Abstract
Background
Ovarian hyperstimulation syndrome (OHSS) is a rare but serious complication of controlled ovarian stimulation. Frozen-embryo transfer (ET) is prompted to be performed in the next menstrual cycles after cancellation of fresh-ET after occurrence of OHSS. However, effects of frozen-ET in the second menstrual cycle have never been investigated. Therefore, this study aimed to assess this in the menstrual cycle after OHSS.
Methods
The OHSS group included 342 women with moderate-severe OHSS who underwent the first frozen-ET in the second menstrual cycle in the First Affiliated Hospital of Anhui Medical University from June 2018 to September 2019. A total of 342 women without OHSS who received frozen-ET in the second menstrual cycle were selected as control group matched by age, body mass index, fertility history, ovulation induction scheme. Uni- and multi-variable conditional logistic regression was used to estimate the association between moderate-severe OHSS and pregnancy outcomes.
Results
There were no significant differences in maternal outcomes (miscarriage, preterm birth and pregnancy complications including gestational diabetes mellitus, pregnancy-induced hypertension, placenta previa, premature rupture of membranes and postpartum hemorrhage) and in neonatal outcome (birth-weight and body length, neonatal congenital diseases and other complications) between the two groups in either uni- or multi-variable models.
Conclusions
Frozen-ET in the menstrual cycle after OHSS has similar maternal and neonatal outcomes as in women without OHSS. This study indicates that frozen-ET could be performed in the second menstrual cycle in women who recovered from moderate-severe OHSS.
Similar content being viewed by others
Background
Ovarian hyperstimulation syndrome (OHSS) is a complication associated with controlled ovarian stimulation (COS) during infertility treatment [1,2,3]. Moderate-severe OHSS occurs approximately in 1-5% of in vitro fertilization (IVF) cycles [4,5,6], whilst mild OHSS is usually unnoticed. OHSS is more severe in China with moderate stage in 3-6% in the Chinese society and severe in 0.2-1% among women undergoing IVF [7, 8]. Moderate OHSS clinical manifestations include abdominal pain, ovarian enlargement with cyst formation, ascites or pleural effusion, and severe cases can be life-threatening [9,10,11]. Studies have found increased odds of multiple pregnancies, gestational diabetes mellitus (GDM), preterm birth and pregnancy-induced hypertension (PIH) among OHSS women compared with women without OHSS [12,13,14,15].
Frozen embryo transfer (frozen-ET) has been shown to reduce the risk of severe OHSS and has been prompted to avoid deterioration of OHSS [14, 16,17,18]. When moderate or severe OHSS is occurring, fresh-ET cycles are cancelled to allow the women to recover from OHSS in clinics [5, 19, 20]. Termination of fresh-ET is frustrating to both infertile couples and clinicians, and earlier embryos transfer is helpful to releave the unhealthy psychology. Although attempts of frozen-ET in the menstrual cycle after embryo retrieval are usually performed among women with OHSS, pregnancy outcomes are unknown. Therefore, this study aimed to assess pregnancy outcomes of frozen-ET in the menstrual cycle after oocytes retrieval among women with moderate-severe OHSS.
Methods
Study population
From the 10.090 women in the Reproductive Center of the First Affiliated Hospital of Anhui Medical University 5.746 women had the first frozen cycle during the period from June 2018 to September 2019. Among them, 705 (12.3%) had moderate-severe OHSS. Inclusion criteria included women ≤40 years who were at high risk of OHSS [21, 22] and frozen-ET in the menstrual cycle after oocytes retrieval. Exclusion criteria were: (1) fresh-ET cycle, (2) refrozen-ET cycle, (3) donor sperm and donor oocyte, (4) preimplantation genetic testing, (5) chromosomal abnormality, (6) clinical data missing, and (7) other diseases that may affect pregnancy outcomes, including diabetes, hypothyroidism, hyperthyroidism, hyperprolactinemia, pituitary tumor, systemic lupus erythematosus, rheumatoid, rheumatic cardiopathy, sicca syndrome and mental diseases.
