Abstract
Objective
To study the combined effect of gestational diabetes mellitus (GDM) and maximum level of maternal serum total bile acid (TBA) on the incidence of adverse pregnancy outcomes in women with intrahepatic cholestasis of pregnancy (ICP).
Methods
This was an observational study with 724 women with ICP. Perinatal outcomes were compared by the presence of GDM. Logistic regression was used to assess the independent and multiplicative interactions of GDM and maximum maternal serum TBA on adverse pregnancy outcomes. Additive interactions were calculated using an Excel sheet developed by Andersson to calculate relative excess risks.
Results
The incidence of GDM in patients with ICP was 21.55%. Maternal age, pre-pregnancy weight, parity, and gravidity were positively correlated with GDM. Hypertensive disorders of pregnancy (HDP) and fetal distress rates were higher in the GDM vs. non-GDM group. There were no significant differences in biochemical outcomes (i.e., Triglyceride (TG), low density lipoprotein (LDL), alanine aminotransferase (ALT), aspartate aminotransferase (AST) and total bile acid (TBA)) between the two groups. In terms of adverse pregnancy outcomes, GDM was only associated with maximum TBA concentration for cesarean section. No additive or pairwise interactions were detected between GDM and maximum TBA concentration and HDP, PPH, preterm delivery, LGA, SGA, and cesarean section.
Conclusion
GDM independently contributes to adverse pregnancy outcomes among women with ICP. However, the combined effects of GDM and maximum TBA concentration on adverse pregnancy outcomes do not appear to be multiplicative or additive.
Similar content being viewed by others
Background
Intrahepatic cholestasis of pregnancy (ICP), also known as pregnancy-specific liver disease, is characterized by unexplained maternal pruritus, elevated serum total bile acid (TBA) levels, and abnormal serum liver tests. Its diagnosis also involves ruling out other liver diseases that may lead to elevated bile acids [1]. The incidence of ICP varies between ethnic groups, ranging from 0.08–27.6% [2]. ICP has a multifactorial etiology with environmental, endocrine, and genetic contributions [3]. ICP is associated with various adverse pregnancy outcomes, including spontaneous preterm labour, fetal hypoxia, meconium-stained liquor, and stillbirth [4]. Moreover, severe ICP (defined as maternal serum bile acid levels > 40 µmol/L) has been reported to lead to complex pregnancy outcomes [5].
Gestational diabetes mellitus (GDM) is a common pregnancy-related metabolic diseases diagnosed when hyperglycemia first appears during pregnancy [6]. The pathogenesis of GDM is unknown. Heredity [7]and environmental [8]factors have been shown to play an important role in the occurrence of GDM, as has chronic inflammation [9], abnormal lipid metabolism [10], and insulin resistance [11]. It is worth noting that GDM has an extensive and far-reaching impact on the health of mothers and offspring [12]. Adverse pregnancy outcomes include macrosomia, neonatal hypoglycemia, and hyperbilirubinemia [13], while long-term effects are related to cardiovascular and metabolic diseases [14].
Research has established that abnormal bile acid levels can lead to glucose and lipid metabolism disorders [15]. Furthermore, a growing body of literature indicates that women with ICP are more likely to develop GDM [16]. Our study aims to explore the interactive effects of GDM and maximum TBA concentration on adverse pregnancy outcomes in women with ICP.
Methods
Patients
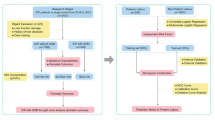
This retrospective and observational study included all women who were followed at the Fujian Maternity and Child Health Hospital (China) between January 2013 and December 2021, with singleton pregnancies that extended to or beyond 24 weeks of gestation. A total of 858 pregnant women with ICP were screened, of which 134 were excluded due to delivery before 28 weeks (n = 5), diagnosis of PGDM which had diabetes before pregnancy (n = 6), and missing key data (n = 123). Thus, our study included 724 women: 156 with ICP and GDM (study group) and 568 with ICP and non-GDM pregnancies (control group). ICP was diagnosed based on new-onset pruritus with a TBA level > 10 µmol/L and the absence of any additional liver diseases. The diagnostic criteria for GDM were based on the National Health and Family Planning Commission of the People’s Republic of China guidelines. When the 75 g OGTT results met or exceeded the following plasma glucose levels at the noted time-points, the women were diagnosed with GDM: 0 h, 5.1 mmol/L; 1 h, 10.0 mmol/L; and 2 h, 8.5 mmol/L. A 75 g OGTT was performed between the 24th and 28th weeks of gestation for all pregnant women who had not previously been diagnosed with diabetes. The study group consisted of all ICP pregnancies with GDM, as demonstrated by a positive OGTT status on antenatal screening and the control group consisted of the ICP pregnancies whose blood glucose was at normal level. Figure 1 shows a flow chart of study from total results to the final inclusion or exclusion.
