 7.9‰-10.2‰), while the ASR for SGA declined from 9.7% to 8.6% (annual decrease: 11.9‰, 95% CI, 11.0‰-12.7‰). These trends are illustrated in Fig. 1.
Specific trends for LBW, PTB, and SGA
To examine how the incidence rates of LBW differed by age and cohort, specific rates were plotted in Fig. 2. Age-specific incidence rates initially fell before age 25 years, then rose, resembling a “U-shaped” curve. Cohort-specific rates declined with the birth cohort, increased thereafter in the 15–24 years and 40–44 years age groups, and showed a steady upward trend in the 25–39 years age group.
Figure 3 showed the trends of PTB in different age, period, and cohort groups. Age-specific incidence rates displayed the same “U-shaped” variation in PTB, whereas cohort-specific rates increased steadily in all age groups, except the 15–19 years age group.
The incidence rates of SGA according to age, period, and cohort were shown in Fig. 4. Overall, age-specific rates initially exhibited a decrease from age 15 to 35 years and then rose slightly after the age of 35 years. Rates among women aged 25–34 years remained stable over the entire period. However, for women in the younger or older age groups, the later the maternal birth cohort, the lower the incidence rate of SGA.
APC effects for LBW, PTB, and SGA
Figure 5 showed the estimated age, period, and birth cohort effects. Maternal age effect (the left curves) showed a changing trend in the incidence rate, while cohort and period effects (the middle and right curves) were illustrated by risk ratios (RR). Variation trend in the three effects indicates that age and birth cohort were the main risk factors for LBW, PTB, and SGA, whereas the period was relatively less impactful. The APC effects were similar among LBW and PTB births but different from those in SGA infants. The effect of age on the trends in LBW and PTB displayed a “U-shaped” curve, reaching its lowest value in the mid-20 s age group. The RR of birth cohort on LBW and PTB increased steadily before 1985 and then remained stable or declined slightly. When compared with the mothers born in 1985, those born in 1960 had the lowest RRs of 0.71 (95% CI, 0.65–0.78) for LBW, and 0.69 (95% CI, 0.64–0.75) for PTB. In contrast, a dramatic reduction in risk was observed in both age and cohort effects in SGA infants. The RRs of SGA decreased from 1.82 (95% CI, 1.72–1.93) to 0.57 (95% CI, 0.54–0.61) during the 40 years' cohort, compared with the reference cohort of those born in 1985. The RR of period effect on LBW, PTB, and SGA fluctuated around 1, without any obvious trend.
Age-period-cohort influences on trends in (A) LBW, (B) PTB, and (C) SGA. The left curve showed the fitted age-specific incidence at the reference cohort (1985), the middle curve was the risk ratios of cohorts relative to the reference cohort (1985), and the right curve was the risk ratios of period conditional on the estimated age and cohort effects
Stratified APC models performed separately by primiparas and multiparas showed the modification effects of parity on LBW, PTB, and SGA (see Additional file). The left curves of LBW incidence among different maternal age groups exhibited a similar “U-shaped” trend between primiparas and multiparas, where the incidence of LBW was reaching its nadir in the mid-20 s age group. When compared to the primiparous mothers born in 1985, the RR of cohort in LBW remarkably increased from 0.57 (95% CI, 0.51–0.65) in 1960 and then remained stable, whereas RR for multiparous mothers increased subtly from 0.88 (95% CI, 0.78–0.99) in the years from 1962 to 1985, but then fell to 0.62 (95% CI, 0.52–0.76) in 2003. The same modification effects of parity on APC models were also observed among PTB delivery. The incidence of PTB dropped to the lowest range around age 25, and the RRs began the rise from 1960 (RR, primiparas: 0.81, 95% CI, 0.73, 0.91; multiparas: 0.68, 95% CI, 0.61, 0.76) in both primiparous and multiparous mothers. When it came to SGA births, the incidence dropped dramatically with maternal age among both primiparous and multiparous mothers, and then increased slightly at advanced age (> 28) only in primiparous mothers. The RR of cohort increased from 0.72 (95% CI, 0.67–0.79) in 1960 before 1985 and then fell to 0.60 (95% CI, 0.56–0.64) in 2004 in primiparous mothers. Meanwhile, the RR of cohort maintained a consecutive decreasing trend from 3.35 (95% CI, 3.03–3.70) in 1960 to 0.46 (95% CI, 0.41–0.52) in 2003 among multiparous mothers. Overall, when compared with primiparous mothers aged over 25 years, the multiparous mothers had a lower incidence rate of SGA at the same age. These findings were presented in Supplementary Fig. 1, Supplementary Fig. 2 and Supplementary Fig. 3, respectively (see Additional file).
