Abstract
Background
SARS-CoV-2 exposure during pregnancy is related to adverse effects for both the mother and the infant. SARS-CoV-2 vaccination has lowered the risk of symptomatic disease substantially. Recently published studies have evaluated the outcomes of women who received the COVID-19 vaccine during pregnancy; systematic evidence regarding vaccination safety is crucial to ensure that COVID-19 vaccination is not associated with adverse pregnancy and neonatal outcomes.
Methods
Pubmed/MEDLINE, EMBASE, Scopus, Web of Science, and Clinicaltrials.gov were searched from each database's inception through April 7, 2022. All interventional and observational studies comparing neonatal or pregnancy outcomes between pregnant women who received COVID-19 vaccines during their pregnancy and unvaccinated pregnant women were included. The random-effects model was used in the meta-analyses.
Results
A total of 11 studies comprising 756,098 pregnant mothers were included. The rate of neonates with 5-min Apgar score ≤ 7 (log RR -0.08 (95% CI: -0.15 to -0.00), (P = 0.03)) and pregnant mothers with preterm birth (log RR -0.11 (95% CI: -0.21 to -0.01), (P = 0.02)) was significantly lower among vaccinated group. No significant difference was observed in adverse neonatal outcomes (log RR -0.07 (95% CI: -0.17 to 0.03)), small for gestational age (log RR -0.06 (95% CI: -0.14 to 0.02)), caesarean delivery (log RR 0.05 (95% CI: -0.05 to 0.15)), postpartum hemorrhage (log RR -0.05 (95% CI: -0.13 to 0.02)), stillbirth (log RR -0.05 (95% CI: -0.54 to 0.45)).
Conclusions and relevance
In this systematic review and meta-analysis, no evident differences were observed when comparing vaccinated pregnant mothers with those who had not received COVID-19 vaccines. Based on low certainty of evidence, vaccination during pregnancy was accompanied by a favorable Apgar score in neonates and fewer preterm births.
Similar content being viewed by others
Background
The world has been struggling with the Coronavirus disease 2019 (COVID-19) pandemic for two and a half years. As of September 21, 2022, there have been more than 610 million cases confirmed, and more than 6.5 million people have lost their lives due to this pandemic [1]. There have been concerns about how COVID-19 could affect pregnant mothers and their neonates. Studies revealed that COVID-19 in pregnant women is accompanied by more severe manifestations compared with non-pregnants [2, 3]. It was also suggested that pregnant women with COVID-19 are at higher risk for preterm birth, preeclampsia, eclampsia, stillbirth, neonatal morbidity, and mortality than pregnant women without COVID-19 [4, 5].
Till now, vaccines are the most reliable option for controlling the severity of this disease [6]. The World Health Organization (WHO) has approved the emergency use of several vaccines, including AstraZeneca/Oxford, Johnson and Johnson, Moderna, Pfizer/BioNTech, Sinopharm, Sinovac, COVAXIN, and Nuvaxoid [7]. Most severe COVID-19 cases in pregnant women were reported from unvaccinated patients [8]. The American College of Obstetricians and Gynecologists (ACOG) recommends the vaccination of pregnant women with one of the mRNA vaccines in the U.S. and states that these vaccines are preferred to the J&J vaccine, whereas WHO recommends vaccination of pregnant women with the Sinopharm vaccine when the benefits outweigh the possible risks [9, 10].
Little data is available regarding the safety of these vaccines during pregnancy in the concept of randomized controlled trials [11, 12]. Although some studies have proven the safety and efficacy of vaccines in pregnant women [13,14,15], there is still a lack of data, and vaccine hesitancy is present among pregnant women [16]. Evidence from systematic reviews and meta-analyses is desperately needed on this topic, and one of the factors affecting the acceptance of vaccination is the certainty of systems to assess vaccine safety [16]; therefore, in this study, we aimed to evaluate the current evidence regarding the safety of vaccination and its possible effect on pregnancy and neonatal outcomes among vaccinated pregnant women compared with unvaccinated pregnant women.
Methods
Search strategy
A systematic review and meta-analysis comparing the possible effect of COVID-19 vaccination on neonatal and pregnancy outcomes was conducted in accordance with Cochrane collaboration procedures [17]. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) was used in this study [18]. The protocol of this study is registered at PROSPERO under the number CRD42022323965.
