Abstract
Background
There is dearth of information on COVID-19’s impact on pregnant women. However, literature reported trends of COVID-19 differ, depending on the presence of clinical features upon presentation.
Objective
This systematic review aimed to assess differences in risk factors, management, complications, and pregnancy and perinatal outcomes in symptomatic vs. asymptomatic pregnant women with confirmed SARS-CoV-2 infection.
Methods
A search was run on electronic databases to identify studies reporting COVID-19 in pregnancy. Meta-analysis was performed and odds ratios and mean difference with 95% confidence intervals were calculated using Review Manager 5.4. Review Prospero registration number CRD42020204662.
Results
We included ten articles reporting data from 3158 pregnancies; with 1900 symptomatic and 1258 asymptomatic pregnant women. There was no significant difference in the mean age, gestational age, and body mass index between the two groups. The meta-analysis suggested that pregnant women who were obese (OR:1.37;95%CI:1.15 to 1.62), hypertensive (OR:2.07;95%CI:1.38 to 3.10) or had a respiratory disorder (OR:1.64;95%CI:1.25 to 2.16), were more likely to be symptomatic when infected with SARS-CoV-2. Pregnant women with Black (OR:1.48;95%CI:1.19 to 1.85) or Asian (OR:1.64;95%CI:1.23 to 2.18) ethnicity were more likely to be symptomatic while those with White ethnicity (OR:0.63;95%CI:0.52 to 0.76) were more likely to be asymptomatic. Cesarean-section delivery (OR:1.40;95%CI:1.17 to 1.67) was more likely amongst symptomatic pregnant women. The mean birthweight(g) (MD:240.51;95%CI:188.42 to 293.51), was significantly lower, while the odds of low birthweight (OR:1.85;95%CI:1.06 to 3.24) and preterm birth (< 37 weeks) (OR:2.10;95%CI:1.04 to 4.23) was higher amongst symptomatic pregnant women. Symptomatic pregnant women had a greater requirement for maternal ICU admission (OR:13.25;95%CI:5.60 to 31.34) and mechanical ventilation (OR:15.56;95%CI:2.96 to 81.70) while their neonates had a higher likelihood for Neonatal Intensive Care Unit admission (OR:1.96;95%CI:1.59 to 2.43). The management strategies in the included studies were poorly discussed, hence could not be analyzed.
Conclusion
The evidence suggests that the presence of risk factors (co-morbidities and ethnicity) increased the likelihood of pregnant women being symptomatic. Higher odds of complications were also observed amongst symptomatic pregnant women. However, more adequately conducted studies with adjusted analysis and parallel comparison groups are required to reach conclusive findings.
Similar content being viewed by others
Introduction
In December 2019, a rising number of cases of ‘pneumonia of unknown etiology’ emerged in Wuhan, Hubei Province, China. Consequently, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was identified as a novel coronavirus in this outbreak [1]. The mode of transmission of this virus is mainly via respiratory droplets, secretions and direct contact [2]. With the rapid spread of the disease worldwide, the novel coronavirus disease (COVID-19) was declared a public health emergency of international concern by the World Health Organization (WHO) [3]. By March 2020, it was declared a pandemic [4], and globally, as of October 21, 2020, a total of 40.2 million cases have been confirmed, with more than 1.1 million deaths [5].
The implications of COVID-19 amongst the vulnerable population, particularly pregnant women, is of utmost concern, as alterations in cell-mediated immunity in pregnancy may increase the susceptibility to intracellular pathogens such as viruses [6]. The anatomical and physiological changes occurring during pregnancy such as the rising transverse diameter of the thorax, elevation of the diaphragm, alterations in lung volumes, and vasodilation with subsequent mucosal edema may lessen the maternal tolerance to hypoxia and later, concur adverse outcomes [7]. Moreover, it has been observed that during pandemics, an increase in severity of the disease expression is recorded amongst the pregnant population. In the 1918 Influenza pandemic, maternal mortality was observed to be 27% amongst those affected by the disease [8]. The clinical outcomes among pregnant women from the previous two coronavirus diseases, the SARS-CoV and Middle East respiratory syndrome coronavirus (MERS- CoV), were less encouraging compared to the non-pregnant women [9,10,11].