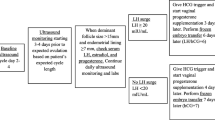
Inclusion criteria were fulfilled in 342 (48.5%) women allocated to the OHSS group. The control group consisted of 342 women, who conceived via the first Frozen-ET cycle during the same time period and did not develop OHSS, matched by age, body mass index (BMI), etiology, fertility history and ovulation induction scheme. The flowchart of the study is summarized in Fig. 1.
Classification of OHSS
Moderate and severe OHSS was classified according to the American Society for Reproductive Medicine criteria [23]. Moderate OHSS is defined as symptoms including a considerable degree of abdominal discomfort, plus nausea, vomiting, and/or diarrhoea, ovaries usually 5 to 12 cm in diameter. Classification of severe OHSS was as follows: ovaries larger than 12 cm in diameter, marked gastrointestinal symptoms, oliguria, ascites, pleural effusion, hypovolemia and hemoconcentration with electrolyte imbalance. Information used to categorize OHSS included size of the ovaries on the day of oocyte collection, ascites, pleural effusion and laboratory data (e.g. Hct, electrolyte, liver enzymes).
Pregnancy outcomes
Data on pregnancy outcomes related to mother and neonate were extracted from the Electronic Medical Records. Maternal outcomes included biochemical pregnancy, clinical pregnancy, ectopic pregnancy, early pregnancy, abortion rate, successful childbirth and pregnancy complications rate (e.g. GDM, PIH, placenta previa, premature rupture of membranes and postpartum hemorrhage). Neonatal outcomes included gestational age at birth, birth-weight and body length, congenital defects rate, jaundice rate and neonatal mortality. Serum human chorionic gonadotropin (HCG) levels on the 14th day after ET were assessed to determine biochemical pregnancy. Clinical pregnancy was defined as a sac visible by ultrasonography with a fetal heartbeat at 7 weeks after ET. Miscarriage with positive heart activity was used to define loss of clinical pregnancy.
Other variables
Demographic and clinical data of the women were retrieved from the Electronic Medical Records including age, years of infertility, types of infertility, number of pregnancies, causes of infertility, BMI, basic sex hormone levels (follicle-stimulating hormone, estradiol2, prolactin, luteinising hormone, testosterone and total dosage of gonadotropin). On trigger day, the following data were extracted: number of follicles, retrieved oocytes, transplantable embryos, high-quality embryos and sex hormone values (estradiol2, luteinising hormone and progesterone). The information of implantation included transfer interval, endometrial thickness on transfer day, number of transferred embryos, clinical pregnancy rate and ectopic pregnancy rate.
Statistical analysis
Continuous data were presented as mean ± standard deviation and count data were described using frequencies and percentages. Normality tests of continuous data were performed by visual inspection of the histogram and distribution curve. Comparison of continuous variables between the groups was conducted using Student’s t-test and analysis of variance. Chi-squared or Fisher exact test were used for comparison of categorical variables as appropriate. Multivariable regression was used to determine the association of moderate-severe OHSS with maternal and neonatal outcomes after controlling for confounders. Potential confounders with P-values < 0.20 in the univariate analysis were included in the multivariate model. Confounders in the multivariable models included BMI, basal serum follicle-stimulating hormone, estradiol2, prolactin, luteinising hormone, total dosage of gonadotropin, total days of gonadotropin, number of follicles, serum estradiol2, luteinising hormone, progesterone on trigger day, number of retrieved oocytes, number of transplantable embryos, number of high-quality embryos. P-values < 0.05 indicated statistically significant differences. Statistical analyses were conducted using SPSS (Windows version 24.0, IBM-SPSS, Chicago, IL).
Results
Women’ characteristics
Baseline characteristics of the moderate-severe OHSS and matched control groups are presented in Table 1. The incidence of severe OHSS was 111/5746 (1.9%) per IVF cycle. Follicle-stimulating hormone level was lower and luteinising hormone higher in the OHSS group as compared to controls. There were no significant differences between the two groups of each 342 women in the ovulation induction program. Total amount of gonadotropins used in the OHSS group was lower (P < 0.05). On trigger day, serum levels of estradiol2 and progesterone were significantly higher and the number of removed oocytes and transplantable embryos were significantly greater in the OHSS group than in the control group (P < 0.05). Regarding perinatal outcomes, 123/304 (40.5%) successful births in the moderate OHSS group, 15/38 (39.5%) in the severe group and 149/342 (43.6%) in the control group (P = 0.478; Fig. 1).