Pubmed/MEDLINE, EMBASE, Scopus, Web of Science, and Clinicaltrials.gov were searched by our reviewers (A.S and O.K and H.R). The following terms with their combinations were searched: SARS-CoV-2, COVID-19, Vaccines, and Pregnanant (Full search strategy is provided in the Supplementary Table 1). All publications published up to April 7, 2022 were retrieved. Additionally, in order to find relevant studies, we hand-searched the reference part of the relevant studies.
Study selection and data extraction
Studies were included based on the following PICOT criteria: 1) Population: adult pregnant women; 2) Exposure: received at least one dose of COVID-19 vaccines (in any types) during their pregnancy; 3) Comparator: unvaccinated pregnant women; 4) Outcome: studies evaluating relative outcomes in both vaccinated and unvaccinated group; and 5) Type of study: all types of original articles were applicabale. We included studies published in English language with accessible full text. Additionally, studies that reported the outcome only in vaccinated group were excluded. The titles and abstracts of the studies were reviewed by three independent reviewers (A.S and O.K and H.R), followed by full text review. An Excel spreadsheet was designed to include the Data extracted from text, tables, figures, graphs, and supplementary materials. Two reviewers (O.K and H.R) independently extracted the following data: author, year of publication, Journal/full paper or abstract, country, population, study type,, number of included patients in the study, type of vaccine, as well as relevant outcome data. To reach an agreement, discepancies were resolved through discussion with a third reviewer (M.T).
Quality assessment
Two reviewers (O.K and H.R) independently assessed the included studies using the National Heart, Lung, and Blood Institute (NHLBI) risk of bias checklist [19]. considering the 14 questions designed to assess the quality of observational cohort and cross-sectional studies,studies with 10 or more yeses are rated as “Good”, 7–9 yeses as “Fair”, and fewer than 7 yeses are rated as “Poor” [20]. We used the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) framework and GRADEpro GDT to evaluate the certainty of evidence for our outcomes [21].
Outcomes
Neonatal outcomes evaluated in this meta-analysis were: 1) adverse neonatal outcomes (ANO), which was defined as neonatal respiratory complications and Neonatal intensive care unit (NICU) admission; 2) 5-min Apgar score ≤ 7, which was defined as the assessment of 5 domains (skin color, heart rate, reflexes, muscle tone, and respiration) in neonates immediately after birth; and 3) small for gestational age (SGA). Pregnancy outcomes were as follow: 4) rate of caesarean delivery; 5) rate of post-partum haemorrhage (PPH); 6) preterm birth defined as gestational age < 37 weeks at delivery; and 7) stillbirth.
Data synthesis and analysis
Since the indicators differed among studies, pooling of our data was carried out using the Restricted-maximum-likelihood random-effects model. A log risk ratio (log RR) was calculated to sum up the overall effects of outcomes. Furthermore, to present the results in forest plots, the log RR was back-transformed to RR for ease of interpretation. A p-value of < 0.05 was considered as the threshold for significance of the effect estimate. We assessed the heterogeneity of the studies using Cochrane Q-test for heterogeneity (cut off point set as < 0.1 showing significant heterogenity) and I2 statistic. Studies were classified into three groups of low, moderate, and high level of heterogeneity based on the respective value of I2 < 50%, 50% to 75%, and > 75%. As the most commonly used variable for measurement of heterogeneity, the I2 value is in direct relationship with the number of included trials, making the comparison of I2 values between analyses challenging. Therefore, both I2 and Tau values for each analysis were reported in our study. Publication bias was appraised using funnel plots inspection and Egger's regression test for funnel plot asymmetry for outcomes. At least ten studies must be included based on the Cochrane handbook's suggestions in order to assess publication bias [9]. To evaluate the effect of individual studies on the pooled results, we performed a leave-one-out sensitivity analysis. The analysis was carried out using R (version 4.1.3) (R Core Team, 2020), the metafor package (version 3.0.2) (Viechtbauer, 2010), and the meta package.
