Abstract
Background
It remains unclear whether polycystic ovary syndrome (PCOS) is an independent risk factor for pregnancy complications in women undergoing assisted reproductive technology (ART) treatment. For the integrative treatment of PCOS patients, it is still important to investigate the pregnancy outcomes of PCOS patients after adjusting for potential biases, such as body mass index, embryo quality and endometrial preparation method.
Methods
This retrospective cohort study ultimately included a total of 336 PCOS patients who conceived after single thawed blastocyst transfer in the PCOS group and 2,325 patients in the control group from January 2018 to December 2020. A propensity score matching (PSM) model was used, and 336 PCOS patients were matched with 336 patients in the control group.
Results
Before PSM, no differences in the miscarriage rate, pregnancy complication rate, preterm birth rate, or live birth rate were found between the PCOS group and the control group. After PSM, the late miscarriage rate of the PCOS group was significantly higher than that of the control group (3.3% vs. 0.6%, P = 0.040), although the early miscarriage rates were similar (14.0% vs. 13.7%). The rates of pregnancy complications, preterm birth and live birth in the PCOS group were comparable to those in the matched control group (P = 0.080, P = 0.105, P = 0.109, respectively). The neonatal weights of male infants and female infants were similar between the two groups (P = 0.219, P = 0.169). Subgroup analysis showed that PCOS patients with homeostasis model assessment of insulin resistance (HOMA-IR) levels ≥ 2.49 had a significantly increased risk of preterm birth compared with those with HOMA-IR levels < 1.26 and 1.26 ≤ HOMA-IR levels < 2.49 (26.0% vs. 6.0% vs. 9.8%, P = 0.005). PCOS patients with total testosterone levels ≥ 0.7 ng/ml had a higher early miscarriage rate but a lower late miscarriage rate than those with total testosterone levels < 0.7 ng/ml (29.4% vs. 12.3%, 0% vs. 3.6%, respectively, P = 0.032).
Conclusions
PCOS is an independent risk factor for late miscarriage in patients conceived after a single thawed blastocyst transfer, even after adjusting for biases. Among PCOS patients, insulin resistance and hyperandrogenism are associated with a higher risk of preterm birth and early miscarriage, respectively.
Similar content being viewed by others
Background
Polycystic ovary syndrome (PCOS) is a prevalent endocrine and metabolic disease in women. According to different definitions, the prevalence rates of PCOS range from 5 to 20% [1]. The characterizing features of women with PCOS usually include clinical or biochemical hyperandrogenism, ovulatory dysfunction, and polycystic ovarian morphology. Women of reproductive age with PCOS usually suffer from obesity and infertility [2, 3]. Assisted reproductive technology (ART) treatment is recommended for patients with PCOS who suffer from infertility.
A growing body of evidence demonstrates that PCOS is associated with pregnancy complications [4, 5]. The risk of premature delivery, gestational diabetes mellitus, and hypertensive disorders of pregnancy in these patients is increased by nearly 2- to 4-fold [5]. High body mass index (BMI) may exacerbate maternal pregnancy complications and neonatal outcomes in PCOS patients [6]. In ART cycles, PCOS patients with high BMI are more prone to suffer from spontaneous abortion than those with normal weight [7]. However, controversies still exist. It has been pointed out that PCOS increases pregnancy complications independent of BMI [8]. In addition, in research investigating the pregnancy outcomes of PCOS patients undergoing IVF, the impact of embryo quality was not considered, which may increase the heterogeneity [9]. Embryo morphology parameters are associated with the pregnancy outcomes of patients undergoing blastocyst transfer [10]. For example, studies have suggested that trophectoderm (TE) quality has an impact on the live birth rate and birth weight [11, 12]. In addition, some studies have suggested that endometrial preparation methods affect pregnancy outcomes, albeit controversy still exists [13,14,15].
To better assess and treat pregnant PCOS patients, more research is needed to investigate the pregnancy outcomes of PCOS patients after adjusting for potential biases. Therefore, to rule out the potential effect of biases, this study aims to analyze the risk of adverse pregnancy outcomes among PCOS women who conceived after single thawed blastocyst transfer, by applying a propensity score matching (PSM) model.
Methods
Study design and subject screening
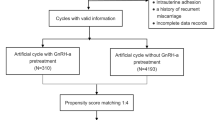
This retrospective cohort study was conducted in the First Affiliated Hospital, Sun Yat-sen University. From January 2018 to December 2020, PCOS patients first diagnosed with clinical pregnancy after a single thawed blastocyst transfer were initially enrolled (n = 431). The inclusion criteria were as follows: i) patients who had a diagnosis of PCOS according to the Rotterdam criteria [16]; ii) patients who underwent IVF/ICSI cycles; and iii) patients for whom a single frozen blastocyst was transferred. Patients who underwent IVF/ICSI due to fallopian tubal factor or male factor infertility were initially enrolled in the control group (n = 3267). The exclusion criteria of both the PCOS group and the control group were as follows: 1) preimplantation genetic diagnosis/screening cycles; 2) history of recurrent miscarriage; 3) endometriosis; 4) luteal phase ovarian stimulation; 5) clomiphene citrate administration; 6) metformin administration; 7) congenital uterine malformations; and 8) intrauterine adhesion. This study ultimately included a total of 336 PCOS patients in the PCOS group and 2,325 patients in the control group.
