Abstract
Background
Acute pancreatitis in pregnancy (APIP) with a high risk of death is extremely harmful to mother and fetus. There are few models specifically designed to assess the severity of APIP. Our study aimed to establish a clinical model for early prediction of severity of APIP.
Methods
A retrospective study in a total of 188 patients with APIP was enrolled. The hematological indicators, IAP (intra-abdominal pressure) and clinical data were obtained for statistical analysis and prediction model construction.
Results
According to univariate and multivariate logistic regression analysis, we found that red cell distribution width (RDW), neutrophil-lymphocyte ratio (NLR) and Intra-abdominal pressure (IAP) are prediction indexes of the severity in APIP (p-value < 0.05). Our novel clinical prediction model was created by based on the above three risk factors and showed superior predictive power in primary cohort (AUC = 0.895) and validation cohort (AUC = 0.863). A nomogram for severe acute pancreatitis in pregnancy (SAPIP) was created based on the three indicators. The nomogram was well-calibrated.
Conclusion
RDW, NLR and IAP were the independent risk factors of APIP. Our clinical prediction model of severity in APIP based on RDW, NLR and IAP with predictive evaluation is accurate and effective.
Similar content being viewed by others
Introduction
Acute pancreatitis in pregnancy (APIP) is a severe disease that affects 1 in 1,000 to 12,000 pregnant women on average, which is more frequently than the general population [1]. Both the mother and the fetus might suffer substantial morbidity as a result of APIP. The APIP still has high rates of maternal and perinatal mortality, 3.3% and 11.6–18.7%, respectively [1].
Acute pancreatitis (AP) is an inflammatory disorder that can damage nearby and distant organs even result in the multiple organ dysfunction. According to the current Atlanta classification based on the presence and duration of organ failure (2012), acute pancreatitis is divided into three types mild acute pancreatitis (MAP), moderately severe acute pancreatitis (MSAP), and severe acute pancreatitis (SAP) [2]. Patients without organ dysfunction and local complications were MAP. Patients with temporary organ dysfunction (≤ 48 h) and/or localized or systemic accompanying diseases were classified as MSAP. SAP is persistent organ dysfunction (> 48 h). The risk of maternal and fetal death is highly associated with the severity of APIP [1, 3]. Reports indicate that SAP in pregnancy (SAPIP) with the maternal mortality rate as high as approximately 20–40% is serious hazardous to the health of pregnant women [4, 5]. Early recognition of APIP severity is critically important for prompt treatment to individual patients. The early stage of AP is usually referred to the first week after the disease onset [6]. Early prediction of the severity of APIP is very important for clinical treatment.
Elevated internal pressure (IAP) is common in critically ill patients. Intraperitoneal hypertension (IAH) has adverse effects on hemodynamics, respiration, and renal function, and may eventually lead to multiple organ failure [7]. It is reported that early recognition of IAH may help in early intervention improving outcomes of acute necrotizing pancreatitis [8]. However, to our knowledge, few studies have been conducted on IAP in APIP.
Currently, several prediction systems are usually used for AP patients. However, the prediction system is not specific for pregnant women, and some do not apply in pregnant women. At present, there is few scoring systems designed for patients with APIP in clinical practice [9]. Therefore, it is very urgent and important to establish a timely, simple and useful clinical prediction model to predict severe acute pancreatitis in pregnancy.
Red cell distribution width (RDW) is a parameter primarily reflecting the volume variability of red blood cells. Recent many studies indicated that RDW relates with the levels of many kinds of inflammatory cytokines in serum [10]. Moreover, RDW has been introduced as a novel inflammatory predictor in various diseases, such as functional bowel conditions [11], autoimmune diseases [12], rheumatoid arthritis [13], degenerative vertebral conditions [14], autoimmune hepatitis [15]. It is proved that RDW can be used to predict mortality and severity of patients with AP [16, 17]. However, few studies focused on the prediction of RDW in the severity of patients with APIP. On the other hand, neutrophil-lymphocyte ratio (NLR) has been introduced as a marker of inflammation in many inflammatory related diseases, such as inflammatory bowel disease [18], diabetes mellitus [19], thyroiditis [20], and AP where NLR can be used to predicting severity [21]. Up to date, it is not clear whether NLR and RDW can be used to construct predictive model of APIP severity. Therefore, we included RDW and NLR in our study, aiming to further determine their predictive role in APIP severity and whether a predictive model of APIP severity can be established.