Results
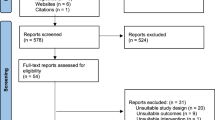
Based on our initial search, we identified 636 studies, removed 172 duplicates, and after the titles and abstracts screening, we reviewed full-texts of 62 articles, and eventually included 11 studies. (Fig. 1).
Characteristics of the included studies
Three studies were conducted in European countries, including Sweden & Norway (n = 1), England (n = 1), and Romania (n = 1); two of the studies were conducted in America, including the United States (n = 1) and Canada (n = 1). There were six studies conducted in Asia, which were all from Israel. mRNA vaccines (including BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna)) were the most common vaccines for which the results regarding their effects have been provided among the included studies. Detailed characteristics of each study are provided in Table 1.
Adverse neonatal outcomes (ANO)
A total of ten studies [4, 6, 22,22,23,24,25,27, 29, 30], including 289,414 pregnant women, reported the rate of NICU admission/newborn respiratory complications among neonates of vaccinated and unvaccinated pregnant mothers (Fig. 2-A). Log RR was -0.07 (95% CI: -0.17 to 0.03) indicating moderate amount of heterogeneity (I2 = 67.7%, Tau = 0.01, p < 0.01). Comparison of adverse neonatal outcomes showed no significant difference between the vaccinated and unvaccinated groups.
Small for gestational age (SGA)
A total of eight studies [4, 22,22,24, 26, 28,28,30], including a total of 196,739 pregnant women, evaluated SGA among neonates of vaccinated and unvaccinated pregnant mothers and their neonates (Fig. 2-B). Log RR was -0.06 (95% CI: -0.14 to 0.02) indicating low amount of heterogeneity (I2 = 30.9%, Tau = 0.00, p = 0.20). There was no significant difference in SGA between the vaccinated and unvaccinated groups.
5-min Apgar score ≤ 7
A total of seven studies [22,22,24, 27,27,28,30], including 269,806 pregnant women, evaluated the Apgar score among neonates of vaccinated and unvaccinated groups (Fig. 2-C). Log RR was -0.08 (95% CI: -0.15 to -0.00) indicating low amount of heterogeneity (I2 = 0.0%, Tau = 0.00, p = 0.64). The rate of neonates with 5-min Apgar score ≤ 7 was significantly lower in the vaccinated group A significantly lower rate of neonates with 5-min Apgar score ≤ 7 was observed in the vaccinated group(p = 0.037).
Caesarean delivery
A total of seven studies [22,22,24, 27,27,28,30], including 112,618 pregnant women, compared the rate of caesarean delivery between vaccinated and unvaccinated groups (Fig. 3-A). Log RR was 0.05 (95% CI: -0.05 to -0.15) indicating moderate amount of heterogeneity (I2 = 61.9%, Tau = 0.01, p = 0.04). No significant difference between groups was observed.
Postpartum hemorrhage (PPH)
A total of seven studies [22,22,24, 27,27,28,30], including 112,618 pregnant women, evaluated the rate of PPH (Fig. 3-B). Log RR was -0.05 (95% CI: -0.13 to 0.02) indicating low amount of heterogeneity (I2 = 0.0%, Tau = 0.00, p = 0.50). There was no significant difference between groups.
Preterm birth
A total of seven studies [6, 23,23,24,26, 28, 29], including 34,782 pregnant women, reported the rate of preterm neonates in vaccinated and unvaccinated groups (Fig. 3-C). Log RR was -0.11 (95% CI: -0.21 to -0.01) indicating low amount of heterogeneity (I2 = 0.0%, Tau = 0.00, p = 0.90). The reate of preterm birth was significantly lower in vaccinated group (p = 0.0282).
Stillbirth
A total of four studies [23, 24, 28, 30], including 9927 pregnant women, reported the rate of stillbirth vaccinated and unvaccinated groups (Fig. 3-D). Log RR was -0.05 (95% CI: -0.54 to 0.45) indicating low amount of heterogeneity (I2 = 0.0%, Tau = 0.00, p = 0.89). Our analysis showed no significant difference between groups.