Pregnancy outcomes between moderate-severe OHSS group and control group
Rates of biochemical pregnancy, clinical pregnancy, extrauterine pregnancy and early abortion were similar in both groups (Table 2). Rates of GDM, PIH, placenta previa, premature rupture of membranes and postpartum hemorrhage were all comparable between two groups (all P > 0.05). Similarly, gestational age at birth, preterm birth rate or caesarean section rate in OHSS group were not statistically different from matched controls. Moreover, none of the maternal and neonatal outcomes were associated with moderate-severe OHSS in either univariable or multivariable regression models.
Pregnancy outcomes among moderate OHSS group, severe OHSS group and controls
Comparisons of women’ characteristics among the moderate OHSS, severe OHSS and control group are shown in Supplementary Table 1. Women with moderate OHSS had the lowest level of basal follicle-stimulating hormone level and the difference was statistically significant compared with controls. The number of follicles was the highest in women with moderate OHSS compared with severe OHSS and controls (P < 0.05). Additionally, numbers of both left and right ovary follicles in severe OHSS were higher than in controls (P < 0.001). Estradiol2 and progesterone levels on trigger day in moderate OHSS were significantly higher than those in the control group (P < 0.001). Hormone levels in severe OHSS were higher than that in control group, however, lower than in moderate OHSS. The number of transplantable and high-quality embryos in severe OHSS was highest compared with the other groups. Supplementary Table 2 summarized pregnancy data for moderate to severe OHSS and controls. Furthermore, there were no significate differences in the odds of pregnancy outcomes among moderate OHSS, severe OHSS and non-OHSS women (Table 3).
Discussion
To the best of our knowledge, this is the first study investigating the effect of frozen-ET in the menstrual cycle after oocyte retrieval on pregnancy outcomes among women with OHSS. We found that there are no differences in the odds of pregnancy outcomes between frozen-ET among women with moderate-severe OHSS compared with controls, and this persisted among women with moderate OHSS, severe OHSS and controls. These findings indicate that frozen-ET in the subsequent menstrual cycle after OHSS has comparable pregnancy outcomes with women without OHSS regardless of the severity of OHSS.
HCG used in COS stimulates the secretion of vascular endothelial growth factor (VEGF). Over-secretion of VEGF, however, increases the capillary permeability resulting in the clinical symptoms of OHSS related to systemic vascular endothelial dysfunction and micro-thromboembolism in GDM and PIH [24,25,26,27,28,29,30]. Treatment strategy is clinically adopted to remit the acute condition, and fresh-ET is prompted to be cancelled. Anticipated conception propels the earliest frozen-ET in the menstrual cycle after OHSS, but the therapeutic effect has never been reported. This study has good homogeneity and comparability, indicated by the indifference in most clinical characteristics between women with and without OHSS. The proportion of OHSS in this study is slightly higher than that in general infertile populations [23, 31]. This could be attributed by the high proportion of women with polycystic ovaries (PCO) (4713/11090, 42.5%) in our reproductive center [32]. The number of retrieved oocytes, number of transplantable embryos and high-quality embryos were higher in the OHSS group, which is in line with previous findings due to hyper-responsiveness of ovarian tissue [33, 34]. The total dosage of gonadotropin for ovarian stimulation was significantly lower in women with OHSS than in those without OHSS, which is expected because women with OHSS tends to be more sensitive to ovarian stimulation [35]. Moreover, levels of estradiol2 on trigger day in both severe and moderate OHSS groups were higher than controls, which is consistent with the theory that circulating high estradiol2 levels could be of predictive value for the occurrence of OHSS [36, 37].
Fresh-ET in women with OHSS has been shown to increase the rate of loss of pregnancy, preterm birth, GDM and PIH compared with women conceived via IVF and did not develop OHSS, whilst we did not find difference in the odds of pregnancy outcomes between women with OHSS and without OHSS [38,39,40]. This suggests the superiority of frozen-ET to fresh-ET for women with OHSS. This could be because the increase of VEGF, IL-1α, IL-6 and TGF-β triggered by COS has been back to physiological level in approximately 20 days after OHSS [36, 41,42,43,44]. This suggests that the pathological consequences of OHSS are eliminated in the subsequent menstrual cycle. Moreover, elimination of the detrimental effects of OHSS on pregnancy outcomes was regardless of the severity of OHSS.