In this study, 188 cases of APIP patients were retrospectively reviewed and classified into two groups by us. MAP and MSAP were included into non-SAPIP groups, and severe acute pancreatitis in pregnancy was included into SAPIP groups. we collected routine laboratory tests data within 48 h after the APIP onset, IAP and other clinical data to assess the predictive ability of these data on the severity of APIP, in order to construct a clinical prediction model of severity in APIP.
Methods
Patients selection
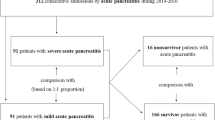
The medical records of patients who were diagnosed with APIP were retrospectively collected at our hospital (The First Affiliated Hospital of University of South China) from January 2008 to December 2021. Patients meeting the following criteria were included: (1) Definite diagnosis of APIP; (2) All cases were first onset and were diagnosed within 48 h of onset. (3) All required information was completed. Exclusion criteria: (1) Pregnancy terminated within 24 h of admission; (2) Acute attack of chronic pancreatitis; (3) Patients complicated with other diseases, such as malignant tumor, comorbidities related to pregnancy or not related to pregnancy, sepsis, hemorrhagic disease (trauma, for example), other Inflammation-related disease, and so on; (4) Use of immunosuppressants, corticosteriod and other drugs; (5) Patients received red blood cells; (7) Patients from obstetrical emergencies as HELLP, preeclampsia, eclampsia etc.; (8) Incomplete information required. The flowchart prensented in Fig. 1. The Ethics Committee of our hospital approved our study, and our study was carried out following the Declaration of Helsinki.
Data collection
We collected the following clinical parameters: age, gestational weeks, red cell distribution width (RDW), alanine aminotransferase (ALT), Albumin, triglyceride (TG), total cholesterol (TC), blood urea nitrogen (BUN), serum creatinine (Scr), Calcium, lactate dehydrogenase (LDH), intra-abdominal pressure (IAP), neutrophil–lymphocyte ratio (NLR). All clinical parameters were tested in the hospital and were collected within 48 h of admission. Among them, hematological test results were obtained on the day of admission, and IAP values were measured within 48 h after admission, the average values of the two highest IAP values were taken, and pressure was measured indirectly through the bladder [22].
Definitions
We diagnosed the APIP patients via the 2012 revised version of the Atlanta criteria [6], the patients were diagnosed with APIP, if they met more than 2 pieces out of the following criteria: (1) characteristic abdominal pain of AP; (2) serum amylase or lipase was more than 3 times of the normal upper limit value; (3) characteristic results of acute pancreatitis from cross-sectional abdominal imaging. We graded the severity of APIP basing on 2012 revised version of the Atlanta criteria, and patients without organ dysfunction and local complications were mild acute pancreatitis (MAP). Patients with temporary organ disorder (≤ 48 h) and/or local or systemic other diseases caused by AP were moderately severe acute pancreatitis (MSAP). Patients with persistent organ dysfunction (> 48 h) were severe acute pancreatitis (SAP). MAP and MSAP were included into NSAPIP group, and severe pancreatitis in pregnancy was included into SAPIP group.