ART procedures
Stimulation protocols of patients undergoing IVF/ICSI cycles included the gonadotropin-releasing hormone (GnRH) antagonist protocol or GnRH agonist protocol. Gonadotropin was administered to stimulate multiple follicles development. The dosage ranged from 75 to 300 IU per day. Transvaginal ultrasound and blood tests of FSH, LH, estradiol, and progesterone levels were required every three to four days to evaluate follicular development. When at least one dominant follicle had a diameter ≥ 18 mm, human chorionic gonadotropin (HCG) was administered to induce oocyte maturation. Oocyte retrieval was performed 36 h later. Different fertilization methods were performed according to sperm quality. Blastocysts were scored based on the Gardner grading system [17]. In this study, the blastocyst expansion stage was divided into two types, 1) expansion stage < 4 and 2) expansion stage ≥ 4, including expanded blastocysts with a thinning zona, full blastocysts or expanding blastocysts. The inner cell mass (ICM) and TE quality were graded according to the number of ICM or TE cells. As patients underwent FET cycles, single thawed blastocyst transfer was performed under transabdominal ultrasound guidance after endometrial preparation. Patients were administered luteal phase support until the 10th week of gestation. The endometrial preparation protocols included hormone replacement treatment (HRT) cycles, natural cycles (NCs), letrozole-stimulated cycles and GnRH agonist (GnRH-a) cycles: (1) Hormone replacement treatment (HRT) cycles: on Days 2–5 of the menstrual cycle, patients were administered 2–8 mg of estradiol valerate daily (Progynova, Bayer Schering Pharma AG, Berlin, Germany) if the serum estradiol level was < 50 pg/ml and the endometrial thickness was < 5 mm. Serum estradiol level measurements and transvaginal ultrasound scans were performed every 3 to 5 days. If the serum estradiol level was > 100 pg/ml and the endometrial thickness was > 8 mm, progesterone (20 mg per ampoule; Shanghai General Pharmaceutical Co., LTD., China) was administered to achieve endometrial transformation. Then, embryo transfer was carried out on Day 6 of progesterone administration. (2) Natural cycles (NCs): on Days 10 to 12 of the menstrual cycle, transvaginal ultrasound scans and LH urine tests were carried out every 3 to 4 days to monitor the development of the dominant follicle. If the dominant follicle was ≥ 18 mm, 10,000 IU of HCG was injected to induce ovulation. An embryo was transferred on Day 6 after ovulation. (3) Letrozole (LE) cycles: patients took two tablets of letrozole (letrozole tablet, 2.5 mg, Zhejiang Hisun Pharmaceutical Co., LTD., China) daily for five days starting from the second to the fifth day of menstruation. Then, 75–150 units of human menopausal gonadotropin (HMG, Livzon Pharmaceutical Group Inc., China) were injected daily. Transvaginal ultrasound monitoring and serum LH, estradiol, and progesterone level measurements were performed every 3 to 4 days. When a dominant follicle with a diameter above 18 mm was observed, 10,000 IU of HCG was injected to induce oocyte maturation, and embryo transfer was performed on Day 6 after ovulation. (4) GnRH agonist (GnRH-a) cycles: GnRH agonist (1.0 mg, triptorelin, Feiling Pharmaceutical Co., LTD., Germany) was subcutaneously injected. Fourteen days after injection, serum FSH, LH and estradiol level measurements and transvaginal ultrasound scans were carried out to confirm the pituitary downregulation effect. HRT treatment was started on Days 3 to 5 of withdrawal bleeding, and the details were the same as those for the HRT cycles.
Outcome measures
The homeostasis model assessment of insulin resistance (HOMA-IR) is calculated as follows: fasting insulin × fasting glucose/22.5 [18]. Fasting insulin, fasting glucose and total testosterone (TT) were tested with chemiluminescence.