Conclusions
This study found no differences in pregnancy outcomes of the first frozen-ET cycle between women with moderate-severe OHSS and those without OHSS. This suggests that once women are recovered from OHSS in the next menstrual cycle, frozen-ET could be performed for anticipated conception. This study provides supportive evidence for the clinical treatment strategy of performing frozen-ET in the menstrual cycle after OHSS.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- OHSS:
-
Ovarian Hyperstimulation Syndrome
- F-ET:
-
Frozen Embryo Transfer
- IVF:
-
In-Vitro Fertilization
- COS:
-
Controlled Ovarian Stimulation
- HCG:
-
Human Chorionic Gonadotropin
- VEGF:
-
Vascular Endothelial Growth Factor
- GDM:
-
Gestational Diabetes Mellitus
- PIH:
-
Pregnancy-Induced Hypertension
- BMI:
-
Body Mass Index
References
Blumenfeld Z. The ovarian Hyperstimulation syndrome. Vitam Horm. 2018;107:423–51.
Schirmer DA, Kulkarni AD, Zhang Y, Kawwass JF, Boulet SL, Kissin DM. Ovarian hyperstimulation syndrome after assisted reproductive technologies: trends, predictors, and pregnancy outcomes. Fertil Steril. 2020;114(3):567–78.
Tobler KJ, Zhao Y, Weissman A, Majumdar A, Leong M, Shoham Z. Worldwide survey of IVF practices: trigger, retrieval and embryo transfer techniques. Arch Gynecol Obstet. 2014;290(3):561–8.
Nastri CO, Ferriani RA, Rocha IA, Martins WP. Ovarian hyperstimulation syndrome: pathophysiology and prevention. J Assist Reprod Genet. 2010;27(2-3):121–8.
Bosch E, De Vos M, Humaidan P. The future of cryopreservation in assisted reproductive technologies. Front Endocrinol (Lausanne). 2020;11:67.
ESHRE Capri Workshop Group. Erratum to: IVF, from the past to the future: the inheritance of the Capri Workshop Group. Hum Reprod Open. 2020;2020(4):hoaa051.
Wu L, Sun Y, Wan J, Luan T, Cheng Q, Tan Y. A proteomic analysis identifies candidate early biomarkers to predict ovarian hyperstimulation syndrome in polycystic ovarian syndrome patients. Mol Med Rep. 2017;16(1):272–80.
Du DF, Li MF, Li XL. Ovarian hyperstimulation syndrome: a clinical retrospective study on 565 inpatients. Gynecol Endocrinol. 2020;36(4):313–7.
Gómez R, Soares SR, Busso C, Garcia-Velasco JA, Simón C, Pellicer A. Physiology and pathology of ovarian hyperstimulation syndrome. Semin Reprod Med. 2010;28(6):448–57.
Luke B, Brown MB, Morbeck DE, Hudson SB, Coddington CC, Stern JE. Factors associated with ovarian hyperstimulation syndrome (OHSS) and its effect on assisted reproductive technology (ART) treatment and outcome. Fertil Steril. 2010;94(4):1399–404.
Kwik M, Maxwell E. Pathophysiology, treatment and prevention of ovarian hyperstimulation syndrome. Curr Opin Obstet Gynecol. 2016;28(4):236–41.
Raziel A, Schachter M, Friedler S, Ron-El R. Outcome of IVF pregnancies following severe OHSS. Reprod BioMed Online. 2009;19(1):61–5.
Haas J, Baum M, Meridor K, et al. Is severe OHSS associated with adverse pregnancy outcomes? Evidence from a case-control study. Reprod BioMed Online. 2014;29(2):216–21.
Dobrosavljevic A, Rakic S. Risk of gestational hypertension in pregnancies complicated with ovarian hyperstimulation syndrome. J Pak Med Assoc. 2020;70(11):1897–900.
Grossman LC, Michalakis KG, Browne H, Payson MD, Segars JH. The pathophysiology of ovarian hyperstimulation syndrome: an unrecognized compartment syndrome. Fertil Steril. 2010;94(4):1392–8.