This retrospective study involving human participants were reviewed and approved by Ethics committee of the Fujian Maternity and Children Hospital (2021KLRD631). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Inclusion and exclusion criteria
The inclusion criteria of this trial were as follows: age ≥ 18 years, singleton pregnancy, and ICP status (as reflected by TBA level on antenatal screening). The exclusion criteria were as follows: patients with pre-existing or a history of medical conditions, including chronic hypertension, diabetes, or other cardiovascular, endocrinological, urogenital, gastrointestinal, autoimmune, or oncological disease; thrombophilia; multiple gestation; or in active labor or patients on ursodeoxycholic acid (UDCA) treatment were excluded from the study. Also excluded were patients who used medications that could interfere with blood glucose, such as glucocorticoids, patients with pre-gestational obesity (body mass index [BMI] > 30 kg/m2), and smokers.
Outcome measurements
We reviewed the records of 724 women who had delivered their infants during the study period and recorded the following parameters: baseline characteristics of the included patients, including maternal age (years), gestational age (weeks; determined from the fetal crown–rump length), gravidity, parity, pre-pregnancy weight (kg), pre-pregnancy BMI (kg/m2) and gestational weight gain (kg); biochemical index before delivery of the included patients including Triglyceride (TG, mmol/L), low density lipoprotein (LDL, mmol/L), alanine aminotransferase (ALT, IU/L), aspartate aminotransferase (AST, IU/L) and total bile acid (TBA, µmol/L). TBA concentration in this study was measured by fasting maternal serum. Maximum level of TBA in maternal serum referred to the highest concentration of TBA during pregnancy, regardless of whether accept medication or not.
The main pregnancy outcomes in this study included: hypertensive disorders of pregnancy (HDP), defined as blood pressure ≥ 140/90 mmHg that occurred after 20 weeks gestation but without proteinuria [17]; preterm premature rupture of membrane (PPROM), spontaneous rupture of membranes before the onset of labor; oligohydramnios, defined as an amniotic fluid index of 5 cm or less; macrosomia, defined as a birth weight of more than 4000 g [18]; Large for gestational age (LGA) or small for gestational age(SGA), defined as a birth weight more than 90th or less than 10th percentile based on gender and gestational age [19]; liver dysfunction, mode of delivery (cesarean section or non-cesarean section); preterm delivery, defined as gestational age at delivery < 37 weeks but > 28 weeks; postpartum hemorrhage (PPH), defined as blood loss of 500 ml or more within 24 h after vaginal birth or 1000 ml or more within 24 h after cesarean section; placental weight (g), 2 h postpartum hemorrhage (mL); fetal distress, defined as a fetus suffering from insufficient oxygen supply, based on abnormal fetal heart rate and movements; gender (male or female), birth weight (g), birth height (cm).
Statistical analyses
SPSS 19.0 (SPSS Inc., Chicago, IL) was used for statistical analyses. Continuous data (i.e., age and biochemical outcomes) are presented as means ± standard deviation (SD) and were analyzed using an independent t-test or a nonparametric test (Kruskal–Wallis test). Dichotomous data (e.g., sex) are presented as percentages and were compared using the χ [2] test or a nonparametric test (Fisher’s exact test). Significance was set at P < 0 0.05. A logistic regression model was first used to calculate the regression coefficients and covariance matrix for two factors. The data were then inputted into an Excel sheet developed by Andersson to calculate relative excess risk due to interaction (RERI), attributable proportion due to interaction (AP), interaction index (synergy index, SI), and 95% CIs [20]. The 95% CI of RERI and AP included “0” and the 95% CI of SI included “1”, indicating the absence of additive interaction.
Results
Baseline characteristics
Participants’ baseline characteristics are displayed in Table 1. The incidence of GDM in patients with ICP was 21.55%. Maternal age (32.24 ± 4.34 versus 29.88 ± 4.20, P < .001), pre-pregnancy weight (54.23 ± 8.23 versus 52.53 ± 7.83, P = .017), and the proportion of gravidity > 1 (64.74% versus 57.57%, P = .011) and multiparity (51.92% versus 41.37%, P = .019) were higher in the GDM group vs. non-GDM group. In contrast, gestational weight gain was lower in the GDM group (12.11 ± 5.47 versus 13.24 ± 4.87, P = .020). There were no significant differences in gestational age at delivery (P = .210) and pre-pregnancy BMI (25.59 ± 3.80 versus 25.26 ± 3.06, P = .319) between the GDM and non-GDM groups.