Development and validation of the prediction model
Variables were screened by univariate logistic regression analysis. To establish our prediction model, we collected all associated factors to carry out the multivariate logistic regression analysis. We evaluated the accuracy of independent prediction factors in the predictive model of SAPIP by the receiver operating characteristic (ROC) curves. A nomogram for SAPIP was produced according to the multivariate logistic regression model. To validate the consistency and accuracy of the model in predicting severity of APIP patients, we applied the internal and external validation sets to assess the consistency, and used the calibration curves, ROC curves to check the accuracy of the prediction model in this study. In the end, decision analysis was applied to further assess the clinical applicability of the predictive model. Decision curve analysis (DCA) was carried out to evaluate the clinical utility of the prediction model. The flowing chart presents in Fig. 2.
Statistical analysis
We presented all the variables as mean ± standard deviation (SD) or median (range), as appropriate. Statistical analyses were conducted by GraphPad Prism 7.0 Software for Windows (GraphPad Software, La Jolla, CA, USA), Service Solutions SPSS Software 25.0 (SPSS, Chicago, IL, USA) and R statistical software (version 4.2.0; https://www.r-project.org/ ). Kolmogorov–Smirnov test was used for normality analysis of the study variables. Student’s t-test was applied to analyse normally distributed continuous variables, and the Mann-Whitney U test was utilized to analyse nonnormally distributed continuous variables. Univariate logistic regression analysis was performed to identify predictive factors of APIP. Predictive factors with p value less than 0.05 in univariate analysis were included in the multivariate analysis. Multivariate logistic regression analysis was conducted to identify independent predictive factors, and check the useful combination of factors that could predict APIP. All p values were two-sided, with statistical significance set at p values less than 0.05.
Results
Basic characteristics of the patients between training cohort and validation cohort
A total of 188 APIP patients were enrolled, and a 7:3 ratio of training cohort (n = 128) to validation cohort (n = 60) were randomly allocated. The detailed baseline characteristics of APIP patients in this investigation were presented in Table 1.
Clinical characteristics between NSAPIP and SAPIP groups
As shown in Fig. 3, we compared and analysed the general conditions between the two groups. Significant differences in some variables were found between NSAPIP and SAPIP groups, including: RDW, TC, TG, Calcium, NLR, IAP(p<0.05). No significant differences in age, gestational weeks, ALT, albumin, BUN, Scr, and LDH were found between the two groups of patients.
Univariate and multivariate logistic regression analysis
As shown in Table 2, the univariate logistic regression analysis indicated that the patients’ RDW [odds-ratio (OR) = 1.683, p = 0.000], TG (OR = 1.072, p = 0.007), TC(OR = 1.182, p = 0.017), IAP(OR = 1.827, p = 0.000), calcium(OR = 0.108, p = 0.001) and NLR(OR = 1.434, p = 0.000) were candidate factors related to the predicting severity of APIP. What’s more, the results of univariate logistic regression analysis indicated that age, ALT, albumin, BUN, Scr, and LDH were not severity predictors of APIP. This result was consistent with the result of variables comparison between NSAPIP and SAPIP groups. These factors which were not severity predictors of APIP were thus excluded from the multivariate logistic regression analysis.The results of the multivariate logistic regression analysis were showed as follows : RDW(OR = 1.450, p = 0.009), IAP(OR = 1.557,p = 0.000), NLR (OR = 1.228, p = 0.017). These results meant that RDW, IAP, NLR are independent prediction marker of severity in APIP patients. Moreover, we also constructed a forest plot of independent predictors of SAPIP with odds-ratio (Fig. 4).
Model construction and validation
The ROC curve of severity prediction constructed based on the potential factors of RDW, IAP and NLR were presented in Fig. 5. The respective areas under the curve (AUC) of RDW and NLR were 0.754 [95% confidence interval (CI) 0.662–0.846], 0.788 (95% CI 0.709–0.866). The cut-off value of RDW for predicting the occurrence of SAPIP was 14.5%, the sensitivity was 0.76, and the specificity was 0.718, the cut-off value of NLR for predicting the occurrence of SAPIP was 12.228, the sensitivity was 0.88, and the specificity was 0.551. Meanwhile, the area under the curve (AUC) of IAP was 0.833 [95% confidence interval (CI) 0.761–0.906], the cut-off value of IAP for predicting the occurrence of SAPIP was 9.55 mmHg, the sensitivity was 0.70, and the specificity was 0.833.