Roque M, Haahr T, Geber S, Esteves SC, Humaidan P. Fresh versus elective frozen embryo transfer in IVF/ICSI cycles: a systematic review and meta-analysis of reproductive outcomes. Hum Reprod Update. 2019;25(1):2–14.
Mizrachi Y, Horowitz E, Farhi J, Raziel A, Weissman A. Ovarian stimulation for freeze-all IVF cycles: a systematic review. Hum Reprod Update. 2020;26(1):118–35.
Singh B, Reschke L, Segars J, Baker VL. Frozen-thawed embryo transfer: the potential importance of the corpus luteum in preventing obstetrical complications. Fertil Steril. 2020;113(2):252–7.
Liu W, Zhang C, Wang L, et al. Successful reversal of ovarian hyperstimulation syndrome in a mouse model by rapamycin, an mTOR pathway inhibitor. Mol Hum Reprod. 2019;25(8):445–57.
Bosdou JK, Venetis CA, Tarlatzis BC, Grimbizis GF, Kolibianakis EM. Higher probability of live-birth in high, but not normal, responders after first frozen-embryo transfer in a freeze-only cycle strategy compared to fresh-embryo transfer: a meta-analysis. Hum Reprod. 2019;34(3):491–505.
Banker M, Garcia-Velasco JA. Revisiting ovarian hyper stimulation syndrome: towards OHSS free clinic. J Hum Reprod Sci. 2015;8(1):13–7.
Leijdekkers JA, van Tilborg TC, Torrance HL, et al. Do female age and body weight modify the effect of individualized FSH dosing in IVF/ICSI treatment? A secondary analysis of the OPTIMIST trial. Acta Obstet Gynecol Scand. 2019;98(10):1332–40.
Practice Committee of the American Society for Reproductive Medicine. Prevention and treatment of moderate and severe ovarian hyperstimulation syndrome: a guideline. Fertil Steril. 2016;106(7):1634–47.
Geva E, Jaffe RB. Role of vascular endothelial growth factor in ovarian physiology and pathology. Fertil Steril. 2000;74(3):429–38.
Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56(4):549–80.
de Zuniga I, Colaci D, Sobral F, et al. Role of vascular endothelial cell growth factor (VEGF) and angiopoietins (ANGPTs) in ovarian hyperstimulation syndrome (OHSS). Hum Reprod. 2011;26:I91.
Miller I, Chuderland D, Grossman H, Ron-El R, Ben-Ami I, Shalgi R. The dual role of PEDF in the pathogenesis of OHSS: negating both Angiogenic and inflammatory pathways. J Clin Endocrinol Metab. 2016;101(12):4699–709.
Soares SR, Gomez R, Simon C, Garcia-Velasco JA, Pellicer A. Targeting the vascular endothelial growth factor system to prevent ovarian hyperstimulation syndrome. Hum Reprod Update. 2008;14(4):321–33.
Herr D, Fraser HM, Konrad R, Holzheu I, Kreienberg R, Wulff C. Human chorionic gonadotropin controls luteal vascular permeability via vascular endothelial growth factor by down-regulation of a cascade of adhesion proteins. Fertil Steril. 2013;99(6):1749–58.
Zhai J, Liu J, Cheng X, et al. Zinc finger gene 217 (ZNF217) promoted ovarian Hyperstimulation syndrome (OHSS) through regulating E synthesis and inhibiting Thrombospondin-1 (TSP-1). Sci Rep. 2017;7(1):3245.
Tomás C, Colmorn L, Rasmussen S, Lidegaard Ø, Pinborg A, Andersen AN. Annual incidence of severe ovarian hyperstimulation syndrome. Dan Med J. 2021;68(2):A12190738.
Swanton A, Storey L, McVeigh E, Child T. IVF outcome in women with PCOS, PCO and normal ovarian morphology. Eur J Obstet Gynecol Reprod Biol. 2010;149(1):68–71.
Mourad S, Brown J, Farquhar C. Interventions for the prevention of OHSS in ART cycles: an overview of Cochrane reviews. Cochrane Database Syst Rev. 2017;1(1):Cd012103.
Maheshwari A, Pandey S, Amalraj Raja E, Shetty A, Hamilton M, Bhattacharya S. Is frozen embryo transfer better for mothers and babies? Can cumulative meta-analysis provide a definitive answer? Hum Reprod Update. 2018;24(1):35–58.