The age-period-cohort effects on the incidence rates of LBW, PTB, and SGA were evaluated using APC Poisson regression model. Comparisons of APC sub-models suggested that the full APC models were optimum, and incidence rates were significantly influenced by age and cohort effects when examining changes in residual deviance (Table 2). Age, period, and cohort effects, and their corresponding 95% Cis were described in Table 3.
Discussion
In this retrospective study based on data obtained from the birth registry in Shanghai from 2004 to 2020, we investigated the prevalence of LBW, PTB, and SGA, and observed the secular trends. We examined the independent effects of maternal age, delivery period, and maternal birth cohort on the trends in LBW, PTB, and SGA births, and further explored the modification effect by parity. The “U-shaped” relationship between maternal age and LBW/PTB was examined in this study. Mothers born before the 1980s had a lower incidence of PTB than those born in more recent years. Meanwhile, the risk of SGA declined with advancing age and in cohorts since 1960. However, there were no obvious fluctuant trends in the three birth outcomes by period, suggesting that the observed temporal changes were mostly influenced by the maternal birth cohort.
The estimated prevalence rates of LBW, PTB, and SGA in Shanghai were lower than the national prevalence, and close to that of other developed cities or regions in China. For instance, in urban districts in Beijing, the percentage of LBW fluctuated around 4.0% [22], and the estimated rate of LBW and PTB in the Guangdong province was 4.14% and 4.16%, respectively [23]. The prevalence of SGA was 10.1% in 13 developed cities in China [24]. Our findings also indicate a significantly rising trend of LBW and PTB, and a declining trend of SGA, which is consistent with previous studies [8, 13, 25].
Our findings on the association between maternal age, birth cohort, and LBW/PTB are also consistent with previous studies [26, 27]. Extremes of maternal age increased the incidence of LBW/PTB, suggesting that natural ageing or social environments, or an interaction of both, should account for the association. Generally, older women were believed to have more obstetric complications, which in turn was a high-risk factor for LBW/PTB. Young women < 18 years old were also more likely to have a higher risk of ABOs because of physical immaturity and irregular prenatal care, especially for teenage pregnancies [28, 29].
We noted that the birth cohort (maternal experience and background of growth) would remarkably affect LBW/PTB. With the postponement of the birth year among women born before 1985, the risk of LBW/PTB increased, which may reflect maternal nutrition, environmental exposure, obstetric interventions, and pregnancy complications [30,31,32,33]. Although the improving maternal socioeconomic status (SES) and nutritional status decreased the risk of LBW/PTB, the consequent air pollutants, obesity and the stresses and strains of life would contribute to the increasing trend [31, 34, 35]. Previous research has demonstrated the casual and dose–response relationship between active/passive maternal smoking and alcohol consumption during pregnancy, and the risks of LBW/PTB [36,37,38,39]. More than an estimated 20% of women of childbearing age (18–39) and 6.5% of pregnant women consumed alcohol in China, and the prevalence of consuming alcohol had increased among both men and women since 2002 [40,41,42]. A nationwide cross-sectional study estimated that, of the pregnant women in China, 0.56% were smokers and 4.43% were ex-smokers [43]. Studies have shown that maternal exposure to fine particulate matter was associated with LBW/PTB [44]. The increasing use of assisted reproductive technology may be another factor contributing to the rise in LBW/PTB [45]. Also, prenatal complications may increase the risk of LBW/PTB [32, 33]. An observational study based on national registry revealed that the proportion of women with prenatal complications and medical diseases increased from 14.4% to 23.8% 66%and from 3.5% to 11.2%, respectively, from 2012 to 2018 [13].