The predictive model was created according to the independent prediction-related factors identified by the multivariate logistic regression analysis and was showed as follows: SAPIP risk = -11.618 + 0.206 × NLR + 0.371 × RDW + 0.443 × IAP. Then, the severity prediction model was visualized by a nomogram. As shown in Fig. 6, we constructed two kinds of nomogram.
A: line-segment static nomograms. Scores for each level of every variable on the nomogram were determined by a vertical dot-line from that factor to the point scale. Therefore, a total point was obtained by summing all the values. Finally, the risk of SAPIP for each patient could be estimated based on the total points. B: Line-segment dynamic nomograms. In R studio, we can click on different characteristics to see the probability of SAPIP in patients with different characteristics
In order to further check the validity of the prediction model, ROC curves were used to assess the discriminative property. The AUC of the predictive model in the training cohort (internal validation) was 0.863 (Fig. 7A ), and was 0.809 in the validation cohort (external validation) (Fig. 7B), indicating that the model has good discriminative ability. From the data in Fig. 8A and B, we knew that the calibration curves of internal (training cohort) and external (validation cohort) were very close to the 45°oblique line, showing that there was a great consistency between the predicted and actual results.
Finally, in order to evaluate the clinical usefulness and applicability of the model, we constructed a decision analysis curve. The decision curve shown in Fig. 9 indicated that the patients used this model can get more net benefit than the patients with complete intervention or no intervention at all. It means the model has potential clinical usefulness as a nomogram.
Discussion
It is still difficult to early diagnose of SAP in the clinical treatment with AP patients [5, 23]. For APIP, early prediction of its severity is more difficult due to the complexity and specificity of pregnancy. At present, the measurement of hematuria amylase and enhanced CT scan are main techniques for AP diagnosis, but as every pancreatologist knows that the level of hematuria amylase is not in direct proportion to the severity of the disease [24, 25]. In addition, CT examination, especially enhanced CT scan, should be prudently selected for pregnant women, and uterine enlargement during pregnancy can lead to some changes in the anatomical position of abdominal organs, moreover, there are few specific predictive models for APIP, so it is relatively difficult to evaluate the severity of APIP. What’s more, APIP is a dangerous disease because of its rapid progression, once it escalates into SAP, it will be very harmful to both mother and fetus [26]. In a word, it is very useful to construct a new multi-factor clinical model to predict the severity of APIP, which will be helpful to deal with risk stratification and management of APIP.
RDW is a commonly used bio-marker to detect the severity of erythrocyte anisocytosis. The higher RDW indicates much more anisocytosis [27]. In addition, there are also many other studies showing that the abnormal elevation of RDW can predict the poor prognosis of patients with septic shock [28], acute myocardial infarction [29], and general trauma patients [30]. The predictive role of RDW in AP has received continuous attention [31, 32]. RDW has several advantages as a predictive marker. Firstly, RDW is a part of the blood routine test which is fairly inexpensive and is a routine test. Additionally, it can be accessed easily and its results can be obtained quickly. Some studies have proved that RDW is an independent risk factor related to the severity of AP [32, 33]. However, the predictive role of severity by RDW in APIP remains unclear. In our study, the higher level of RDW in SAPIP patients indicated that RDW maybe a potential predictor of SAPIP. After univariate and multivariate logistic regression analysis, we found RDW is an independent predictive factor of severity in APIP.