Due to previously noted maternal and neonatal complications with SARs and MERS; the concern of increased risk of maternal and fetal complications with COVID-19 has been high and since the start of the pandemic, multiple studies have focused on the clinical features and outcomes of pregnant women with COVID-19 [12, 13]. New data on pregnant women affected by COVID-19 is emerging with each passing day, but it is imperative to evaluate the differences in the risk factors, management, and pregnancy and perinatal outcomes between pregnant women having laboratory-confirmed coronavirus with varying clinical presentation. Therefore, this systematic review aims to assess the difference, if any, in the risk factors (co-morbidities and ethnicity), management, and pregnancy and perinatal outcomes between symptomatic and asymptomatic COVID-19 confirmed pregnant women. This shall enable healthcare professionals to plan out management for any obstetric patient affected by COVID-19 infection and take timely decisions.
Methodology
This systematic review has been registered with the International Prospective Register of Systematic Reviews (PROSPERO) database under the Registration number CRD42020204662. It follows the guidelines recommended by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [14] (Supplementary Table 1).
We conducted an electronic search using PubMed, Embase, the WHO COVID-19 Database, and Google Scholar until February 25, 2021. Preprint databases, namely MedRxiv and BioRxiv were also explored using keywords. The following terms and their variants were used in our search strategy: “Coronavirus” OR “COVID-19” OR “SARS-CoV-2” AND “Pregnancy” OR “Pregnant women”. The full search strategy is attached in supplementary Table 2.
We included observational studies (cohort, case-control, cross-sectional, and case series, but excluded case reports) including consecutive patients and with a comparison of symptomatic and asymptomatic confirmed cases of pregnant women with COVID-19. The outcomes included risk factors, management, pregnancy outcomes, and perinatal outcomes. We excluded studies that only reported the number of symptomatic and asymptomatic pregnant women with COVID-19 without reporting outcomes separately for each group, studies that grouped asymptomatic with mild COVID-19 cases and compared it to moderate and severe COVID-19. Studies that included data from similar settings and during the same time period were assessed for data overlap. Where information was unclear, authors were contacted to confirm the center of data collection and their time period to ensure there was no repetition of data. Identified overlapping papers were further assessed and the studies with inclusion of more variables, bigger sample size, and better quality of assessment were chosen as shown in supplementary Table 3. We did not apply any language restrictions while screening articles.
Two reviewers (DSK and LH) independently screened titles and abstracts, full texts were then reviewed for relevant data and examined for fulfilling the inclusion criteria. Any discrepancies were resolved by discussion with the senior reviewer (ZSL). Two review authors independently assessed the risk of bias for each study using the National Heart, Lung, and Blood Institute (NHLBI) quality assessment tool for cohort and case-series studies [15]. This tool helps evaluate the internal validity of a study, hence ensuring that the results are truly due to the exposure being evaluated. Two reviewers (DSK and LH) independently extracted data from relevant articles on the following variables: author’s name, study design, location (center, city, and country of data collection), time period of data collection, sample size, management (intensive care unit (ICU) management), pregnancy outcomes (mode of delivery) and perinatal outcomes (e.g. preterm birth, mean birthweight, low birth weight (LBW), APGAR scores at 1 and 5 min, neonatal intensive care unit (NICU) admission, perinatal mortality (including stillbirths and neonatal death), and SARS-CoV-2 infection in neonates, etc.).
The analysis was carried out using Review Manager (RevMan) version 5.4 [16]. Continuous data were reported as mean difference (MD) with 95% confidence interval (CI) whereas dichotomous data were reported using odds ratio (OR) with 95% CI. Heterogeneity between the studies was explored using the p-value of chi-square and I2 statistic and a random effect model was used.