Sensitivity analysis and publication bias
Based on a leave-one out method, we evaluate the effect of removing individual study on the pooled results. We evaluated the effect of individual studies on the pooled results by employing a leave-one out method. The results of the sensitivity analysis for ANO, caesarean delivery, PPH, and preterm birth showed the pooled effect size was remained non-significant. The pooled results for three outcomes differed after omitting an individual study: 1) When excluding Rottenstreich et al. the risk of SGA for vaccinated group was significantly lower compring the unvaccinated (p = 0.01); 2) no significant difference was shown between the two groups after excluding Magnus et al. (p = 0.07) and Fell (p = 0.26) et al., respectively, in terms of the risk of 5-min Apgar < 7; and 3.Exclusion of Goldshtein (p = 0.48) and Dick (p = 0.51) et al.did not cause any significant difference between the two groups in terms of the risk of preterm birth (Table 2).
Regarding publication bias, there was only one outcome with at least ten studies to evaluate ANO. A funnel plot of the ANO estimates is shown in Supplementary Fig. 1. The regression test demonstrated no sign of funnel plot asymmetry (p = 0.8377).
Quality assessment and certainty of evidence
According to NHLBI checklist, most of the included studies were juded to be of Good/Fair quality. The majority of included studies did not provide details regarding the blinding of outcome assessors to the participants' exposure status or assessing the exposure in more than one study (Further details are available in the Supplementary Table 2). Moreover, the certainty of evidence for study outcomes are available in Table 3.
Discussion
It is well-known from the experience of influenza and pertussis that prevention of infections through vaccination is effective in decreasing both maternal and prenatal undesirable outcomes [31]. The current systematic review and meta-analysis was conducted with the aim of evaluating the effect of COVID-19 vaccination during pregnancy on neonatal and pregnancy outcomes. Our review included 11 observational studies with 756,098 participants. Several outcomes were assessed, including postpartum hemorrhage, preterm birth, stillbirth, caesarean delivery, and a low 5-min Apgar score (< 7). No significant differences were found regarding adverse neonatal outcomes, small for gestational age, caesarean delivery, postpartum hemorrhage, and stillbirth. However, our analyses showed that COVID-19 vaccination during pregnancy significantly decreases the incidence of preterm birth and low 5-min Apgar score [7] compared to the unvaccinated group with low certainty of evidence. Further research, including studies with larger sample sizes from different countries and sociodemographic diversity, are required to confirm our findings.
Current evidence shows that SARS-Cov-2 infection during pregnancy is associated with a higher risk of developing COVID-19 complications. The risk of maternal hospitalization, ICU admission, need for mechanical ventilation, and even death is higher among pregnant patients compared to their non-pregnant counterparts. Furthermore, they showed significantly higher rates of adverse pregnancy outcomes such as preterm birth and stillbirth [5, 32]. Therefore, it is crucial for this group to get vaccinated to prevent possible complications caused by the disease affecting both the mother and the fetus. Early vaccine trials only included non-pregnant women. On the other hand, due to physical alterations of the human body during pregnancy, special attention should be given to the safety measures of the vaccines for pregnant women. As pregnant women are more cautious about receiving a new vaccine, they are reluctant to receive vaccines. Therefore, they should be provided with adequate information available regarding this issue to enable them to make informed decisions regarding vaccination [33].
There are studies suggesting that maternal vaccination with the proper transfer of neutralizing antibodies through the placenta could potentially induce offspring immunity. This is particularly beneficial since neonates and infants are more susceptible to severe illness caused by COVID-19 compared to their older pediatric counterparts, especially, when there is no current approved vaccine used in children younger than two years old [34, 35]. Beharier et al. showed that antenatal BNT162b2 mRNA vaccination induces a robust maternal immune response that is followed by an effective transfer of protective antibodies and a rise in their amount in the fetal circulation, emphasizing the importance of vaccination against COVID-19 during pregnancy [6].
Studies included in our review reported that there is no significant association between SARS-CoV-2 vaccination during pregnancy and an increased risk of adverse pregnancy outcomes. Rottenstreich et al. stated that based on their adjusted multivariable logistic regression analysis, the rate of composite adverse neonatal outcomes was lower among the vaccinated group. However, none of the individual neonatal outcomes were different between the two groups [36]. Magnus et al. showed that the risk of neonatal care admission and low Apgar scores was modestly decreased following vaccination during the third trimester [4]. In Dick et al.’s study, an increased rate of preterm birth was observed among pregnant women vaccinated during the second trimester in comparison with unvaccinated pregnant women [28]. Additionally, Goldshtein et al. observed that the rate of congenital malformation in the vaccinated group was not higher than the unvaccinated group and was similar to prepandemic reports [26].