NLR is a widely-used marker of bodily inflammation, easily obtained from calculation of the parameters usually supplied in a full blood count report. NLR is the ratio of neutrophils to lymphocytes, which combines two different parts of the immune pathway and shows the balance between inflammatory activator neutrophils and inflammatory regulator lymphocytes. In addition, higher NLR values represent a more unbalanced inflammatory state [34]. There were some studies indicated that NLR is related to the severity of AP. Li et al. performed a retrospective study and found that NLR is the most significant biomarker of overall survival in the AP patient group [35]. Jeon et al. found that higher NLR value is closely associated with severe acute pancreatitis and organ failure [36]. In our study, we found the higher level of NLR in SAPIP patients indicated that NLR maybe a potential predictor of SAPIP. After univariate and multivariate logistic regression analysis, we determined that NLR is an independent predictive factor of severity in APIP.
Many studies have led to an increasing interest in the measurement of IAP as a indicator of prognostic or predictive severity in patients with acute pancreatitis [37,38,39]. Intra-abdominal hypertension (IAH) refers to a repeated pathological high IAP with the value of greater than or equal to 12 mmHg. It is commonly believed that IAH in AP with organ dysfunction indicates visceral oedema because of the inflammatory process [40]. More studies have been showed that it is important to monitor IAP in patients with AP as it can reflect severity and potential influence about management. Besides, studies also proved that IAP is related with organ dysfunction and mortality of pancreatitis [40, 41]. However, few studies have evaluate the value of IAP in APIP. Therefore, it remains unclear whether IAP can predict the severity of APIP. Our study determined that IAP is an independent severity predictor in APIP.
ROC curve analysis showed that RDW, IAP and NLR have a great predictive value in SAPIP. Moreover, in our study, a new predictive model consisting of three risk factors (RDW, IAP, NLR) was constructed. In addition, our new predictive model of severity in APIP based on the three risk factors (RDW, IAP and NLR) has good predictive value. Morever, we created a model-based prediction nomogram that provides a convenient metric for predicting the severity of APIP. The results can be obtained very quickly and accurately without replacing the numbers into the equation. Of note, the model showed great accuracy and consistency in both external and internal validation. The major advantage of our clinical model is that all variables can be obtained easily and quickly, providing a fast and reliable tool for clinical prediction of APIP. Yang’s team analyzed 190 cases of APIP and established the prediction model for moderately severe and severe acute pancreatitis based on lactate dehydrogenase, triglyceride, cholesterol, and albumin levels [42]; Sheng’s team constructed a nomogram for POF with APIP based on four indicators: lactate dehydrogenase, triglycerides, serum creatinine, and procalcitonin [43]. However, the above prediction models of APIP require more indicators, and some indicators cannot be obtained quickly, so patients’ conditions can’t be timely and accurately judged. Our blood indicators can be obtained from the blood routine, which takes only half an hour, and we can also quickly measure the IAP and get the value.About the clinical utility of the study findings, via this prediction model based on blood test and measurement of IAP, the doctors can early recognize APIP severity in the clinical work, which will be very helpful to deal with risk stratification and management of APIP.
Our study has several drawbacks. First off, our research was limited to a single-center retrospective design., so further multi-center studies are needed to support the results. Secondly, we only collected the data of 188 patients, further validation with a larger sample of data is required, and this is also our research plan to carry out in the future.
Conclusion
In conclusion, we established and validated a new predictive nomogram model of severity according to RDW, IAP and NLR in APIP patients, which presents superior accuracy and accessibility. It is very useful to apply this model to stratify APIP patients for primary management and early intervention to improve prognosis.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available because they contain the patients’ personal information, but are available from the corresponding author on reasonable request.
Abbreviations
- Alb:
-
Albumin
- ALT:
-
Alanine aminotransferase
- AP:
-
Acute pancreatitis
- APIP:
-
Acute pancreatitis in pregnancy
- BUN:
-
Blood urea nitrogen
- Ca:
-
Calcium
- DCA:
-
Decision curve analysis
- IAP:
-
Intra-abdominal pressure
- LDH:
-
Lactate dehydrogenase
- MSAP:
-
Moderately severe acute pancreatitis
- NLR:
-
Neutrophil-lymphocyte ratio
- RDW:
-
Red cell distribution width
- ROC:
-
Curves:receiver operating characteristic curves
- SAP:
-
Severe acute pancreatitis
- Scr:
-
Serum creatinine
- SD:
-
Standard deviation
- TC:
-
Total cholesterol
- TG:
-
Triglyceride
References
Luo L, Zen H, Xu H, Zhu Y, Liu P, Xia L, He W, Lv N. Clinical characteristics of acute pancreatitis in pregnancy: experience based on 121 cases. Arch Gynecol Obstet. 2018;297(2):333–9.