Supplemental Table 1 shows the definitions of the main outcomes. Clinical pregnancy was defined as the presence of one gestational sac at 7 weeks of gestation after FET [19]. Early or late miscarriages were defined as pregnancy losses before 12 weeks or at 12–24 weeks of gestation, respectively [20]. Pregnancy complications mainly included preeclampsia (defined as gestational hypertension [blood pressure ≥ 140/90 mmHg] or proteinuria plus organ dysfunction after 20 weeks of gestation) [21], pregnancy-induced hypertension syndrome (defined as hypertension [blood pressure ≥ 140/90 mmHg] after 20 weeks of gestation, but resolving up to 12 weeks postpartum) [22], gestational diabetes mellitus (defined as the occurrence or discovery of abnormal glucose metabolism during pregnancy) [23], placenta previa (referred to the lower placental edge overlapping or within 2 cm of the internal cervical orifice in late pregnancy) [24], premature rupture of membrane (the rupture of membranes prior to delivery) [25], fetal distress (referred to the presence of fetal heart trace, with or without fetal acidosis) [26], oligohydramnios (referred to the maximum vertical depth of the amniotic fluid pool or amniotic fluid < 2 cm) [27], and macrosomia (defined as a birth weight ≥ 4000 g) [28]. Preterm birth was defined as delivery after 28 weeks of gestation but before 37 weeks of gestation [29]. Live birth was defined as cycles with at least one live-born baby [19].
Statistical analysis
All data were analyzed with SPSS software (version 26.0, Inc., Chicago, USA). Continuous variables with a normal distribution are described as the mean and standard deviation. Nonnormally distributed variables are expressed as medians and interquartile ranges. Student’s t test or the Mann‒Whitney U test was used to compare differences between the two groups. Categorical variables, expressed as frequencies and percentages, were analyzed with the chi-square test or Fisher’s exact test. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. P < 0.05 was considered statistically significant.
With the propensity score matching (PSM) extension of SPSS, a PSM model was performed to minimize bias and increase comparability between the PCOS group and the control group. Four covariates were selected to calculate the propensity score: female age, TE grade, BMI, and the endometrial preparation method. A caliper value was set as 0.02 to ensure matching accuracy. Patients in the PCOS group were matched 1:1 to patients in the control group using the nearest-neighbor random matching algorithm. The sampling method was set as without replacement, and the random number seed was 1 to 6.
Results
Baseline characteristics of the population
A total of 336 PCOS patients were ultimately enrolled in the PCOS group. In addition, 2,325 patients were included in the control group. After 1:1 matching, 336 PCOS patients were successfully matched with 336 patients in the control group. The baseline characteristics of the PCOS group and the control group before and after PSM are shown in Tables 1 and 2, respectively. Before PSM, patients in the PCOS group were younger (30 vs. 32, P < 0.001), had a higher BMI (21.6 vs. 20.8, P < 0.001), and had a higher level of basal TT (0.42 vs. 0.29, P < 0.001) than those in the control group. In addition, the PCOS group had a lower proportion of transferring a C TE grade blastocyst than the control group (5.1% vs. 9.3%, P = 0.021). The distribution of endometrial preparation protocols differed between the PCOS group and the control group (P < 0.001). In the PCOS group, 75.6% of the patients underwent HRT cycles, which was 1.75 times higher than that in the control group. In addition, 8.3% of the PCOS patients underwent the NC protocol, whereas 44.0% of the non-PCOS patients underwent the NC protocol. After PSM, no differences in maternal age, BMI or TE quality were found, but the endometrial preparation protocol was still different in the two matched groups (P < 0.001).
Clinical outcomes before and after PSM
Before PSM, the rates of miscarriage, pregnancy complications, preterm birth and live birth in the PCOS group were similar to those in the control group (shown in Table 1). The pregnancy outcomes after PSM are presented in Table 2. The late miscarriage rate of the PCOS group was significantly higher than that of the control group (3.3% vs. 0.6%, P = 0.040), although the early miscarriage rates were similar (14.0% vs. 13.7%). The pregnancy complication rate of the PCOS group was slightly higher than that of the control group, but no significant difference was found (25.6% vs. 19.9%, P = 0.080; OR 1.38, 95% CI 0.96–1.99). There were no significant differences in the rates of preterm birth and live birth between the PCOS group and the control group (10.7% vs. 7.1%, P = 0.105, OR 1.56, 95% CI 0.91–2.68; 79.5% vs. 84.2%, P = 0.109, OR 0.73, 95% CI 0.49–1.08, respectively). The neonatal weights in the PCOS group were similar to those in the control group (female infants: 3150 vs. 3200, P = 0.169; male infants: 3300 vs. 3345, P = 0.219).
Considering that the endometrial preparation protocol might affect clinical outcomes, the Cochran‒Mantel‒Haenszel test was applied (shown in Supplemental Table 2). The results showed that after adjusting for endometrial preparation protocols, the rates of miscarriage, pregnancy complications, preterm birth and live birth were comparable between the two matched groups (P = 0.577, P = 0.089, P = 0.238, and P = 0.228, respectively).
Subgroup analysis in PCOS patients
We further performed subgroup analysis in the PCOS group according to the levels of HOMA-IR, TT and BMI. Supplemental Table 3 displays the pregnancy outcomes of the PCOS patients with different HOMA-IR levels. A total of 202 of the 336 PCOS patients underwent fasting glucose and fasting insulin tests. The HOMA-IR level was stratified into quartiles (25% quartile: 1.26; 75% quartile: 2.49). Compared with the PCOS patients with HOMA-IR levels < 1.26 and 1.26 ≤ HOMA levels < 2.49, the PCOS patients with HOMA-IR levels ≥ 2.49 had a significantly higher preterm birth rate (26.0% vs. 6.0% vs. 9.8%, P = 0.005). However, the rates of miscarriage, pregnancy complications and live birth were comparable (P = 0.772, P = 0.440, P = 0.861, respectively).