Haas J, Yinon Y, Meridor K, Orvieto R. Pregnancy outcome in severe OHSS patients following ascitic/plerural fluid drainage. J Ovarian Res. 2014;7:56.
Carosso AR, Canosa S, Gennarelli G, et al. Luteal Support with very Low Daily Dose of Human Chorionic Gonadotropin after Fresh Embryo Transfer as an Alternative to Cycle Segmentation for High Responders Patients Undergoing Gonadotropin-Releasing Hormone Agonist-Triggered IVF. Pharmaceuticals. 2021;14:228.
Miller D, Vukina J. Recent advances in clinical diagnosis and treatment of male factor infertility. Postgrad Med. 2020;132(sup4):28–34.
Pereira N, Petrini AC, Hancock KL, Rosenwaks Z. Fresh or frozen embryo transfer in in vitro fertilization: an update. Clin Obstet Gynecol. 2019;62(2):293–9.
Vuong LN, Nguyen LK, Le AH, et al. Fresh embryo transfer versus freeze-only after in vitro maturation with a pre-maturation step in women with high antral follicle count: a randomized controlled pilot study. J Assist Reprod Genet. 2021;38(6):1293–302.
Davenport MJ, Vollenhoven B, Talmor AJ. Gonadotropin-releasing hormone-agonist triggering and a freeze-all approach: the final step in eliminating ovarian Hyperstimulation syndrome? Obstet Gynecol Surv. 2017;72(5):296–308.
Abramov Y, Barak V, Nisman B, Schenker JG. Vascular endothelial growth factor plasma levels correlate to the clinical picture in severe ovarian hyperstimulation syndrome. Fertil Steril. 1997;67(2):261–5.
Fang L, Li Y, Wang S, et al. TGF-β1 induces VEGF expression in human granulosa-lutein cells: a potential mechanism for the pathogenesis of ovarian hyperstimulation syndrome. Exp Mol Med. 2020;52(3):450–60.
Melincovici CS, Boşca AB, Şuşman S, et al. Vascular endothelial growth factor (VEGF) - key factor in normal and pathological angiogenesis. Romanian J Morphol Embryol. 2018;59(2):455–67.
Peitsidis P, Agrawal R. Role of vascular endothelial growth factor in women with PCO and PCOS: a systematic review. Reprod BioMed Online. 2010;20(4):444–52.
Acknowledgements
We are thankful to the First Affiliated Hospital of Anhui Medical University providing the required technical and administrative support. Furthermore, without the efforts of our members, this research would not have been possible.
Funding
To date this work has been supported by the Natural Science Foundation of Anhui Province (No.1908085MH244), National Key Research and Development Program of China (2016YFC1000204-3 and 2016YFC1000204-2) and National Natural Science Foundation of China (NSFC-82000399). The funders had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript. Open access funding provided by the First Affiliated Hospital of Anhui Medical University.
Author information
Authors and Affiliations
Contributions
YY, DZ and QW contributed to data collection, analysis and interpretation of data and drafted manuscript; CM contributed to analysis and interpretation of data and critical revision of manuscript; DL, JW, PZ and ZW contributed to data collection and quality control; XP, YC and XX: project conception and study design, interpretation of data and critical revision of manuscript. All authors have read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the ethics committee of Anhui Medical University (20160270). All participants have consented and signed to use their information for research and publications without disclosure of their private information. All authors have read and approved the submission of the manuscript; the manuscript has not been submitted elsewhere nor published elsewhere in whole or in part. All research procedures were carried out in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no potential conflicts of interest, including no financial competing interests, personal competing interests and professional competing interests relevant to the subject of this manuscript.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Table 1.
Basic data of moderate-severe OHSS group and matched Control
Additional file 2: Supplementary Table 2.
Ovulation induction and transplant information of moderate-severe OHSS and control.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, Y., Zhu, D., Wang, Q. et al. Frozen embryo transfer in the menstrual cycle after moderate-severe ovarian hyperstimulation syndrome: a retrospective analysis. BMC Pregnancy Childbirth 22, 907 (2022). https://doi.org/10.1186/s12884-022-05239-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12884-022-05239-0