Kryvoruchko IA, Kopchak VM, Usenko O, Honcharova NM, Balaka SM, Teslenko SM, Andreieshchev SA. Classification of an acute pancreatitis: Revision by international consensus in 2012 of classification, adopted in Atlanta. Klin Khir. 2014;(9):19–24.
Sun L, Li W, Geng Y, Shen B, Li J. Acute pancreatitis in pregnancy. Acta Obstet Gynecol Scand. 2011;90(6):671–6.
Li J, Chen J, Tang W. The consensus of integrative diagnosis and treatment of acute pancreatitis-2017. J Evid Based Med. 2019;12(1):76–88.
Peng T, Peng X, Huang M, Cui J, Zhang Y, Wu H, Wang C. Serum calcium as an indicator of persistent organ failure in acute pancreatitis. Am J Emerg Med. 2017;35(7):978–82.
Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, Tsiotos GG, Vege SS. Acute Pancreatitis classification Working G. classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102–11.
Trikudanathan G, Vege SS. Current concepts of the role of abdominal compartment syndrome in acute pancreatitis - an opportunity or merely an epiphenomenon. Pancreatology. 2014;14(4):238–43.
Verma S, Rana SS, Kang M, Gorsi U, Gupta R. Computed tomography features predictive of intra-abdominal hypertension in acute necrotizing pancreatitis: a prospective study. Indian J Gastroenterol. 2021;40(3):326–32.
Yang Z, Guo G, Li H. Predicting fetal loss in severe acute pancreatitis during pregnancy: a 5-year single-tertiary-center retrospective analysis. Postgrad Med. 2020;132(5):473–8.
He Y, Liu C, Zeng Z, Ye W, Lin J, Ou Q. Red blood cell distribution width: a potential laboratory parameter for monitoring inflammation in rheumatoid arthritis. Clin Rheumatol. 2018;37(1):161–7.
Aktas G, Alcelik A, Tekce BK, Tekelioglu V, Sit M, Savli H. Red cell distribution width and mean platelet volume in patients with irritable bowel syndrome. Prz Gastroenterol. 2014;9(3):160–3.
Aktas G, Sit M, Dikbas O, Tekce BK, Savli H, Tekce H, Alcelik A. Could red cell distribution width be a marker in Hashimoto’s thyroiditis? Exp Clin Endocrinol Diabetes. 2014;122(10):572–4.
Al-Rawi ZS, Gorial FI, Al-Bayati AA. Red cell distribution width in rheumatoid arthritis. Mediterr J Rheumatol. 2018;29(1):38–42.
Dagistan Y, Dagistan E, Gezici AR, Halicioglu S, Akar S, Ozkan N, Gulali A. Could red cell distribution width and mean platelet volume be a predictor for lumbar disc hernias? Ideggyogy Sz. 2016;69(11–12):411–4.
Ustaoglu M, Aktas G, Avcioglu U, Bas B, Bahceci BK. Elevated platelet distribution width and red cell distribution width are associated with autoimmune liver diseases. Eur J Gastroenterol Hepatol. 2021;33(1S Suppl 1):e905–8.
Cheng T, Liu BF, Han TY, Pan P, Liu JZ, Yu H. Efficiency of red cell distribution width in predicting severity and mortality of patients with acute pancreatitis: a protocol for systematic review and meta-analysis. Med (Baltim). 2021;100(6):e24658.
Kilic MO, Celik C, Yuksel C, Yildiz BD, Tez M. Correlation between Ranson score and red cell distribution width in acute pancreatitis. Ulus Travma Acil Cerrahi Derg. 2017;23(2):112–6.