Considering that the mean TT level of PCOS patients in other studies was nearly 0.7 ng/ml [30, 31], the cutoff value was set as 0.7 in the TT subgroup analysis. We divided the PCOS patients into two subgroups: the TT level < 0.7 ng/ml subgroup (low TT subgroup) and TT level ≥ 0.7 ng/ml subgroup (high TT subgroup). As presented in Table 3, the high TT subgroup had a significantly higher early miscarriage rate but a lower late miscarriage rate than the low TT subgroup (29.4% vs. 12.3%, 0% vs. 3.6%, P = 0.032). Correspondingly, the prevalence of pregnancy complications (14.7% vs. 26.8%, P = 0.125; OR 0.47, 95% CI 0.18–1.26), preterm birth (5.9% vs. 11.3%, P = 0.337; OR 0.49, 95% CI 0.11–2.15) and live birth (70.6% vs. 80.5%, P = 0.177; OR 0.58, 95% CI 0.26–1.29) in the high TT subgroup was comparable to that in the low TT subgroup.
Table 4 shows the association between BMI and pregnancy outcomes in the PCOS group. The BMI level was stratified into quartiles (25% quartile: 20.12; 75% quartile: 23.92). BMI quartiles were not significantly associated with the rates of miscarriage, pregnancy complications, preterm birth or live birth (P = 0.702, P = 0.410, P = 0.326, P = 0.595, respectively).
Discussion
In this propensity-matched retrospective study, we found that PCOS patients who conceived after a single thawed blastocyst transfer had a higher risk of late miscarriage than patients without PCOS. Additionally, IR significantly increased the risk of preterm birth in PCOS patients. PCOS patients with a higher level of TT were at increased risk of early miscarriage, and those with a lower level of TT were prone to suffer from late miscarriage.
Previous studies considered PCOS to be an independent risk factor for miscarriage [32, 33]. Due to the impact of anovulation, obesity, IR and androgen excess, sex-hormone receptors, enzymatic pathways and metabolic pathways change abnormally [34, 35]. Hence, the endometrial function of PCOS patients is impaired, which may compromise their pregnancy outcomes. Indeed, a large cohort study found that the miscarriage rate in PCOS patients was 20% higher than that in patients without PCOS [36]. However, other studies have shown inconsistent conclusions. A retrospective cohort study revealed that PCOS patients aged 35 years or older had a higher cumulative pregnancy rate and live birth rate but a miscarriage rate similar to that of age- and BMI-matched control patients [37]. Another study concluded that PCOS patients who conceived through IVF had a similar clinical miscarriage rate compared with patients without PCOS [38]. In contrast, our data demonstrated that PCOS patients were prone to late miscarriage compared with the matched control group. Our results indicated that PCOS was a risk factor for late miscarriage in patients who conceived after FET after adjusting for the biases of age, BMI and embryo quality. Consistent with our results, Cai et al. [32] applied multivariable analysis and found that PCOS increased the risk of late miscarriage, albeit no significant difference was found. They adjusted several important factors, such as age, embryo transfer number and BMI, but did not adjust for embryo quality.
The distribution of endometrial preparation methods differed between the PCOS group and the control group, even after PSM. We found that 75.6% of the PCOS patients underwent HRT cycles, which may be attributed to the oligoovulation and/or anovulation characteristics of PCOS. With the Cochran‒Mantel‒Haenszel test, we found that after PSM, the preparation protocols did not increase pregnancy complications in the PCOS group or the matched control group. Mackens et al. [15] pointed out that there is insufficient evidence to support which endometrial preparation protocol is optimal for FET. However, whether endometrial preparation methods affect reproductive outcomes is still controversial. A randomized clinical trial suggested that the NC protocol was superior to HRT cycles [13]. Moreover, Li et al. [14] reported that the NC protocol was associated with a decreased risk of obstetric and perinatal complications compared with HRT ovarian stimulation protocols. More prospective randomized clinical trials are required to determine the optimal endometrial preparation method for patients with PCOS.
Notably, we found that PCOS patients with preconception HOMA-IR levels ≥ 2.49 had a higher risk of preterm birth. A large population-based cohort study found that PCOS increased the risk of preterm birth among mothers with type 2 diabetes or GDM, but HOMA-IR levels were not included in this study [39]. IR is detrimental to the decidualization process and glucose homeostasis of the endometrium [35], which may compromise the pregnancy outcomes of PCOS patients. Future research is needed to further validate whether IR increases the risk of preterm birth in PCOS patients and explore the underlying mechanism.
