Abstract
Background
Amniotic fluid (AF) provides vital information on fetal development, which is also valuable in identifying fetal abnormalities during pregnancy. However, the relationship between the metabolic profile of AF in the second trimester of a normal pregnancy with several maternal–fetal parameters remains poorly understood, which therefore limits its application in clinical practice. The aim of this study was to explore the association between the metabolic profile of AF with fetal gender, maternal age, and gestational week using an untargeted metabolomics method.
Methods
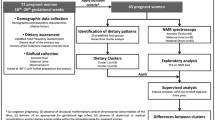
A total of 114 AF samples were analyzed in this study. Clinical data on fetal gender, maternal age, and gestational week of these samples were collected. Samples were analyzed by gas chromatography/time-of-flight-mass spectrometry (GC-TOF/MS). Principal component analysis(PCA), orthogonal partial least square discrimination analysis(OPLS-DA) or partial least square discrimination analysis (PLS-DA) were conducted to compare metabolic profiles, and differential metabolites were obtained by univariate analysis.
Results
Both PCA and OPLS-DA demonstrated no significant separation trend between the metabolic profiles of male and female fetuses, and there were only 7 differential metabolites. When the association between the maternal age on AF metabolic profile was explored, both PCA and PLS-DA revealed that the maternal age in the range of 21 to 40 years had no significant effect on the metabolic profile of AF, and only four different metabolites were found. There was no significant difference in the metabolic profiles of AF from fetuses of 17–22 weeks, and 23 differential metabolites were found.
Conclusions
In the scope of our study, there was no significant correlation between the AF metabolic profile and the fetal gender, maternal age and gestational week of a small range. Nevertheless, few metabolites appeared differentially expressed.
Similar content being viewed by others
Background
Amniotic fluid (AF) is the fluid that surrounds the fetus in the amniotic cavity, which plays a key role in protecting and providing nutrients to the fetus. Numerous studies have shown that AF contains DNA, RNA, and metabolites that have vital biological functions in fetal development, which can be used to identify any fetal developmental abnormalities [1,2,3].
Metabolomics is a rapidly advancing field in research and clinical applications following genomics, transcriptomics, and proteomics [4]. The concept of metabolomics was firstly proposed and defined by Nicholson et al. [5] as “the quantitative measurement of the dynamic multiparametric metabolic response of living systems to pathophysiological stimuli or genetic modification”. It explores the changes of endogenous small molecule metabolites(< 1000 Da), such as amino acid, carbohydrate, and organic acid upon the interaction with factors including heredity, environment, diet, drugs, disease, etc. [6, 7]. In the case of organisms, DNA, RNA and proteins are the material basis of biological events or processes, but the event may or may not occur, and the presence of metabolites reflects what has already happened in the course of life. Therefore, the metabolome is more reflective of a person's phenotype.
Metabolomics can be divided into targeted and non-targeted metabolomics according to the pertinence of detection methods. Targeted metabolomics accurately determines specific or several metabolites with similar properties, while untargeted metabolomics conducts a systematic and comprehensive analysis of the entire metabolome of an organism without bias, thus obtaining substantial small-molecule metabolite data [8, 9]. Common detection methods include nuclear magnetic resonance(NMR), liquid chromatography mass spectrometry(LC–MS) and gas chromatography-mass spectrometry(GC–MS) [10]. Numerous types of samples can be analyzed by metabolomics, including blood, urine, amniotic fluid, stool, tissue, cells, dried blood spot, etc. [11,12,13,14]. In recent years, metabolomics has helped in making remarkable advancements and achievements in many fields, including biomarker screening, disease diagnosis, determining disease pathogenesis, and drug development [15, 16].
Studies have shown that the composition of metabolites in biological samples may be influenced by factors such as gender, age, diet, lifestyle, environmental, etc. [17,18,19,20]. Considering this, AF as a biological sample undergoes changes throughout the gestational week as the fetus develops. However, to date, few studies have examined the factors affecting the metabolomics of AF. The study by Orczyk-Pawilowicz et al. [21] demonstrated that the AF during the transition from the 2nd (15.4 ± 0.96 weeks) to the 3rd (37.7 ± 1.68 weeks) trimester was associated with elevated levels of creatinine, succinate, pyruvate, choline, N,N-dimethylglycine, and urocanate, while a reduction in the levels of amino acids, glucose, and carnitine was observed. However, the impacts of gestational week, fetal gender and maternal age on AF metabolic profile warrant further exploration.
In this study, untargeted metabolomics based on GC-TOF/MS was applied to explore the relationship between fetal gender, maternal age, and gestational week with the metabolic profile of AF in the second trimester, in order to identify potential influencing factors that may provide a foundation for further AF-related metabolomics research in the future.