Results
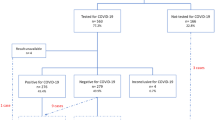
There were a total of 4347 articles identified after running the search strategy on all electronic databases. After screening titles and abstracts, 4179 were excluded. Of the 168 studies retrieved for full-text review, 158 studies were excluded, of which 42 were authors’ perspectives or reviews, 32 were guidelines or guidance papers based on other coronavirus strains, 45 studies compared SARS-CoV-2 infected pregnant women with non-infected individuals or SARS-CoV-2 infected non-pregnant women, 32 studies did not report any outcome of interest and seven studies were excluded due to overlap as they were conducted at a similar center during the same time period. A total of ten studies with 3158 participants that met the eligibility were included in this review (Fig. 1).
All the ten studies were observational; with six cohort studies (n = 3032) [17,18,19,20,21,22]; three case-series (n = 36) [23,24,25], with only one having a sample size greater than 10 [24] and one case-control study (n = 45) [26]. The data from included cases were collected between December 2019 to November 2020 and all manuscripts were published between the years 2020 and 2021. Three studies were conducted in Wuhan, China [23,24,25], three in different states across the United States of America [20,21,22], one in London, United Kingdom [19], one in Muscat, Oman [18], one in Hamadan province, Iran [26] and one was a multi-country study that included cases from 73 centers of 22 countries [17]. The countries included were Argentina, Australia, Belgium, Brazil, Colombia, Czech Republic, Finland, Germany, Greece, Israel, Italy, North Macedonia, Peru, Portugal, Republic of Kosovo, Romania, Russia, Serbia, Slovenia, Spain, Turkey, and United States. Five of the included studies were from a single-center [18, 22,23,24,25] whereas four were multicenter studies [19,20,21, 26], and one was a multicountry multicenter study [17]. The quality assessment for cohort studies revealed all studies to have described their objectives and study subjects well. They all had individuals recruited from the same populations and had uniform criteria for measuring exposure. However, none of the studies assessed a varying level of exposure, nor was the exposure measured at different time intervals as there was no follow-up. The quality assessment for the case series revealed all studies to have adequate individual case descriptions with clear objectives and methods of analysis except one study [25]. The quality assessment for a single study with a case-control study design specified objective and study subjects with controls recruited from the same population. Quality assessment for each study is depicted in Supplementary Tables 4, 5, and 6.
The number of enrolled individuals in each study ranged from seven to 1219. Six studies had a sample size of less than 100 participants [18, 22,23,24,25,26], two studies had a sample between 100 to 500 participants [17, 20], and two studies had a sample size greater than 1000 but less than 1300 participants [19, 21]. Eight of the studies used RT-PCR as the method of confirming SARS-CoV-2 infection [17, 18, 20, 22,23,24,25,26], one used molecular or antigen test [21], and one study had not specified the testing technique/s used [19]. The characteristics of included studies are reported in Table 1 and their methodological quality in Supplementary Tables 4, 5, and 6.
All of the participants included in this review were SARS-CoV-2 infected pregnant women with 1900 symptomatic individuals and 1258 asymptomatic individuals. The meta-analysis did not find any significant difference in mean age, gestation age, and BMI between symptomatic and asymptomatic COVID-19 affected pregnant women as reported in Table 2. There was no statistically significant difference between symptomatic and asymptomatic pregnant women who were nulliparous. The comparison according to different ethnic groups revealed the odds of being symptomatic was greater amongst Black (OR 1.48; 95% CI: 1.19 to 1.85; 2 studies, 2367 participants) and Asian (OR 1.64; 95% CI: 1.23 to 2.18; 1 study, 1148 participants) ethnicities. However, individuals from the White ethnicity were more likely to be asymptomatic (OR 0.63; 95% CI: 0.52 to 0.76; 2 studies, 2367 participants) (Table 2).