We further applied subgroup analysis in the PCOS group according to different TT levels. We found that a TT level ≥ 0.7 ng/ml was associated with the occurrence of early miscarriage instead of late miscarriage. Chen et al. [40] reported that PCOS patients undergoing fresh embryo transfer had a higher risk of late pregnancy loss than PCOS patients undergoing frozen embryo transfer. In contrast, our results showed that among PCOS patients undergoing single thawed blastocyst transfer, a TT level ≥ 0.7 ng/ml increased the risk of early miscarriage. The mechanisms of miscarriage in patients with PCOS vary. High androgen levels decrease the expression of homeoboxA 10 and integrin, and inhibit glucose transport and angiogenesis of the endometrium among PCOS patients, which may affect endometrial receptivity and further contribute to the occurrence of miscarriage [34]. A high androgen level is detrimental to oocyte and embryo quality [41, 42]. Therefore, the alleviation of hyperandrogenism may be beneficial to patients with PCOS in the early pregnancy stage, but further evidence is needed. In addition, chromosomal abnormalities might contribute to the occurrence of miscarriage in PCOS patients, but the existing evidence is inconsistent [43,44,45].
In addition, BMI is considered to be associated with compromised pregnancy outcomes. A retrospective cohort study revealed that PCOS patients with a BMI ≥ 30 kg/m2 had reduced rates of implantation and live birth and an increased risk of early miscarriage [46]. Chen et al. discovered that overweight/obese PCOS patients had a late abortion rate that was nearly three times that of normal-weight PCOS patients after FET [47]. However, it remains inconclusive whether PCOS confers an increased risk of pregnancy complications independent of high BMI [6, 11, 30]. A population-based study [30] found that women with PCOS had a higher prevalence of adverse pregnancy complications, such as GDM, preeclampsia and gestational hypertension, after controlling for obesity and other confounding factors. Similarly, our data suggested that the PCOS group had an increased risk of late miscarriage compared with the matched group. Furthermore, we stratified the PCOS group into three subgroups according to the 25% quartile and 75% quartile of BMI. Previous studies showed that PCOS patients with high BMI had suboptimal pregnancy outcomes [46, 47]. Inconsistently, we found that the pregnancy outcomes were comparable in PCOS patients across different BMI levels. The discrepancy may be due to the limited size of our study.
This cohort study has certain limitations. First, other studies have suggested that metabolic syndrome exacerbates the probability of pregnancy complications in women with or without PCOS [48,49,50]. The impact of HOMA-IR levels on pregnancy outcomes of PCOS patients was evaluated in our study. However, whether metabolic syndrome confers the risk of adverse pregnancy outcomes could not be analyzed due to the lack of serum lipid profile and waist circumference data. Further investigating the influence of metabolic factors on the pregnancy outcomes of PCOS patients is important and is worthy of further study in the future. In addition, the phenotypes of PCOS may affect pregnancy outcomes. The oocyte competence of PCOS patients varies in different phenotypes [51]. Furthermore, Palomba et al. [52] found that in PCOS patients, trophoblast invasion and macroscopic placental lesions were different across PCOS phenotypes. However, we were unable to perform stratified analysis based on different phenotypes in our study because the PCOS patients were not subdivided by phenotype. Future studies are warranted to evaluate the associations between PCOS phenotypes and pregnancy complications.
Conclusion
In conclusion, PCOS was an independent risk factor for late miscarriage after matching for age, BMI, TE grade and endometrium preparation method. PCOS patients with IR have an increased risk of preterm birth. In addition, PCOS patients with hyperandrogenism are prone to early miscarriage. These results suggest that PCOS patients with IR and hyperandrogenism require more intensive monitoring when they are pregnant.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due to the hospital policy. However, the datasets are available from the corresponding author on reasonable request and with the permission of the hospital.
References
Azziz R. Polycystic Ovary Syndrome. Obstet Gynecol. 2018;132(2):321–36. https://doi.org/10.1097/AOG.0000000000002698.
Homburg R, Berkowitz D, Levy T, Feldberg D, Ashkenazi J, Ben-Rafael Z. In vitro fertilization and embryo transfer for the treatment of infertility associated with polycystic ovary syndrome. Fertil Steril. 1993;60(5):858–63. https://doi.org/10.1016/s0015-0282(16)56287-6.
Peigné M, Dewailly D. Long term complications of polycystic ovary syndrome (PCOS). Ann Endocrinol (Paris). 2014;75(4):194–9. https://doi.org/10.1016/j.ando.2014.07.111.
Boomsma CM, Eijkemans MJ, Hughes EG, Visser GH, Fauser BC, Macklon NS. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum Reprod Update. 2006;12(6):673–83. https://doi.org/10.1093/humupd/dml036.
Palomba S, de Wilde MA, Falbo A, Koster MP, La Sala GB, Fauser BC. Pregnancy complications in women with polycystic ovary syndrome. Hum Reprod Update. 2015;21(5):575–92. https://doi.org/10.1093/humupd/dmv029.