Biochemical tests
The results of biochemical tests performed during pregnancy are displayed in Table 2. There were no significant differences in mean TG (3.64 ± 2.25 versus 3.42 ± 1.59, P = .137), LDL (2.95 versus 3.06, P = .138), ALT (14.20 versus 17.20, P = .220), AST (18.85 versus 20.15, P = .673), and TBA (28.84 ± 24.69 versus 27.22 ± 20.83, P = .409) levels between the GDM and non-GDM groups.
Perinatal outcomes
Maternal outcomes are displayed in Table 3. Compared with the non-GDM group, the proportion of HDP was higher in the GDM group (15.38% versus 8.80%, P = .016). There were no significant differences in PPROM (16.03% versus 18.84%, P = .420), oligohydramnios (1.92% versus 4.23%, P = .179), liver dysfunction (26.92% versus 21.48%, P = .128), delivery mode (cesarean section: 62.18% versus 56.87%, vaginal delivery: 37.82% versus 43.13%, P = .234), preterm delivery (23.08% versus 17.96%, P = .149), PPH (0.64% versus 1.76%, P = .311), placental weight (572.01 ± 123.94 versus 599.62 ± 125.92, P = .158), and 2-hour postpartum hemorrhages (340.07 ± 161.44 versus 325.89 ± 160.37, P = .329) between the GDM and the non-GDM groups.
Fetal outcomes are displayed in Table 4. Compared with the non-GDM group, the GDM group had higher rates of fetal distress (1.92% versus 0.35%, P = .036). No significant differences were found in LGA (3.21% versus 1.76%, P = .262), SGA (3.85% versus 5.46%, P = .418), gender (male: 49.36% versus 53.17%, female: 50.64% versus 46.83%, P = .416), birth weight (2964.22 ± 654.98 versus 3058.06 ± 549.77, P = .071), and birth height (48.45 ± 2.61 versus 48.22 ± 3.37, P = .373) between the GDM and the non-GDM groups.
Interactions
The associations between GDM, maximum TBA concentration, and adverse pregnancy outcomes are shown in Table 5. Only maximum TBA concentration was associated with cesarean section.
The pairwise interaction between GDM and maximum TBA concentration on adverse pregnancy outcomes is displayed in Table 6. No pairwise interactions were detected between GDM and maximum TBA concentration and HDP, PPH, preterm delivery, LGA, SGA, and cesarean section. The additive interactions between GDM and maximum TBA concentration and adverse pregnancy outcomes are displayed in Table 7. No additive interactions were detected between GDM and maximum TBA concentration and HDP, PPH, preterm delivery, LGA, SGA, and cesarean section.
Discussion
In this population-based study, we found that GDM was associated with differences in maternal and fetal outcomes in women with ICP. We also explored the interactive effects of GDM and maximum TBA concentration on adverse pregnancy outcomes in women with ICP. We determined that the incidence of GDM in patients with ICP was 21.55%, which was similar to the 27.45% reported by Majewska [21]. We also found that maternal age, pre-pregnancy weight, parity, and gravidity were positively associated with GDM. This latter finding is novel, as many researchers have reported that age, parity, and gravidity are significantly higher among patients with GDM as compared with healthy controls [22]. ICP can be divided into mild and severe, and according to the guidelines, severe ICP is the reason for a planned cesarean section [23]. This study did not find a difference in gestational age at delivery and pre-pregnancy BMI in pregnant women with vs. without GDM. Further research remains to be done.
This study determined that the proportion of HDP and fetal distress was higher among women with GDM than those without GDM. Further, no significant between-group differences were found in PPROM, oligohydramnios, liver dysfunction, delivery mode, preterm delivery and PPH, placental weight, 2-hour postpartum hemorrhage, LGA, SGA, gender, birth weight, and birth height. Axelsen et al. found that significantly more women with GDM and ICP developed preeclampsia during pregnancy as compared with women with only GDM [24]. Similarly, a meta-analysis conducted by Zhang et al. determined that ICP significantly increased the risk of both PE and GDM [25]. These findings are consistent with the results of our study.