Interestingly, advanced maternal age was associated with lower rates of SGA in this study, while prior studies have reported conflicting results [18, 46]. The debatable association could be attributed to several reasons: (1) Classifying SGA by various fetal growth curves or birth weight percentiles would lead to differences in the prevalence of SGA [24]. The birth weight percentiles based on healthy populations, could be more effective for recognizing neonates with intrauterine growth restriction or infant mortality [47, 48]. (2) Both domestic and international studies have indicated that maternal age over 40 years was associated with higher risk of SGA, while relatively less advanced maternal age (30–39) was associated with lower risk, compared with maternal age of those in their twenties [49, 50].The small proportion of pregnant women aged over 40 years in our study made it difficult to observe the independent effect of advanced maternal age on SGA. (3) Ethnic differences in the risk factors for SGA across populations [18, 51].
Although it is difficult to explain the decreased trend of SGA, there are some theories to explain this. Firstly, we hypothesised that mothers could have benefited from accessible prenatal interventions and nutritional improvements. Due to the rapid developments in SES, the nutritional and health status of urban residents has greatly improved, and more fertility policies have been promoted [13]. Previous studies in China showed that the decreasing trend of SGA was accompanied by a significant reduction in caesareans and an increasing frequency of antenatal visits over the past decade, meaning that women born in more recent cohorts were unlikely to have SGA births [13]. Secondly, the specific contribution of factors associated with SGA have changed over time [52]. For instance, income inadequacy and being a recent immigrant were risk factors unique to SGA [34, 53], and poor maternal mental health was a risk factor specific to LBW/PTB [54]. Meanwhile, maternal education and parity were associated with both SGA and PTB, and the effect on SGA was greater than PTB [53, 55], which could be the interpretation of the different trend observed in primiparous mothers. Finally, SGA was essentially a different conception from other ABOs. LBW, the traditionally used metric of intrauterine nutrition, overlapped a great deal with PTB, and those infants heavier than 2500 g might also be premature [56]. Hence, identifying newborn babies with intrauterine growth restriction by means of LBW/PTB could be arbitrary [56]. In settings with a high proportion of SGA births but neither LBW nor PTB and the high mortality risk of term-SGA in Asia, SGA would be more sensitive and suitable for identifying intrauterine growth restriction and tracking neonatal health [3, 56, 57]. In summary, SGA and LBW/PTB were distinct but related pregnancy outcomes, and the risk factors related to these outcomes had both differences and similarities, which could account for the different trends of SGA.
It is worth noting that parity might play a role in the association between maternal age and SGA, which has been reported in prior related studies [50, 53]. The slight rising rates in advanced primiparas was consistent with a study conducted in America, which identified that, among primiparous mothers, maternal age ≥ 30 had higher rates of SGA compared with those aged 20–29 years [50]. In contrast to LBW and PTB, we found a reduced incidence rate of SGA in multiparas at the same age, compared to primiparas over 25 years of age, suggesting a different pathogenesis for LBW, PTB and SGA. A retrospective study conducted in China also found that, compared with primiparas aged 25–29, multiparas aged ≥ 35 were at lower risk, examining the combined effects of maternal age and parity on SGA [53]. One potential explanation was that LBW and PTB were more likely to link with placental and oocyte defectivity, whereas SGA might mainly attribute to intrauterine nutritional deficiency [53]. To sum up, our results suggested that parity may affect advanced maternal age and the risk of SGA, which might be due to the lower placenta blood stream and smaller uterine cavity in primiparous mothers [58, 59]. Regarding birth cohort, however, the rising trend beginning from 1960 to 1985 in primiparas is more difficult to explain, and additional studies are needed to explore it.