Bahri Khomami M, Joham AE, Boyle JA, Piltonen T, Arora C, Silagy M, et al. The role of maternal obesity in infant outcomes in polycystic ovary syndrome-a systematic review, meta-analysis, and meta-regression. Obes Rev. 2019;20(6):842–58. https://doi.org/10.1111/obr.12832.
Sun YF, Zhang J, Xu YM, Cao ZY, Wang YZ, Hao GM, et al. High BMI and insulin resistance are risk factors for spontaneous abortion in patients with polycystic ovary syndrome undergoing assisted reproductive treatment: a systematic review and meta-analysis. Front Endocrinol (Lausanne). 2020;11:592495. https://doi.org/10.3389/fendo.2020.592495.
Bahri Khomami M, Joham AE, Boyle JA, Piltonen T, Silagy M, Arora C, et al. Increased maternal pregnancy complications in polycystic ovary syndrome appear to be independent of obesity-a systematic review, meta-analysis, and meta-regression. Obes Rev. 2019;20(5):659–74. https://doi.org/10.1111/obr.12829.
Lin J, Guo H, Wang B, Chen Q, Zhu Q. Neonatal outcomes in women with polycystic ovary syndrome after frozen-thawed embryo transfer. Fertil Steril. 2021;115(2):447–54. https://doi.org/10.1016/j.fertnstert.2020.08.1435.
Van den Abbeel E, Balaban B, Ziebe S, Lundin K, Cuesta MJ, Klein BM, et al. Association between blastocyst morphology and outcome of single-blastocyst transfer. Reprod Biomed Online. 2013;27(4):353–61. https://doi.org/10.1016/j.rbmo.2013.07.006.
Rees DA, Jenkins-Jones S, Morgan CL. Contemporary reproductive outcomes for patients with polycystic ovary syndrome: a retrospective observational study. J Clin Endocrinol Metab. 2016;101(4):1664–72. https://doi.org/10.1210/jc.2015-2682.
Sterling L, Liu J, Okun N, Sakhuja A, Sierra S, Greenblatt E. Pregnancy outcomes in women with polycystic ovary syndrome undergoing in vitro fertilization. Fertil Steril. 2016;105(3):791-797.e2. https://doi.org/10.1016/j.fertnstert.2015.11.019.
Pan Y, Li B, Wang Z, Wang Y, Gong X, Zhou W, et al. Hormone replacement versus natural cycle protocols of endometrial preparation for frozen embryo transfer. Front Endocrinol (Lausanne). 2020;11:546532. https://doi.org/10.3389/fendo.2020.546532.
Li C, He YC, Xu JJ, Wang Y, Liu H, Duan CC, et al. Perinatal outcomes of neonates born from different endometrial preparation protocols after frozen embryo transfer: a retrospective cohort study. BMC Pregnancy Childbirth. 2021;21(1):341. https://doi.org/10.1186/s12884-021-03791-9.
Mackens S, Santos-Ribeiro S, van de Vijver A, Racca A, Van Landuyt L, Tournaye H, et al. Frozen embryo transfer: a review on the optimal endometrial preparation and timing. Hum Reprod. 2017;32(11):2234–42. https://doi.org/10.1093/humrep/dex285.
Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25. https://doi.org/10.1016/j.fertnstert.2003.10.004.
Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73(6):1155–8. https://doi.org/10.1016/s0015-0282(00)00518-5.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. https://doi.org/10.1007/BF00280883.
Barnhart KT. Live birth is the correct outcome for clinical trials evaluating therapy for the infertile couple. Fertil Steril. 2014;101(5):1205–8. https://doi.org/10.1016/j.fertnstert.2014.03.026.
Giakoumelou S, Wheelhouse N, Cuschieri K, Entrican G, Howie SE, Horne AW. The role of infection in miscarriage. Hum Reprod Update. 2016;22(1):116–33. https://doi.org/10.1093/humupd/dmv041.
Hypertension in pregnancy. Report of the American college of obstetricians and gynecologists’ task force on hypertension in pregnancy. Obstet Gynecol. 2013;122(5):1122–31. https://doi.org/10.1097/01.AOG.0000437382.03963.88.
Watanabe K, Naruse K, Tanaka K, Metoki H, Suzuki Y. Outline of definition and classification of “pregnancy induced hypertension (PIH).” Hypertension Research in Pregnancy. 2013;1(1):3–4.
ACOG Practice Bulletin No. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131(2):e49–64. https://doi.org/10.1097/AOG.0000000000002501.
D’Antonio F, Bhide A. Ultrasound in placental disorders. Best Pract Res Clin Obstet Gynaecol. 2014;28(3):429–42. https://doi.org/10.1016/j.bpobgyn.2014.01.001.
American College of Obstetricians and Gynecologists’ Committee on Practice Bulletin—Obstetrics. Practice Bulletin No. 172: Premature Rupture of Membranes. Obstet Gynecol. 2016;128(4):e165–77. https://doi.org/10.1097/AOG.0000000000001712.