We did not find any significant differences in biochemical outcomes (i.e., TG, LDL, ALT, AST and TBA) between the GDM and non-GDM groups. In terms of adverse pregnancy outcomes, GDM was only associated with maximum TBA concentration for cesarean section. No additive or pairwise interactions were detected between GDM and maximum TBA concentration and HDP, PPH, preterm delivery, LGA, SGA, and cesarean section. These findings are consistent with the results of Majewska and others, which did not find any significant correlations between the blood glucose levels of pregnant women with ICP and ALT, AST, and perinatal outcomes [21]. However, Majewska et al. believe that the decrease in serum TBA levels in patients with ICP is related to the presence of GDM [21]. In contrast, our study suggests that there are no significant differences in the serum TBA levels of women with and without GDM. In a separate study, Gao et al. found that the serum levels of 8 bile acids were elevated among women with GDM as compared with healthy controls [26]. Further, Jin et al. reported that compared with a healthy control group, every increase in TG level in the third trimester of pregnancy increased the risk of GDM, while increasing LDL levels reduced the risk of GDM [27]. Future studies should explore the effects of GDM on the blood lipid levels of women with ICP.
There are some limitations of our study. First, as a retrospective design, confirmation of causal association is limited. Second, there is no detailed analysis of the specific treatment methods of pregnant women with GDM. Third, the measurement of TBAs is very method-dependent and can also be negatively influenced by drugs such as UDC. Also, in our cohort, we did not divide ICP into severe group (TBA ≥ 100 µmol/l) and mild group (TBA < 100 µmol/l) for further study. Although these limitations, our study is the first time to study the combined effect of GDM and maximum level of maternal serum TBA on the incidence of adverse pregnancy outcomes in women with ICP. The second strength is that we adjusted for the potential mediating effect and considered results reliable.
Conclusion
GDM independently contributes to adverse pregnancy outcomes among women with ICP. However, the combined effects of GDM and maximum TBA concentration on adverse pregnancy outcomes do not appear to be multiplicative or additive.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- BMI:
-
Body mass index
- GDM:
-
Gestational diabetes mellitus
- ICP:
-
Intrahepatic cholestasis of pregnancy
- LDL:
-
Low-density lipoprotein
- TBA:
-
Total bile acid
- TG:
-
Triglyceride
References
Ovadia C, Williamson C. Intrahepatic cholestasis of pregnancy: recent advances. Clin Dermatol. 2016;34(3):327–34.
Geenes V, Williamson C. Intrahepatic cholestasis of pregnancy. World J Gastroenterol. 2009;15(17):2049-66.
Sebiha Ozkan Y, Ceylan OV, Ozkan, et al. Review of a challenging clinical issue:Intrahepatic cholestasis of pregnancy. World J Gastroenterol. 2015;21(23):7134–41.
Victoria G. Lucy, Association of severe intrahepatic cholestasis of pregnancy with adverse pregnancy outcomes: A prospective population-based case-control study. Hepatology, 2014.
Xu M, Liu B, Wu MF, et al. Relationships among acylation-stimulating protein, insulin resistance, lipometabolism, and fetal growth in gestational diabetes mellitus women. J Obstet Gynecol. 2015;35(4):341–5.
Julia M, Potter PE, Hickman et al. Strict Preanalytical Oral Glucose Tolerance Test Blood Sample Handling Is Essential for Diagnosing Gestational Diabetes Mellitus. Diabetes care,2020.
Huang Y, Ying Z, Quan W, et al. The clinical significance of Neutrophil-to-lymphocyte ratio and monocyte-to-lymphocyte ratio in Guillain-Barré syndrome. Int J Neurosci. 2018;128(9):1–22.
Rammah A, Whitworth KW, Symanski E. Particle air pollution and gestational diabetes mellitus in Houston, Texas. Environ Res. 2020;190:109988.
Wang N, Dong X, Shi D, et al. Cryptotanshinone ameliorates placental oxidative stress and inflammation in mice with gestational diabetes mellitus. Arch Pharm Res. 2020;43:235–7.
Bogda Skowrońska M, Fichna P, Fichna. The role of adipose tissue in the endocrine system. Chem Res Chin Univ. 2005;31(6):1–6.
Feng Y, Feng Q, Lv Y, et al. The relationship between iron metabolism, stress hormones, and insulin resistance in gestational diabetes mellitus. Volume 10. Nutrition & Diabetes; 2020. p. 17. 1.
Yu Yongfu, Arah Onyebuchi A, Liew, Zeyan et al. Maternal diabetes during pregnancy and early onset of cardiovascular disease in offspring: population based cohort study with 40 years of follow-up. BMJ (Clinical research ed.),2019,367.
Bogdanet D, Egan A, Reddin C et al. ATLANTIC DIP: Despite insulin therapy in women with IADPSG diagnosed GDM, desired pregnancy outcomes are still not achieved. What are we missing?. Diabetes Res Clin Pract, 2018:S016882271731625X.