With regard to past medical history (existing co-morbidities); hypertensive pregnant women with COVID-19 were more likely to be symptomatic (OR 2.07; 95% CI 1.38 to 3.10; 3 studies, 2427 participants) as were pregnant women with respiratory disease (OR 1.64; 95% CI: 1.25 to 2.16; 3 studies, 2516 participants). However, there was no statistical difference in diabetic and hypothyroid pregnant women or pregnant women with chronic cardiac disease for being symptomatic or asymptomatic. Obese pregnant women with COVID-19 had greater odds of being symptomatic (OR 1.37; 95% CI 1.15 to 1.62; 3 studies, 2516 participants). Our meta-analysis also reports pregnant women who smoked to have lower odds of being symptomatic (OR 0.50; 95% CI: 0.36 to 0.71; 2 studies, 2367 participants).
There was no difference between gestational diabetes mellitus (GDM), premature rupture of membranes, or intrauterine growth retardation (IUGR) with a predilection for either being symptomatic or asymptomatic amongst pregnant women with COVID-19. However, the odds of being symptomatic were higher in pregnant women with preeclampsia or pregnancy-induced hypertension (OR 1.84; 95% CI 1.01 to 3.38; 5 studies, 1465 participants). A summary of forest plots has been presented in Fig. 2 and individual forest plots can be accessed in the supplementary file as Supplementary Figs. 1 to 8.
The odds were greater for symptomatic pregnant women with COVID-19 to deliver via a cesarean section (OR 1.40; 95% CI 1.17 to 1.67; 8 studies, 2982 participants), while there was no difference in the odds of vaginal delivery across the two groups. Our meta-analysis reported mean birthweight to be lower in neonates of symptomatic pregnant women with COVID-19 (MD 240.96 g; 95% CI 188.42 to 293.51; 4 studies, 1488 participants). Similarly, the odds of having an LBW newborn (OR 1.85; 95% CI 1.06 to 3.24; 3 study, 401 participants) and preterm birth less than 37 weeks (OR 2.10; 95% CI 1.04 to 4.23; 6 studies, 1760 participants) was higher amongst symptomatic women. There was no difference in mean APGAR score at 1-min, neonatal SARS-CoV-2 infection, and neonatal death between either group. Neonatal Intensive Care Unit (NICU) admissions were more likely amongst neonates of symptomatic mothers with COVID-19 (OR 1.96; 95% CI 1.59 to 2.43; 4 studies, 2637 participants). The odds of maternal ICU admission (OR 13.25; 95% CI 5.60 to 31.34; 3 studies, 1756 participants) and mechanical ventilation requirement (OR 15.56; 95% CI 2.96 to 81.70, 2 studies, 443 participants) were also higher in symptomatic pregnant women. A summary of forest plots has been presented in Fig. 3 and individual forest plots can be accessed in the supplementary file as Supplementary Figs. 9 to 15. The management strategies could not be compared between the symptomatic and asymptomatic pregnant women infected with COVID-19 due to lack of available data.
Discussion
Since the start of the COVID-19 pandemic, healthcare workers have been at the forefront to gather enough data in order to understand this disease better. This review primarily focused on pregnant women with COVID-19 and summarizes the differences in their risk factors, management along with their pregnancy and neonatal outcomes between symptomatic and asymptomatic pregnant women. To the best of our knowledge, this meta-analysis is the first of its kind to analyse the difference in pregnancy and perinatal outcomes that could potentially be affected, depending on the severity of COVID-19 disease.
In the present meta-analysis, we found that being symptomatic varied across different ethnicities with Black and Asian pregnant women having a higher likelihood of being symptomatic while pregnant women of White ethnicity had a higher likelihood of being asymptomatic. We also found that obese, hypertensive, and asthmatic pregnant women with COVID-19 have a higher likelihood of being symptomatic. Symptomatic pregnant women with COVID-19 had higher chances of delivering via cesarean-section while asymptomatic pregnant women with COVID-19 had a higher chance of having a vaginal delivery. The mean birthweight was lower among neonates of symptomatic pregnant women and the odds of LBW was also higher among symptomatic women. The likelihood of NICU admission was higher amongst neonates from symptomatic mothers with COVID-19. Women with preeclampsia or pregnancy-induced hypertension had a higher likelihood of being symptomatic. Symptomatic pregnant women with COVID-19 also had a greater requirement for ICU admission and mechanical ventilation. There was inadequate data regarding management strategies used for each subgroup due to which differences in management practices could not be compared.