Parer JT, Livingston EG. What is fetal distress? Am J Obstet Gynecol. 1990;162(6):1421–7. https://doi.org/10.1016/0002-9378(90)90901-i.
Cunningham FG, Leveno KJ, Bloom SL, Spong CY, Dashe JS. Williams Obstetrics. 24rd ed. New York: McGraw–Hill; 2014.
Pan XF, Tang L, Lee AH, Binns C, Yang CX, Xu ZP, et al. Association between fetal macrosomia and risk of obesity in children under 3 years in Western China: a cohort study. World J Pediatr. 2019;15(2):153–60. https://doi.org/10.1007/s12519-018-0218-7.
Chen Y, Li G, Ruan Y, Zou L, Wang X, Zhang W. An epidemiological survey on low birth weight infants in China and analysis of outcomes of full-term low birth weight infants. BMC Pregnancy Childbirth. 2013;13:242. https://doi.org/10.1186/1471-2393-13-242.
Mills G, Badeghiesh A, Suarthana E, Baghlaf H, Dahan MH. Polycystic ovary syndrome as an independent risk factor for gestational diabetes and hypertensive disorders of pregnancy: a population-based study on 9.1 million pregnancies. Hum Reprod. 2020;35(7):1666–74. https://doi.org/10.1093/humrep/deaa099.
Zaeemzadeh N, Sadatmahalleh SJ, Ziaei S, Kazemnejad A, Mottaghi A, Mohamadzadeh N, et al. Prevalence of metabolic syndrome in four phenotypes of PCOS and its relationship with androgenic components among Iranian women: a cross-sectional study. Int J Reprod Biomed. 2020;18(4):253–64. https://doi.org/10.18502/ijrm.v13i4.6888.
Cai H, Mol BW, Gordts S, Wang H, Wang T, Li N, et al. Early and late pregnancy loss in women with polycystic ovary syndrome undergoing IVF/ICSI treatment: a retrospective cohort analysis of 21 820 pregnancies. BJOG. 2021;128(7):1160–9. https://doi.org/10.1111/1471-0528.16590.
Liu S, Mo M, Xiao S, Li L, Hu X, Hong L, et al. Pregnancy outcomes of women with polycystic ovary syndrome for the first in vitro fertilization treatment: a retrospective cohort study with 7678 patients. Front Endocrinol (Lausanne). 2020;11:575337. https://doi.org/10.3389/fendo.2020.575337.
Guo F, Huang Y, Fernando T, Shi Y. Altered Molecular Pathways and Biomarkers of Endometrial Receptivity in Infertile Women with Polycystic Ovary Syndrome. Reprod Sci. 2022;29(1):1–11. https://doi.org/10.1007/s43032-022-00845-x.
Palomba S, Piltonen TT, Giudice LC. Endometrial function in women with polycystic ovary syndrome: a comprehensive review. Hum Reprod Update. 2021;27(3):584–618. https://doi.org/10.1093/humupd/dmaa051.
Bu Z, Hu L, Su Y, Guo Y, Zhai J, Sun YP. Factors related to early spontaneous miscarriage during IVF/ICSI treatment: an analysis of 21,485 clinical pregnancies. Reprod Biomed Online. 2020;40(2):201–6. https://doi.org/10.1016/j.rbmo.2019.11.001.
Mai Z, Liu M, Pan P, Li L, Huang J, Chen X, et al. Comparison of cumulative live birth rate between aged PCOS women and controls in IVF/ICSI cycles. Front Endocrinol (Lausanne). 2021;12:724333. https://doi.org/10.3389/fendo.2021.724333.
Liu L, Tong X, Jiang L, Li TC, Zhou F, Zhang S. A comparison of the miscarriage rate between women with and without polycystic ovarian syndrome undergoing IVF treatment. Eur J Obstet Gynecol Reprod Biol. 2014;176:178–82. https://doi.org/10.1016/j.ejogrb.2014.02.041.
Chen X, Gissler M, Lavebratt C. Association of maternal polycystic ovary syndrome and diabetes with preterm birth and offspring birth size: a population-based cohort study. Hum Reprod. 2022;37(6):1311–23. https://doi.org/10.1093/humrep/deac050.
Chen ZJ, Shi Y, Sun Y, Zhang B, Liang X, Cao Y, et al. Fresh versus frozen embryos for infertility in the polycystic ovary syndrome. N Engl J Med. 2016;375(6):523–33. https://doi.org/10.1056/NEJMoa1513873.
Lebbe M, Woodruff TK. Involvement of androgens in ovarian health and disease. Mol Hum Reprod. 2013;19(12):828–37. https://doi.org/10.1093/molehr/gat065.
Wissing ML, Bjerge MR, Olesen AI, Hoest T, Mikkelsen AL. Impact of PCOS on early embryo cleavage kinetics. Reprod Biomed Online. 2014;28(4):508–14. https://doi.org/10.1016/j.rbmo.2013.11.017.