Posul E, Yilmaz B, Aktas G, Kurt M. Does neutrophil-to-lymphocyte ratio predict active ulcerative colitis? Wien Klin Wochenschr. 2015;127(7–8):262–5.
Duman TT, Aktas G, Atak BM, Kocak MZ, Erkus E, Savli H. Neutrophil to lymphocyte ratio as an indicative of diabetic control level in type 2 diabetes mellitus. Afr Health Sci. 2019;19(1):1602–6.
Aktas G, Sit M, Dikbas O, Erkol H, Altinordu R, Erkus E, Savli H. Elevated neutrophil-to-lymphocyte ratio in the diagnosis of Hashimoto’s thyroiditis. Rev Assoc Med Bras (1992). 2017;63(12):1065–68.
Zhou H, Mei X, He X, Lan T, Guo S. Severity stratification and prognostic prediction of patients with acute pancreatitis at early phase: a retrospective study. Med (Baltim). 2019;98(16):e15275.
Chun R, Baghirzada L, Tiruta C, Kirkpatrick AW. Measurement of intra-abdominal pressure in term pregnancy: a pilot study. Int J Obstet Anesth. 2012;21(2):135–9.
Zhou CL, Zhang CH, Zhao XY, Chen SH, Liang HJ, Hu CL, Chen NW. Early prediction of persistent organ failure by serum apolipoprotein A-I and high-density lipoprotein cholesterol in patients with acute pancreatitis. Clin Chim Acta. 2018;476:139–45.
Hong W, Geng W, Chen B, Basharat Z, Wu Q, Zimmer V, Zhou M. Predictors of acute pancreatitis with low elevation of serum amylase. Ther Clin Risk Manag. 2017;13:1577–84.
Ismail OZ, Bhayana V. Lipase or amylase for the diagnosis of acute pancreatitis? Clin Biochem. 2017;50(18):1275–80.
Tang M, Xu JM, Song SS, Mei Q, Zhang LJ. What may cause fetus loss from acute pancreatitis in pregnancy: analysis of 54 cases. Med (Baltim). 2018;97(7):e9755.
O’Connell RM, Boland MR, O’Driscoll J, Salih A, Arumugasamy M, Walsh TN, Allen MJ, Beddy DJ. Red cell distribution width and neutrophil to lymphocyte ratio as predictors of outcomes in acute pancreatitis: a retrospective cohort study. Int J Surg. 2018;55:124–7.
Wang TH, Hsu YC. Red cell distribution width as a prognostic factor and its comparison with lactate in patients with sepsis. Diagnostics (Basel). 2021;11(8):1474.
Hu GX, Zhang J, Tian YG, Li YH, Mou L, Qiao LJ. Diagnostic value of joint detection of homocysteine and RDW CV on acute myocardial infarction. Eur Rev Med Pharmacol Sci. 2017;21(20):4472.
Brown M, Nassoiy S, Plackett T, Luchette F, Posluszny J. Jr. Red blood cell distribution width and outcome in trauma patients. J Osteopath Med. 2021;121(2):221–8.
Karabuga B, Gemcioglu E, Konca Karabuga E, Baser S, Ersoy O. Comparison of the predictive values of CRP, CRP/albumin, RDW, neutrophil/lymphocyte, and platelet/lymphocyte levels in determining the severity of acute pancreatitis in patients with acute pancreatitis according to the BISAP score. Bratisl Lek Listy. 2022;123(2):129–35.
Zhang T, Liu H, Wang D, Zong P, Guo C, Wang F, Wu D, Tang M, Zhou J, Zhao Y. Predicting the Severity of Acute Pancreatitis with Red cell distribution width at early admission stage. Shock. 2018;49(5):551–5.
Yalcin MS, Tas A, Kara B, Olmez S, Saritas B. New predictor of acute necrotizing pancreatitis: red cell distribution width. Adv Clin Exp Med. 2018;27(2):225–8.