To the best of our knowledge, several studies have identified maternal APC effect on PTB and SGA [18, 26, 60, 61]. Although our study aimed to analyse the temporal influence of LBW, PTB, and SGA births, several important limitations should be considered. First, due to the lack of other determinants of ABOs, including maternal smoking, gestational weight gain, pregnancy complications, and paternal factors, we were unable to elucidate the mechanisms of maternal age and cohort effects on ABOs [36, 62]. Second, the estimated gestational age, based on the first date of a woman's last menstrual period and not on ultrasound-based methods, may not accurately classify PTB/SGA infants. Although misclassification might influence the results mentioned above, it was unlikely to contribute to the temporal trends entirely. Third, the data was collected from a single birth registry database, which does not represent the nationwide population. However, Shanghai is a megacity with a large population (almost 25 million), which could be representative of the other developed cities in China and other developed Asian countries.
Our study demonstrates independent effects of maternal age, delivery period, and maternal birth cohort on trends in LBW, PTB, and SGA. Within the context of the universal 2-child policy, more women of advanced age prefer to raise a second child in China [63]. Although older women obtained better education and higher SES through social selection, they were more likely to suffer from obstetric complications. Both young and advanced mothers are more likely to have LBW/PTB; accordingly, more prenatal care and public education should be provided to younger and older pregnant women.
Conclusions
In summary, we found strong maternal age and birth cohort effects on LBW, PTB, and SGA, suggesting that younger and older pregnant women should be key target population groups for perinatal care and treatment. Moreover, there was a continuous increase in the incidence rates of LBW and PTB, encouraging the need to formulate public health intervention and prevention policies in the developed areas of China. Among women in the same age groups, those born in more recent years had a lower risk of SGA. More knowledge of how these trends were associated with LBW, PTB, and SGA in China is required.
Availability of data and materials
The datasets used and analysed in the study are available from the corresponding author upon reasonable request.
Abbreviations
- ABOs:
-
Adverse birth outcomes
- LBW:
-
Low birth weight
- PTB:
-
Preterm birth
- SGA:
-
Small for gestational age
- SCDC:
-
Shanghai Municipal Centre for Disease Control and Prevention
- APC:
-
Age-period-cohort
- CI:
-
Confidence interval
- RR:
-
Risk ratio
- SDs:
-
Standard deviations
- SES:
-
Socioeconomic status
References
Blencowe H, Krasevec J, de Onis M, Black RE, An X, Stevens GA, Borghi E, Hayashi C, Estevez D, Cegolon L, et al. National, regional, and worldwide estimates of low birthweight in 2015, with trends from 2000: a systematic analysis. Lancet Glob Health. 2019;7(7):e849–60.
Chawanpaiboon S, Vogel JP, Moller AB, Lumbiganon P, Petzold M, Hogan D, Landoulsi S, Jampathong N, Kongwattanakul K, Laopaiboon M, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health. 2019;7(1):e37–46.
Katz J, Lee AC, Kozuki N, Lawn JE, Cousens S, Blencowe H, Ezzati M, Bhutta ZA, Marchant T, Willey BA, et al. Mortality risk in preterm and small-for-gestational-age infants in low-income and middle-income countries: a pooled country analysis. Lancet (London, England). 2013;382(9890):417–25.
Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, Adler A, Vera Garcia C, Rohde S, Say L, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet (London, England). 2012;379(9832):2162–72.
Kaforau LSK, Tessema GA, Jancey J, Dhamrait G, Bugoro H, Pereira G. Prevalence and risk factors of adverse birth outcomes in the Pacific Island region: A scoping review. Lancet Reg Health West Pac. 2022;21: 100402.
Lawn JE, Blencowe H, Oza S, You D, Lee AC, Waiswa P, Lalli M, Bhutta Z, Barros AJ, Christian P, et al. Every Newborn: progress, priorities, and potential beyond survival. Lancet (London, England). 2014;384(9938):189–205.
Chen Y, Wu L, Zhang W, Zou L, Li G, Fan L. Delivery modes and pregnancy outcomes of low birth weight infants in China. J Perinatol. 2016;36(1):41–6.
He H, Miao H, Liang Z, Zhang Y, Jiang W, Deng Z, Tang J, Liu G, Luo X. Prevalence of small for gestational age infants in 21 cities in China, 2014–2019. Sci Rep. 2021;11(1):7500.