Weghofer A, Munne S, Chen S, Barad D, Gleicher N. Lack of association between polycystic ovary syndrome and embryonic aneuploidy. Fertil Steril. 2007;88(4):900–5. https://doi.org/10.1016/j.fertnstert.2006.12.018.
Wang Q, Luo L, Lei Q, Lin MM, Huang X, Chen MH, et al. Low aneuploidy rate in early pregnancy loss abortuses from patients with polycystic ovary syndrome. Reprod Biomed Online. 2016;33(1):85–92. https://doi.org/10.1016/j.rbmo.2016.04.006.
Li Y, Wang L, Xu J, Niu W, Shi H, Hu L, et al. Higher chromosomal aberration rate in miscarried conceptus from polycystic ovary syndrome women undergoing assisted reproductive treatment. Fertil Steril. 2019;111(5):936–943.e2. https://doi.org/10.1016/j.fertnstert.2019.01.026.
Qiu M, Tao Y, Kuang Y, Wang Y. Effect of body mass index on pregnancy outcomes with the freeze-all strategy in women with polycystic ovarian syndrome. Fertil Steril. 2019;112(6):1172–9. https://doi.org/10.1016/j.fertnstert.2019.08.009 (Published correction appears in Fertil Steril. 2020 Nov;114(5):1122).
Chen R, Chen S, Liu M, He H, Xu H, Liu H, et al. Pregnancy outcomes of PCOS overweight/obese patients after controlled ovarian stimulation with the GnRH antagonist protocol and frozen embryo transfer. Reprod Biol Endocrinol. 2018;16(1):36. https://doi.org/10.1186/s12958-018-0352-z.
Grieger JA, Bianco-Miotto T, Grzeskowiak LE, Leemaqz SY, Poston L, McCowan LM, et al. Metabolic syndrome in pregnancy and risk for adverse pregnancy outcomes: a prospective cohort of nulliparous women. PLoS Med. 2018;15(12):e1002710. https://doi.org/10.1371/journal.pmed.1002710.
Chatzi L, Plana E, Daraki V, Karakosta P, Alegkakis D, Tsatsanis C, et al. Metabolic syndrome in early pregnancy and risk of preterm birth. Am J Epidemiol. 2009;170(7):829–36.
Arya S, Hansen KR, Peck JD, Wild RA, National Institute of Child Health and Human Development Reproductive Medicine Network. Metabolic syndrome in obesity: treatment success and adverse pregnancy outcomes with ovulation induction in polycystic ovary syndrome. Am J Obstet Gynecol. 2021;225(3):280.e1-280.e11. https://doi.org/10.1016/j.ajog.2021.03.048.
Palomba S, Daolio J, La Sala GB. Oocyte competence in women with polycystic ovary syndrome. Trends Endocrinol Metab. 2017;28(3):186–98. https://doi.org/10.1016/j.tem.2016.11.008.
Palomba S, Falbo A, Chiossi G, Tolino A, Tucci L, La Sala GB, et al. Early trophoblast invasion and placentation in women with different PCOS phenotypes. Reprod Biomed Online. 2014;29(3):370–81. https://doi.org/10.1016/j.rbmo.2014.04.010.
Acknowledgements
Not applicable.
Funding
This study is supported by Guangdong Provincial Key Laboratory of Reproductive Medicine [No: 2020B1212090029] and Clinical Medical Research of China Medical Sciences - Stem Cell Basic Research Project [No: 19020010780].
Author information
Authors and Affiliations
Contributions
Can-Quan Zhou is the corresponding author responsible for designing the study and revising the paper. Hui-Ying Jie was responsible for statistical analysis and drafting the manuscript. Xiu Zhou and Min-Hu contributed to the discussion part. Qing-Yun Mai and Ming-Peng Zhao helped with manuscript revision. All authors reviewed and approved the final manuscript as submitted.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the First Affiliated Hospital, Sun Yat-sen University [No. IIT-2022–157]. All procedures were performed in accordance with the Declaration of Helsinki. Since this retrospective study analyzed the medical records obtained in the past clinical diagnosis and treatment, the Ethics Committee of the First Affiliated Hospital, Sun Yat-sen University agreed to waive the patient's written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplemental Table 1.
The definitions of clinical outcomes.
Additional file 2: Supplemental Table 2.
The pregnancy outcomes in the PCOS group and the matched control group with a Cochran-Mantel-Haenszel test adjusting for endometrial preparation methods.
Additional file 3: Supplemental Table 3.
The pregnancy outcomes in PCOS patients after stratification by HOMA-IR quartiles.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jie, HY., Zhou, X., Zhao, MP. et al. Pregnancy outcomes in patients with polycystic ovary syndrome who conceived after single thawed blastocyst transfer: a propensity score-matched study. BMC Pregnancy Childbirth 22, 718 (2022). https://doi.org/10.1186/s12884-022-05011-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12884-022-05011-4