A wide spectrum of disease severity exists amongst pregnant women with COVID-19 disease, with 86% exhibiting mild disease, 9.3% severe, and 4.7% critical [27]. This percentage is similar to those calculated from the non-pregnant adult population (with 80% mild, 15% severe, and 5% critical disease) [28]. However, information regarding the reason for progression to critical disease, depending on the basis of clinical features of COVID-19, is still lacking in the literature.
Previously, the risk of delivery via cesarean section has been reported to be higher amongst pregnant women with COVID-19 disease compared to the general pregnant population [29]. However, whether the presence of symptomatic disease predisposed pregnant women infected with SARS-CoV-2 to a certain mode of delivery is unknown. Our meta-analysis found cesarean section to be more likely amongst symptomatic pregnant women and vaginal delivery to be more likely amongst asymptomatic pregnant women.
A direct link has been established in the literature between SARS-CoV-2 infection and premature labour [30]. A higher rate (37.7%) of preterm birth is seen in pregnant women with COVID-19 as compared to the general pregnant population (12%) [29]. Another systematic review specified that preterm birth before 37 weeks gestation was prevalent in 21.8% of the pregnant women affected by COVID-19 [30]. In terms of assessing the risk of preterm delivery in symptomatic versus asymptomatic pregnant women, our meta-analysis found significant differences between the two groups i.e. higher preterm births in symptomatic pregnant women and similarly, lower birth weight among those infants. Ideally we would have done a subgroup analysis for preterm births but separating the data for term and preterm births was not possible.
Newborns from symptomatic pregnant women were more likely to have lower birthweight. These findings from our review are in line with most studies reporting neonatal outcomes of pregnant women with COVID-19 [27].
The limitations for this review included: (i) a limited number of included studies, (ii) smaller sample size per study, (iii) lack of data on management strategies for both groups, (iv) lack of data on other variables including demographics and co-morbidities from all studies, (v) few studies reporting adjusted analysis and (vi) dearth of evidence from low- and middle-income settings. To obtain conclusive results, more detailed data is required, especially on areas like demographics (co-morbidity, ethnicity, etc.) and maternal outcomes (including those occurring prior to or after COVID-19 diagnosis, e.g. GDM, preeclampsia, etc.). Multivariable analysis to identify factors associated with management, pregnancy, and perinatal outcomes in symptomatic versus asymptomatic pregnant women with COVID-19 could not be done due to insufficient data. However, if data on individual patients is provided in the future, then individual patient data meta-analysis (IPD-MA) would be the ideal approach to provide insights into recognizing and managing COVID-19 disease based on symptoms.
In conclusion, the findings of this study summarize the risk factors, pregnancy, and perinatal outcomes amongst pregnant women based on whether they were symptomatic at presentation. According to this review, obese, hypertensive pregnant women with COVID-19 or those with the respiratory disorder were more likely to be symptomatic. Black or Asian pregnant women with COVID-19 were more likely to be symptomatic while White pregnant women were more likely to be asymptomatic. Delivery via c-section was more likely amongst symptomatic pregnant women while vaginal delivery was more likely amongst asymptomatic pregnant women. Lower mean birthweight was reported among neonates of symptomatic pregnant women and their odds of having LBW babies and preterm births was also higher. Symptomatic pregnant women had a greater requirement for maternal ICU admission and mechanical ventilation and their neonates had a higher likelihood for NICU admission.