There are some sociodemographic factors that contribute to a disparity between populations in terms of vaccination rates. Studies reported that older age, higher level of maternal education, higher socioeconomic position, conceiving following fertility treatment, having sufficient prenatal care, and lower gravidity are associated with increased rates of vaccination [22, 23].
The current findings should give people and clinicians confidence that vaccination against COVID-19 protects individuals from maternal SARS-CoV-2 infection and is not associated with adverse pregnancy and neonatal outcomes. Efforts should be made to improve awareness of vaccine safety among pregnant women and health providers and to address the issue of vaccine hesitancy. There are some strengths in our study. As far as we are aware, the current study is the most comprehensive systematic review and meta-analysis conducted to evaluate the association of COVID-19 vaccination with pregnancy outcomes. Ma et al. conducted a systematic review and meta-analysis on this subject, including six observational studies [37]. De Rose et al.’s systematic review summarized the current knowledge about pregnancy outcomes related to vaccination during pregnancy and breastfeeding [38]. The findings of these studies are in line with ours. However, our review is more comprehensive when considering both the number of included studies and whether a meta-analysis was performed. We performed a thorough database search to obtain the most comprehensive set of underlying studies and achieve accurate results. For three of the outcomes, we included seven studies in the meta-analysis, with the rest including six and four studies. Most of the included studies had adjusted for confounding variables.Our study has limitations. Since the majority of the analyzed studies were cohorts, they might be potentially biased due to their retrospective design. Most of our data were extracted from observational studies of high-income countries, limiting us regarding the diversity of participants in terms of sociodemographic characteristics. Furthermore, vaccines used in the studies were primarily mRNA vaccines, and little data was available regarding other types of vaccines approved by the WHO and used worldwide, such as Sinopharm, Sinovac, COVAXIN, and Nuvaxoid. Therefore, further research is required to determine the safety of administering these vaccines during pregnancy. Most vaccines were administered in the second or third trimester of pregnancy. Even though few studies have examined the safety of vaccines during the first trimester, the authors call for more data on the precise time of vaccine administration and its safety to inform maternal, pregnancy, and infant outcomes.
Conclusion
Our analyses show that vaccination against SARS-CoV-2 during pregnancy is not associated with a higher risk of adverse pregnancy and neonatal outcomes. Further research, including studies with larger sample sizes, more diverse populations, different types of vaccines, and variable timings for the administration of vaccines, is required to reach a solid conclusion regarding this issue.
Availability of data and materials
Data sharing is available by contacting corresponding author.
Abbreviations
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- COVID-19:
-
Coronavirus disease 2019
- WHO:
-
World Health Organization
- NHLBI:
-
National Heart Lung and Blood Institute
- NICU:
-
Neonatal intensive care unit
- ANO:
-
Adverse neonatal outcomes
- SGA:
-
Small for gestational age
- PPH:
-
Postpartum hemorrhage
References
WHO Coronavirus (COVID-19) Dashboard. Available from: https://covid19.who.int/.
DeSisto CLWB, Simeone RM, et al. Risk for stillbirth among women with and without COVID-19 at delivery hospitalization — United States, March 2020–September 2021. MMWR Morb Mortal Wkly Rep. 2021;2021(70):1640–5.
Delahoy MJ, Whitaker M, O’Halloran A, Chai SJ, Kirley PD, Alden N, et al. Characteristics and maternal and birth outcomes of hospitalized pregnant women with laboratory-confirmed COVID-19 - COVID-NET, 13 States, March 1-August 22, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(38):1347–54.
Magnus MC, Örtqvist AK, Dahlqwist E, Ljung R, Skår F, Oakley L, et al. Association of SARS-CoV-2 vaccination during pregnancy with pregnancy outcomes. JAMA. 2022;327(15):1469.
Villar J, Ariff S, Gunier RB, Thiruvengadam R, Rauch S, Kholin A, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr. 2021;175(8):817–26.