Venkatraghavan L, Tan TP, Mehta J, Arekapudi A, Govindarajulu A, Siu E. Neutrophil lymphocyte ratio as a predictor of systemic inflammation - A cross-sectional study in a pre-admission setting. F1000Res. 2015;4:123.
Li Y, Zhao Y, Feng L, Guo R. Comparison of the prognostic values of inflammation markers in patients with acute pancreatitis: a retrospective cohort study. BMJ Open. 2017;7(3):e013206.
Jeon TJ, Park JY. Clinical significance of the neutrophil-lymphocyte ratio as an early predictive marker for adverse outcomes in patients with acute pancreatitis. World J Gastroenterol. 2017;23(21):3883–9.
Kurdia KC, Irrinki S, Chala AV, Bhalla A, Kochhar R, Yadav TD. Early intra-abdominal hypertension: a reliable bedside prognostic marker for severe acute pancreatitis. JGH Open. 2020;4(6):1091–5.
Ke L, Ni HB, Tong ZH, Li WQ, Li N, Li JS. Intra-abdominal pressure and abdominal perfusion pressure: which is a better marker of severity in patients with severe acute pancreatitis. J Gastrointest Surg. 2011;15(8):1426–32.
Fei Y, Gao K, Tu J, Wang W, Zong GQ, Li WQ. Predicting and evaluation the severity in acute pancreatitis using a new modeling built on body mass index and intra-abdominal pressure. Am J Surg. 2018;216(2):304–9.
Aitken EL, Gough V, Jones A, Macdonald A. Observational study of intra-abdominal pressure monitoring in acute pancreatitis. Surgery. 2014;155(5):910–8.
van Brunschot S, Schut AJ, Bouwense SA, Besselink MG, Bakker OJ, van Goor H, Hofker S, Gooszen HG, Boermeester MA, van Santvoort HC. Dutch pancreatitis study G. Abdominal compartment syndrome in acute pancreatitis: a systematic review. Pancreas. 2014;43(5):665–74.
Yang DJ, Lu HM, Liu Y, Li M, Hu WM, Zhou ZG. Development and validation of a prediction model for moderately severe and severe acute pancreatitis in pregnancy. World J Gastroenterol. 2022;28(15):1588–600.
Sheng C, Xu Z, Wang J. Nomogram for Predicting Persistent Organ failure with Acute Pancreatitis in pregnancy. Front Endocrinol (Lausanne). 2022;13:863037.
Acknowledgements
Not applicable.
Funding
This work was supported by Hunan Provincial Natural Science Fund (No.2021JJ40495, 2022JJ30530, 2022JJ30526), Scientific Research Fund Project of Hunan Provincial Health Commission( No.202105010225, 20201950), Clinical Medical Technology Innovation Guidance Project from Department of Science and Technology of Hunan Province (No.2020SK5181, 2021SK51812) and Science and Technology Research Project (Basic Applied Research Program) from Department of Science and Technology of Hengyang City (No.202150063929).
Author information
Authors and Affiliations
Contributions
WL and CD conceived and designed this study. GT, JH, CY, and SQ collected clinical data. WL, XL, JH, XY, CF, XJ, XD, SQ and GC analysed the data with relevant statistics and provided discussion points in the study. CD reviewed and provided crucial interpretation of study results. WL writing of this paper, CD revise the article. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Our study was approved by the Ethics Committee of the First Affiliated Hospital of University of South China. All methods were performed in accordance with the relevant guidelines and regulations. Furthermore, informed consent was obtained from all participants before the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liao, W., Tao, G., Chen, G. et al. A novel clinical prediction model of severity based on red cell distribution width, neutrophil-lymphocyte ratio and intra-abdominal pressure in acute pancreatitis in pregnancy. BMC Pregnancy Childbirth 23, 189 (2023). https://doi.org/10.1186/s12884-023-05500-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12884-023-05500-0