The findings of this study, though not very robust can aid to increase the understanding of the course of the disease amongst the two subsets of pregnant women with COVID-19 disease, and its impact on perinatal and neonatal outcomes. This may enable the health care providers to impart better care for the mother and the fetus. However, more studies comparing asymptomatic and symptomatic pregnant women and reporting adjusted pregnancy and birth outcomes are required from varying contexts.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–33.
Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. New Engl J Med. 2020;382:1199–207.
WHO. Public health emergency of international concern (PHEIC): Who; 2020.
Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta. Biomedica. 2020.
WHO. WHO COVID-19 Dashboard [Internet]. 2020. Available from: https://covid19.who.int/
Jamieson DJ, Theiler RN, Rasmussen SA. Emerging infections and pregnancy. Emerg Infect Dis. 2006.
Liu W, Wang Q, Zhang Q, Chen L, Chen J, Zhang BM, et al. Title: coronavirus disease 2019 (COVID-19) during pregnancy: a case series. 2020.
Rasmussen SA, Jamieson DJ, Bresee JS. Pandemic influenza and pregnant women. Emerg Infect Dis. 2008;14(1):95–100 Available from: http://wwwnc.cdc.gov/eid/article/14/1/07-0667_article.htm.
Wong S, Chow K, Leung T, Ng W, et al. TN-A journal of, 2004 U. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome: Elsevier; 2020.
Alfaraj SH, Al-Tawfiq JA, Memish ZA. Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection during pregnancy: Report of two cases & review of the literature. J Microbiol Immunol Infect. 2019;52(3):501–3 Available from: https://linkinghub.elsevier.com/retrieve/pii/S168411821830152X.
Favre G, Pomar L, Musso D, (London DB-L, England) U, 2020 U. 2019-nCoV epidemic: what about pregnancies? ncbi.nlm.nih.gov. 2020;
Chen L, Li Q, Zheng D, Jiang H, Wei Y, Zou L, et al. Clinical characteristics of pregnant women with Covid-19 in Wuhan, China. New Engl J Med. 2020;382:E100.
Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020.
Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D, Antes G, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009.
Services UD of H and H. Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. Bethesda, MD: Natl Institutes Heal Dep Heal Hum Serv; 2014.
Review manager (RevMan) [computer program]. Version 5.4, The Cochrane Collaboration, 2020.
Saccone, Gabriele, Sen Cihat MD. Maternal and Perinatal Outcomes of Pregnant Women with SARS-COV-2 infection. 2020;
Santhosh J, Al Salmani M, Khamis F, Ali Al Ubaidani S, Al-Zakwani I. Clinical characteristics of COVID-19 in pregnant women: a retrospective descriptive single-center study from a tertiary hospital in Muscat, Oman. Int J Gynecol Obstet. 2021;152(2):270–4 Available from: https://onlinelibrary.wiley.com/doi/10.1002/ijgo.13427.
Vousden N, Bunch K, Morris E, Simpson N, Gale C, O’Brien P, et al. The incidence, characteristics and outcomes of pregnant women hospitalized with symptomatic and asymptomatic SARS-CoV-2 infection in the UK from march to September 2020: a national cohort study using the UK obstetric surveillance system (UKOSS). medRxiv. 2021.
Verma S, Bradshaw C, Auyeung NSF, Lumba R, Farkas JS, Sweeney NB, et al. Outcomes of maternal-newborn dyads after maternal SARS-CoV-2. Pediatrics. 2020;146(4):e2020005637 Available from: http://pediatrics.aappublications.org/lookup/doi/10.1542/peds.2020-005637.
Metz TD, Clifton RG, Hughes BL, Sandoval G, Saade GR, Grobman WA, et al. Disease severity and perinatal outcomes of pregnant patients with coronavirus disease 2019 (COVID-19). Obstet Gynecol. 2021;137(4):571–80 Available from: https://journals.lww.com/10.1097/AOG.0000000000004339.
London V, McLaren R, Atallah F, Cepeda C, McCalla S, Fisher N, et al. The relationship between status at Presentation and outcomes among pregnant women with COVID-19. Am J Perinatol. 2020.