Beharier O, Plitman Mayo R, Raz T, Nahum Sacks K, Schreiber L, Suissa-Cohen Y, et al. Efficient maternal to neonatal transfer of antibodies against SARS-CoV-2 and BNT162b2 mRNA COVID-19 vaccine. J Clin Invest. 2021;131(13):e150319.
Coronavirus Disease (COVID-19); 2021. Available from: https://extranet.who.int/pqweb/vaccines/covid-19-vaccines.
Engjom H, van den Akker T, Aabakke A, Ayras O, Bloemenkamp K, Donati S, et al. Severe COVID-19 in pregnancy is almost exclusively limited to unvaccinated women – time for policies to change. Lancet Reg Health Eur. 2022;13:100313.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from: www.training.cochrane.org/handbook.
The Sinopharm COVID-19 vaccine: what you need to know 2021 [updated 15 March 2022. Available from: https://www.who.int/news-room/feature-stories/detail/the-sinopharm-covid-19-vaccine-what-you-need-to-know.
Smith DD, Pippen JL, Adesomo AA, Rood KM, Landon MB, Costantine MM. Exclusion of pregnant women from clinical trials during the Coronavirus Disease 2019 pandemic: a review of international registries. Am J Perinatol. 2020;37(8):792–9.
Van Spall HGC. Exclusion of pregnant and lactating women from COVID-19 vaccine trials: a missed opportunity. Eur Heart J. 2021;42(28):2724–6.
Falsaperla R, Leone G, Familiari M, Ruggieri M. COVID-19 vaccination in pregnant and lactating women: a systematic review. Expert Rev Vaccines. 2021;20(12):1619–28.
Goldshtein I, Nevo D, Steinberg DM, Rotem RS, Gorfine M, Chodick G, et al. Association between BNT162b2 vaccination and incidence of SARS-CoV-2 infection in pregnant women. JAMA. 2021;326(8):728–35.
Dagan N, Barda N, Biron-Shental T, Makov-Assif M, Key C, Kohane IS, et al. Effectiveness of the BNT162b2 mRNA COVID-19 vaccine in pregnancy. Nat Med. 2021;27(10):1693–5.
Salmon DA, Dudley MZ, Glanz JM, Omer SB. Vaccine hesitancy: causes, consequences, and a call to action. Vaccine. 2015;33:D66–71.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions. 2nd Edition. Chichester (UK): Wiley; 2019.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. https://doi.org/10.1136/bmj.n71.
Ma LL, Wang YY, Yang ZH, Huang D, Weng H, Zeng XT. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Mil Med Res. 2020;7(1):7.
Shafiee A, Teymouri Athar MM, Nassar M, Seighali N, Aminzade D, Fattahi P, Rahmannia M, Ahmadi Z. Comparison of COVID-19 outcomes in patients with Type 1 and Type 2 diabetes: A systematic review and meta-analysis. Diab Metab Syndr. 2022 16(6):102512. https://doi.org/10.1016/j.dsx.2022.102512.
Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ; GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6. https://doi.org/10.1136/bmj.39489.470347.AD.
Wainstock T, Yoles I, Sergienko R, Sheiner E. Prenatal maternal COVID-19 vaccination and pregnancy outcomes. Vaccine. 2021;39(41):6037–40.
Theiler RN, Wick M, Mehta R, Weaver AL, Virk A, Swift M. Pregnancy and birth outcomes after SARS-CoV-2 vaccination in pregnancy. Am J Obstet Gynecol MFM. 2021;3(6):100467.
Rottenstreich M, Sela HY, Rotem R, Kadish E, Wiener-Well Y, Grisaru-Granovsky S. Covid-19 vaccination during the third trimester of pregnancy: rate of vaccination and maternal and neonatal outcomes, a multicentre retrospective cohort study. BJOG. 2022;129(2):248–55.
Mayo RP, Raz T, David BB, Meir G, Barr H, Solmesky LJ, Chen R, Idelson A, Zorzetti L, Gabbay-Benziv R, Moshkovich YJ. Waning of the Humoral Response to SARS-CoV-2 in Pregnancy is Variant-Dependent. medRxiv. 2021.