Vogel JP, Chawanpaiboon S, Moller AB, Watananirun K, Bonet M, Lumbiganon P. The global epidemiology of preterm birth. Best Pract Res Clin Obstet Gynaecol. 2018;52:3–12.
Wilcox AJ, Cortese M, McConnaughey DR, Moster D, Basso O. The limits of small-for-gestational-age as a high-risk category. Eur J Epidemiol. 2021;36(10):985–91.
Cutfield W, Ayyavoo A. The auxological and metabolic consequences for children born small for gestational age. Indian J Pediatr. 2021;88(12):1235–40.
Popkin BM, Du S. Dynamics of the nutrition transition toward the animal foods sector in China and its implications: a worried perspective. J Nutr. 2003;133(11 Suppl 2):3898s–906s.
Deng K, Liang J, Mu Y, Liu Z, Wang Y, Li M, Li X, Dai L, Li Q, Chen P, et al. Preterm births in China between 2012 and 2018: an observational study of more than 9 million women. Lancet Glob Health. 2021;9(9):e1226–41.
Xu H, Dai Q, Xu Y, Gong Z, Dai G, Ding M, Duggan C, Hu Z, Hu FB. Time trends and risk factor associated with premature birth and infants deaths due to prematurity in Hubei Province, China from 2001 to 2012. BMC Pregnancy Childbirth. 2015;15:329.
Weng YH, Yang CY, Chiu YW. Risk assessment of adverse birth outcomes in relation to maternal age. PLoS ONE. 2014;9(12): e114843.
Zhang C, Yan L, Qiao J. Effect of advanced parental age on pregnancy outcome and offspring health. J Assist Reprod Gen. 1969;2022:39.
Reither EN, Hauser RM, Yang Y. Do birth cohorts matter? Age-period-cohort analyses of the obesity epidemic in the United States. Soc Sci Med. 2009;69(10):1439–48.
Margerison-Zilko C. The contribution of maternal birth cohort to term small for gestational age in the United States 1989–2010: an age, period, and cohort analysis. Paediatr Perinat Epidemiol. 2014;28(4):312–21.
Mikolajczyk RT, Zhang J, Betran AP, Souza JP, Mori R, Gülmezoglu AM, Merialdi M. A global reference for fetal-weight and birthweight percentiles. Lancet (London, England). 2011;377(9780):1855–61.
Carstensen B. Age-period-cohort models for the Lexis diagram. Stat Med. 2007;26(15):3018–45.
Carstensen B, Plummer M, Laara E, Hills M (2021). Epi: A Package for Statistical Analysis in Epidemiology. R package version 2.44, https://CRAN.R-project.org/package=Epi.
Shan X, Chen F, Wang W, Zhao J, Teng Y, Wu M, Teng H, Zhang X, Qi H, Liu X, et al. Secular trends of low birthweight and macrosomia and related maternal factors in Beijing, China: a longitudinal trend analysis. BMC Pregnancy Childbirth. 2014;14:105.
Miao H, Li B, Li W, Yao F, Chen Y, Chen R, Lin J, Wu Y, Guo P, Zhao Q. Adverse birth outcomes in Guangdong province, China, 2014–2017: a spatiotemporal analysis of 2.9 million births. BMJ Open. 2019;9(11):e030629.
Zhang YQ, Li H, Zong XN, Wu HH. Comparison of updated birth weight, length and head circumference charts by gestational age in China with the INTERGROWTH-21st NCSS charts: a population-based study. World J Pediatr : WJP. 2023;19(1):96–105.
Li C, Liang Z, Bloom MS, Wang Q, Shen X, Zhang H, Wang S, Chen W, Lin Y, Zhao Q, et al. Temporal trends of preterm birth in Shenzhen, China: a retrospective study. Reprod Health. 2018;15(1):47.
Lu J, Wei D, Shen S, Xia X, He J, Sun Y, Lam KBH, Bao W, Xia H, Qiu X. Increasing trends in incidence of preterm birth among 2.5 million newborns in Guangzhou, China, 2001 to 2016: an age-period-cohort analysis. BMC Public Health. 2020;20(1):1653.