Cao D, Yin H, Chen J, Tang F, Peng M, Li R, et al. Clinical analysis of ten pregnant women with COVID-19 in Wuhan, China: A retrospective study. Int J Infect Dis. 2020.
Wu X, Sun R, Chen J, Xie Y, Zhang S, Wang X. Radiological findings and clinical characteristics of pregnant women with COVID-19 pneumonia. Int J Gynecol Obstet. 2020.
Hu X, Gao J, Luo X, Feng L, Liu W, Chen J, et al. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vertical Transmission in Neonates Born to Mothers With Coronavirus Disease 2019 (COVID-19) Pneumonia. Obstet Gynecol. 2020; Available from: https://journals.lww.com/10.1097/AOG.0000000000003926.
Jenabi E, Bashirian S, Khazaei S, Masoumi SZ, Ghelichkhani S, Goodarzi F, et al. Pregnancy outcomes among symptomatic and asymptomatic women infected with COVID-19 in the west of Iran: a case-control study. J Matern Neonatal Med. 2020:1–3 Available from: https://www.tandfonline.com/doi/full/10.1080/14767058.2020.1861599.
Breslin N, Baptiste C, Gyamfi-Bannerman C, Miller R, Martinez R, Bernstein K, et al. Coronavirus disease 2019 infection among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of new York City hospitals. Am J Obstet Gynecol MFM. 2020;2(2):100118 Available from: https://linkinghub.elsevier.com/retrieve/pii/S2589933320300483.
Wu Z, Jama JM-. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese. jamanetwork.com. 2020.
Matar R, Alrahmani L, Monzer N, Debiane LG, Berbari E, Fares J, et al. Clinical Presentation and Outcomes of Pregnant Women With Coronavirus Disease 2019: A systematic review and Meta-analysis. Clin Infect Dis. 2020; Available from: https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciaa828/5861636.
Khalil A, Kalafat E, Benlioglu C, O’Brien P, Morris E, Draycott T, et al. SARS-CoV-2 infection in pregnancy: a systematic review and meta-analysis of clinical features and pregnancy outcomes. EClinicalMedicine. 2020;100446.
Acknowledgements
Not applicable.
Funding
This research did not receive any funding.
Author information
Authors and Affiliations
Contributions
All authors contributed adequately towards this systematic review and meta-analysis and have been provided authorship on that basis. DSAK, LH and ZSL conducted literature search, full text review, analysis and wrote the manuscript. AA worked on analysis and figures for the study. JKD and RAS edited the manuscript and provided feedback. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not Applicable. As this is a systematic review, ethics approval or consent was not warranted.
Consent for publication
Not Applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Table 1
: PRISMA Checklist. Supplementary Table 2: Search Strategy. Supplementary Table 3: Overlapping studies. Supplementary Table 4: NHLBI Quality assessment tool for Cohort studies. Supplementary Table 5: NHBLI Quality Assessment tool for Case-control studies. Supplementary Table 6: NHLBI Quality assessment tool for case-series. Figure 1: Smoking. Figure 2: Co-morbidity. Figure 3: Obesity. Figure 4: Hypertension. Figure 5: Cardiovascular disease. Figure 6: Respiratory disease. Figure 7: Diabetes Mellitus. Figure 8: Hypothyroid. Figure 9: Cesarean Section. Figure 10: Vaginal Delivery. Figure 11: Preterm Birth < 37 weeks. Figure 12: Preterm Birth < 34 weeks. Figure 13: Maternal ICU admission. Figure 14: Maternal Mechanical Ventilation. Figure 15: NICU admission.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Khan, D.S.A., Hamid, LR., Ali, A. et al. Differences in pregnancy and perinatal outcomes among symptomatic versus asymptomatic COVID-19-infected pregnant women: a systematic review and meta-analysis. BMC Pregnancy Childbirth 21, 801 (2021). https://doi.org/10.1186/s12884-021-04250-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12884-021-04250-1