Daly Barbara, Toulis Konstantinos A, Thomas Neil et al. Correction: Increased risk of ischemic heart disease, hypertension, and type 2 diabetes in women with previous gestational diabetes mellitus, a target group in general practice for preventive interventions: A population-based cohort study. PLoS medicine,2019,16(7).
Zhu B, Yin P, Ma Z, et al. Characteristics of bile acids metabolism profile in the second and third trimesters of normal pregnancy. Metabolism: clinical and experimental; 2019.
Arafa A, Dong JY. Association between intrahepatic cholestasis of pregnancy and risk of gestational diabetes and preeclampsia: a systematic review and meta-analysis. Hypertension in Pregnancy; 2020.
Fatima SA, Latha JM, Vani N, et al. A comparative study of serum lipids and Lipoprotein- A levels of women with pregnancy Induced Hypertension (PIH) and normotensive pregnant women. Int J Clin Biochem Res. 2021;7:488–96.
Alexander G, Kogan M, Himes J. 1994–1996 U.S. Singleton Birth Weight Percentiles for Gestational Age by Race, hispanic origin, and gender. Matern Child Health J. 1999;3:225–31.
Villar J, Cheikh Ismail L, Victora C, et al. International Standards for Newborn Weight, length, and head circumference by gestational age and sex: the newborn cross-sectional study of the INTERGROWTH-21st Project. Lancet (London England). 2014;384:857–68.
Andersson T, Alfredsson L, Kllberg H, et al. Calculating measures of Biological Interaction. Eur J Epidemiol. 2005;20:575–9.
Majewska A, Godek B, Bomba-Opon D, et al. Association between intrahepatic cholestasis in pregnancy and gestational diabetes mellitus. A retrospective analysis. Ginekol Pol. 2019;90(8):458–63.
Bentley-Lewis R, Huynh J, Xiong G et al. Metabolomic profiling in the prediction of gestational diabetes mellitus. Diabetologia 2015;58(6):1329–32.
Zhang Y, Lu L, Victor DW, et al. Ursodeoxycholic acid and S-adenosylmethionine for the treatment of intrahepatic cholestasis of pregnancy: a Meta-analysis. Hepat Mon. 2016;23(8):e38558.
Axelsen SM, Kampmann U, Koefoed AS, McIntyre D, Ovesen PG, Fuglsang J. Intrahepatic cholestasis of pregnancy: Association with glycaemic control in gestational diabetes. Diabet Med. 2021;38(8):e14574.
Zhang L, Tang C, Ye C et al. Intrahepatic cholestasis of pregnancy can increase the risk of metabolic disorders: A meta-analysis. J Med Biochem 2022;41(4):549–58.
Gao J, Xu B, Zhang X et al. Association between serum bile acid profiles and gestational diabetes mellitus: A targeted metabolomics study. Clin Chim Acta 2016;459:63–72.
Jin WY, Lin SL, Hou RL et al. Associations between maternal lipid profile and pregnancy complications and perinatal outcomes: a population-based study from China. BMC Pregnancy Childbirth 2016;16:60.
Acknowledgements
The authors would like to express their gratitude to EditSprings (https://www.editsprings.cn ) for the expert linguistic services provided.
Funding
Guide Fund for the Development of Local Science and Technology from the Central Government (2020L3019), Joint Funds for the Innovation of Science and Technology, Fujian Province (2020Y9134), Fujian Provincial Health Technology Project (2021CXA034), Joint Funds for the Innovation of Science and Technology, Fujian Province (2020Y9161) and Joint Funds for the Innovation of Science and Technology, Fujian Province (2021Y9134).
Author information
Authors and Affiliations
Contributions
Material preparation, data collection and analysis were performed by Tingting Liao, Xia Xu, Yulong zhang and Jianying Yan. As Xia Xu and Tingting Liao contributed equally to the article, we decided to list them as co-first authors. The first draft of the manuscript was written by Tingting Liao, Xia Xu, Yulong Zhang Jianying Yan and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Ethics Committee of Fujian Maternity and Child Health Hospital approved all protocols (2021KLRD631). The study was conducted in accordance with the Declaration of Helsinki and informed consent was obtained from all subjects and/or their legal guardian(s).
Consent for publication
Not applicable.
Competing interests
The authors report no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liao, T., Xu, X., Zhang, Y. et al. Interactive effects of gestational diabetes mellitus and maximum level of total bile acid in maternal serum on adverse pregnancy outcomes in women with intrahepatic cholestasis of pregnancy. BMC Pregnancy Childbirth 23, 326 (2023). https://doi.org/10.1186/s12884-023-05621-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12884-023-05621-6