Ananth CV, Misra DP, Demissie K, Smulian JC. Rates of preterm delivery among Black women and White women in the United States over two decades: an age-period-cohort analysis. Am J Epidemiol. 2001;154(7):657–65.
Sebayang SK, Dibley MJ, Kelly PJ, Shankar AV, Shankar AH. Determinants of low birthweight, small-for-gestational-age and preterm birth in Lombok, Indonesia: analyses of the birthweight cohort of the SUMMIT trial. Tro Med Int Health : TM & IH. 2012;17(8):938–50.
Honorato DJP, Fulone I, Silva MT, Lopes LC. Risks of adverse neonatal outcomes in early adolescent pregnancy using group prenatal care as a strategy for public health policies: a retrospective cohort study in Brazil. Front Public Health. 2021;9: 536342.
Keen CL, Clegg MS, Hanna LA, Lanoue L, Rogers JM, Daston GP, Oteiza P, Uriu-Adams JY. The plausibility of micronutrient deficiencies being a significant contributing factor to the occurrence of pregnancy complications. J Nutr. 2003;133(5 Suppl 2):1597s–605s.
Shah PS, Balkhair T. Air pollution and birth outcomes: a systematic review. Environ Int. 2011;37(2):498–516.
Lei LL, Lan YL, Wang SY, Feng W, Zhai ZJ. Perinatal complications and live-birth outcomes following assisted reproductive technology: a retrospective cohort study. Chin Med J. 2019;132(20):2408–16.
He M, Sun X, Wang C, Sui Y. Analysis of the risk of complications during pregnancy in pregnant women with assisted reproductive technology: a retrospective study using registry linkage from 2013 to 2018 in Shanghai, China. BMC Pregnancy Childbirth. 2022;22(1):526.
Bushnik T, Yang S, Kaufman JS, Kramer MS, Wilkins R. Socioeconomic disparities in small-for-gestational-age birth and preterm birth. Health Rep. 2017;28(11):3–10.
Kozuki N, Katz J, Lee AC, Vogel JP, Silveira MF, Sania A, Stevens GA, Cousens S, Caulfield LE, Christian P, et al. Short maternal stature increases risk of small-for-gestational-age and preterm births in low- and middle-income countries: individual participant data meta-analysis and population attributable fraction. J Nutr. 2015;145(11):2542–50.
Chan A, Keane RJ, Robinson JS. The contribution of maternal smoking to preterm birth, small for gestational age and low birthweight among Aboriginal and non-Aboriginal births in South Australia. Med J Aust. 2001;174(8):389–93.
McCowan LM, Dekker GA, Chan E, Stewart A, Chappell LC, Hunter M, Moss-Morris R, North RA. Spontaneous preterm birth and small for gestational age infants in women who stop smoking early in pregnancy: prospective cohort study. BMJ. 2009;338: b1081.
Hamadneh S, Hamadneh J. Active and passive maternal smoking during pregnancy and birth outcomes: a study from a developing country. Ann Glob Health. 2021;87(1):122.
Patra J, Bakker R, Irving H, Jaddoe VW, Malini S, Rehm J. Dose-response relationship between alcohol consumption before and during pregnancy and the risks of low birthweight, preterm birth and small for gestational age (SGA)-a systematic review and meta-analyses. BJOG. 2011;118(12):1411–21.
Fang YH, He YN, Bai GY, Zhao WH. Prevalence of alcohol drinking and influencing factors in female adults in China, 2010–2012. Zhonghua Liu Xing Bing Xue Za Zhi. 2018;39(11):1432–7.
Popova S, Lange S, Probst C, Gmel G, Rehm J. Estimation of national, regional, and global prevalence of alcohol use during pregnancy and fetal alcohol syndrome: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(3):e290–9.
Zhang J, Casswell S, Cai H. Increased drinking in a metropolitan city in China: a study of alcohol consumption patterns and changes. Addiction (Abingdon, England). 2008;103(3):416–23.