Goldshtein I, Steinberg DM, Kuint J, Chodick G, Segal Y, Shapiro Ben David S, et al. Association of BNT162b2 COVID-19 vaccination during pregnancy with neonatal and early infant outcomes. JAMA Pediatr. 2022;176(5):470.
Fell DB, Dhinsa T, Alton GD, Török E, Dimanlig-Cruz S, Regan AK, et al. Association of COVID-19 vaccination in pregnancy with adverse peripartum outcomes. JAMA. 2022;327(15):1478.
Dick A, Rosenbloom JI, Gutman-Ido E, Lessans N, Cahen-Peretz A, Chill HH. Safety of SARS-CoV-2 vaccination during pregnancy- obstetric outcomes from a large cohort study. BMC Pregnancy Childbirth. 2022;22(1):166.
Citu IM, Citu C, Gorun F, Sas I, Tomescu L, Neamtu R, et al. Immunogenicity following administration of BNT162b2 and Ad26.COV2.S COVID-19 vaccines in the pregnant population during the third trimester. Viruses. 2022;14(2):307.
Blakeway H, Prasad S, Kalafat E, Heath PT, Ladhani SN, Le Doare K, et al. COVID-19 vaccination during pregnancy: coverage and safety. Am J Obstet Gynecol. 2022;226(2):236.e1-e14.
Madhi SA, Nunes MC. Experience and challenges on influenza and pertussis vaccination in pregnant women. Hum Vaccin Immunother. 2018;14(9):2183–8.
Karasek D, Baer RJ, McLemore MR, Bell AJ, Blebu BE, Casey JA, et al. The association of COVID-19 infection in pregnancy with preterm birth: a retrospective cohort study in California. Lancet Reg Health Am. 2021;2.
Skirrow H, Barnett S, Bell S, Riaposova L, Mounier-Jack S, Kampmann B, Holder B. Women's views on accepting COVID-19 vaccination during and after pregnancy, and for their babies: a multi-methods study in the UK. BMC Pregnancy Childbirth. 2022;22(1):33. https://doi.org/10.1186/s12884-021-04321-3.
Munoz FM. Can We Protect Pregnant Women and Young Infants From COVID-19 Through Maternal Immunization? JAMA Pediatr. 2021;175(6):561–2.
Flannery DD, Gouma S, Dhudasia MB, Mukhopadhyay S, Pfeifer MR, Woodford EC, et al. Assessment of maternal and neonatal cord blood SARS-CoV-2 antibodies and placental transfer ratios. JAMA Pediatr. 2021;175(6):594–600.
Rottenstreich M, Sela H, Rotem R, Kadish E, Grisaru SG. Uptake and outcomes of Covid-19 vaccination during the third trimester of pregnancy: a multicenter study. Am J Obstet Gynecol. 2022;226(1):S401–2.
Ma Y, Deng J, Liu Q, Du M, Liu M, Liu J. Effectiveness and safety of COVID-19 vaccine among pregnant women in real-world studies: a systematic review and meta-analysis. Vaccines. 2022;10(2):246.
De Rose DU, Salvatori G, Dotta A, Auriti C. SARS-CoV-2 vaccines during pregnancy and breastfeeding: a systematic review of maternal and neonatal outcomes. Viruses. 2022;14(3):539.
Acknowledgements
Not applicable.
Funding
The authors declare no funding information.
Author information
Authors and Affiliations
Contributions
AS: Conceptualization, Investigation, Project administration, Writing- original draft, Writing- review & editing; OK and MT: Conceptualization, Writing- original draft; HF and MG: Investigation, Writing- review & editing; SM: Project administration, Writing- review & editing. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Table 1.
Databases searched and search strategies employed. Supplementary Table 2. NIH Quality Assessment Checklist. Supplementary Figure 1. Funnel Plot of Studies Included in the Meta-analysis of adverse neonatal outcomes (ANO).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shafiee, A., Kohandel Gargari, O., Teymouri Athar, M.M. et al. COVID-19 vaccination during pregnancy: a systematic review and meta-analysis. BMC Pregnancy Childbirth 23, 45 (2023). https://doi.org/10.1186/s12884-023-05374-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12884-023-05374-2