Hu K, Zou S, Zhang CJ, Wu H, Akinwunmi B, Wang Z, Ming WK. Health-related quality of life among pregnant women with pre-pregnancy smoking and smoking cessation during pregnancy in China: national cross-sectional study. JMIR Public Health Surveill. 2022;8(1): e29718.
Liu A, Qian N, Yu H, Chen R, Kan H. Estimation of disease burdens on preterm births and low birth weights attributable to maternal fine particulate matter exposure in Shanghai, China. Sci Total Environ. 2017;609:815–21.
Yu H, Liang Z, Cai R, Jin S, Xia T, Wang C, Kuang Y. Association of adverse birth outcomes with in vitro fertilization after controlling infertility factors based on a singleton live birth cohort. Sci Rep. 2022;12(1):4528.
Rotem R, Rottenstreich M, Prado E, Baumfeld Y, Yohay D, Pariente G, Weintraub AY. Trends of change in the individual contribution of risk factors for small for gestational age over more than 2 decades. Arch Gynecol Obstet. 2020;302(5):1159–66.
Ferdynus C, Quantin C, Abrahamowicz M, Platt R, Burguet A, Sagot P, Binquet C, Gouyon JB. Can birth weight standards based on healthy populations improve the identification of small-for-gestational-age newborns at risk of adverse neonatal outcomes? Pediatrics. 2009;123(2):723–30.
Ding G, Tian Y, Zhang Y, Pang Y, Zhang JS, Zhang J. Application of a global reference for fetal-weight and birthweight percentiles in predicting infant mortality. BJOG. 2013;120(13):1613–21.
L SI, H B, Eo O, M CB. Maternal risk factors for small-for-gestational-age newborns in Mexico: analysis of a nationwide representative cohort. Front Public Health. 2021;9:707078. https://doi.org/10.3389/fpubh.2021.707078.
Palatnik A, De Cicco S, Zhang L, Simpson P, Hibbard J, Egede LE. The association between advanced maternal age and diagnosis of small for gestational age. Am J Perinatol. 2020;37(1):37–43.
Garcia R, Ali N, Guppy A, Griffiths M, Randhawa G. Ethnic differences in risk factors for adverse birth outcomes between Pakistani, Bangladeshi, and White British mothers. J Adv Nurs. 2020;76(1):174–82.
Heaman M, Kingston D, Chalmers B, Sauve R, Lee L, Young D. Risk factors for preterm birth and small-for-gestational-age births among Canadian women. Paediatr Perinat Epidemiol. 2013;27(1):54–61.
Lin L, Lu C, Chen W, Li C, Guo VY. Parity and the risks of adverse birth outcomes: a retrospective study among Chinese. BMC Pregnancy Childbirth. 2021;21(1):257.
Voit FAC, Kajantie E, Lemola S, Räikkönen K, Wolke D, Schnitzlein DD. Maternal mental health and adverse birth outcomes. PLoS ONE. 2022;17(8): e0272210.
Ruiz M, Goldblatt P, Morrison J, Kukla L, Švancara J, Riitta-Järvelin M, Taanila A, Saurel-Cubizolles MJ, Lioret S, Bakoula C, et al. Mother’s education and the risk of preterm and small for gestational age birth: a DRIVERS meta-analysis of 12 European cohorts. J Epidemiol Community Health. 2015;69(9):826–33.
Lee AC, Katz J, Blencowe H, Cousens S, Kozuki N, Vogel JP, Adair L, Baqui AH, Bhutta ZA, Caulfield LE, et al. National and regional estimates of term and preterm babies born small for gestational age in 138 low-income and middle-income countries in 2010. Lancet Glob Health. 2013;1(1):e26-36.
Lee AC, Kozuki N, Cousens S, Stevens GA, Blencowe H, Silveira MF, Sania A, Rosen HE, Schmiegelow C, Adair LS, et al. Estimates of burden and consequences of infants born small for gestational age in low and middle income countries with INTERGROWTH-21(st) standard: analysis of CHERG datasets. BMJ. 2017;358: j3677.
Prefumo F, Bhide A, Sairam S, Penna L, Hollis B, Thilaganathan B. Effect of parity on second-trimester uterine artery Doppler flow velocity and waveforms. Ultrasound Obstetr Gynecol. 2004;23(1):46–9.
Canteiro R, Bahamondes MV, dos Santos FA, Espejo-Arce X, Marchi NM, Bahamondes L. Length of the endometrial cavity as measured by uterine sounding and ultrasonography in women of different parities. Contraception. 2010;81(6):515–9.
Li H, Shi Y, Ahmed Z, Khan A, Xu K, Yin X. Nawsherwan, Zhang H: Secular trends and age-period-cohort effect on adverse perinatal outcomes in Hubei, China (2011–2019). Sci Rep. 2022;12(1):22558.
Ananth CV, Balasubramanian B, Demissie K, Kinzler WL. Small-for-gestational-age births in the United States: an age-period-cohort analysis. Epidemiology. 2004;15(1):28–35.
Li J, Qiu J, Lv L, Mao B, Huang L, Yang T, Wang C, Liu Q. Paternal factors and adverse birth outcomes in Lanzhou, China. BMC Pregnancy Childbirth. 2021;21(1):19.
Li HT, Xue M, Hellerstein S, Cai Y, Gao Y, Zhang Y, Qiao J, Blustein J, Liu JM. Association of China’s universal two child policy with changes in births and birth related health factors: national, descriptive comparative study. BMJ. 2019;366: l4680.
Acknowledgements
The authors are grateful to all those who contributed to project implementation, including researchers, project coordinators, data collectors, and data clerks.
The authors would like to thank Editage (www.editage.cn) for English language editing.
Funding
This work was funded by the National Natural Science Foundation of China (82003486), the World Health Organization and the US National Institute on Aging through Interagency Agreements (OGHA 04034785; YA1323-08-CN-0020; Y1-AG-1005-01) and through a research grant (R01-AG034479), the National Key R &D Program of China (2022YFC3600801), and the Technical standard project of Shanghai Scientific and Technological Innovation Action Plan (22DZ2206000). The funding sources had no influence on the content of and no role in the writing of this work.
Author information
Authors and Affiliations
Contributions
Study concept and design: R.Z. and H.Y.; Methodology: H.Y. and R.Z.; Formal Analysis: R.Z. and H.Y.; Data Curation: H.Y., R.C. and S.J.; Writing—Original Draft: H.Y.; Writing—Review & Editing: H.Y., R.Z., N.Q. and L.C.; Project administration: C.W. and F.W.; Funding acquisition: H.Y. and C.W.; Critical revision of the manuscript for important intellectual content: All authors.
Authors’ information
Rongfei Zhou, 20211020005@fudan.edu.cn
Huiting Yu, yuhuiting@scdc.sh.cn.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was a retrospective, population-based cohort study approved by the Ethics Review Committee of the Shanghai Municipal Centre for Disease Control and Prevention (SCDC), and all methods were carried out following relevant guidelines and regulations. As only de-identified routinely collected surveillance data were used, the requirement to obtain informed consent was waived.
Name of the ethics committee: Shanghai Municipal Centre for Disease Control and Prevention Ethics Review Committee.
The relevant legislation: Informed consent may be waived if information and/or biological specimens obtained from health surveillance are reused without further tracking of subject information.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Table 1.
Birthweight percentiles by sex and gestational age. Supplementary Figure 1. Flowchart of study population selection. Supplementary Figure 2. Age-period-cohort influences on trends in LBW by parity. Supplementary Figure 3. Age-period-cohort influences on trends in PTB by parity. Supplementary Figure 4. Age-period-cohort influences on trends in SGA by parity.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhou, R., Yu, H., Qian, N. et al. Secular trends of low birth weight, preterm birth, and small for gestational age in Shanghai from 2004 to 2020: an age-period-cohort analysis. BMC Pregnancy Childbirth 23, 540 (2023). https://doi.org/10.1186/s12884-023-05799-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12884-023-05799-9