Methods
Samples
From January 2012 to December 2018, a total of 1,859 AF samples were collected prospectively, from pregnant women of similar ethnic backgrounds in the Jiangsu Province. These were residual AF following clinical molecular diagnostic tests in our prenatal diagnostic center, which were stored at -800C. Of these, 114 AF samples were selected for this study, with the inclusion criteria as follows: singleton pregnancy; amniocentesis performed due to advanced age or high risk for Down’s Syndrome following a serological screening in the second trimester of pregnancy; analysis of AF showed normal karyotypes; and no abnormality was identified after birth during the postnatal follow-ups. AF samples were excluded if they were from pregnant women with pregnancy-related disorders, such as hypertension and diabetes.
Chemical materials and instruments for untargeted metabolomics
Pyridine, methoxyamine HCl, anhydrous sodium sulfate, and fatty acid methyl ester standards (C7–C30, FAMEs) were purchased from Sigma-Aldrich (St. Louis, MO, USA), while N-methyl-N(trimethylsilyl)trifluoroacetamide (MSTFA) with 1% (vol/vol) trimethylchlorosilane (TMCS), dichloromethane, hexane, chloroform, methanol (Optima LC–MS), acetonitrile (Optima LC–MS) and acetone were purchased from Thermo-Fisher Scientific (FairLawn, NJ, USA). Ultrapure water was obtained through a Milli-Q reference system (Millipore, Billerica, MA, USA). GC-TOF/MS (Pegasus HT, Leco Corp., St. Joseph, MO, USA) was equipped with an Agilent 7890B gas chromatography, a Gerstel multipurpose sample MPS2 (Gerstel, Muehlheim, Germany), and a Rxi-5 ms capillary column (30 m × 250 μm i.d., 0.25 μm film thickness, Restek Corporation, Bellefonte, PA, USA).
Sample processing
The frozen AF samples stored at -80 °C were thawed on ice. Then, they were mixed well and centrifuged at 1,000 g for 3 min at 4 °C. Each 100 μL of AF sample was aliquoted into a pre-cooled Eppendorf tube and 10 μL of the internal standard solution was added. For metabolite extraction, each 200 μL of pre-cooled methanol: chloroform (3:1, v/v) was used. After centrifugation (13,000 g, 20 min, 4 °C), the supernatant was transferred into an auto-sampler vial (Agilent Technologies, Foster City, CA, USA). Quality control (QC) samples were prepared by mixing the remaining supernatant from each AF sample. To remove the chloroform solvent, all the samples were centrifuged for 5 min in a vacuum centrifuge concentrator (Labconco, Kansas City, MO, USA), and then transferred to a freeze dryer (Labconco, Kansas City, MO, USA) and completely lyophilized. Dichloromethane was added to ensure complete dryness of the samples, followed by high-purity nitrogen (Parker Balston, Lancaster, NY, USA) filling in the dried powder at room temperature. Untargeted metabolite analysis was performed on the XploreMET platform (Metabo-Profile, Shanghai, China). Briefly, 50 μL of methoxyamine (20 mg/mL in pyridine) was added to each dried sample at 30 °C for 2 h and then mixing with 50 μL of MSTFA (1% TMCS) containing FAMEs at 37.5 °C for 1 h.
Chromatographic conditions
Helium (99.9999%) was used as the carrier gas at the constant flow rate of 1.0 mL/min. The injection volume was 1 μL, and the injection and transfer interface temperatures were both at 270 °C. The GC temperature programming was set to 2 min of isothermal heating at 80 °C, followed by 12 °C/min oven temperature ramps at 80–300 °C, 4.5 min at 300 °C, 40 °C/min at 300–320 °C, and 1 min of final maintenance at 320 °C.
Mass spectrometry conditions
In the full scan mode (m/z 50–500), metabolites were measured using electron impact ionization (70 eV), and the ion source was set at 220 °C. The acquisition rate was 25 spectra/s, and the mass range was 50–500 Da.
Data analysis
Peak picking, automated baseline denoising, deconvolution, and signal alignment were processed by XploreMET 3.0 software [22, 23]. Each data set was transformed into the comparable data vectors, and MetaboAnalyst 5.0, an open software, was used for multivariate statistical analysis, including the PCA, OPLS-DA and PLS-DA. PCA is an unsupervised classification model, which continuously reduces the dimensions of multi-dimensional data into several main components (PCs) to describe the characteristics of the original data to the furthest extent possible [24]. On the other hand, the PLS-DA model and OPLS-DA model are supervised classification models, which can maximize the differences between groups according to the predefined classification and achieve a better separation effect than PCA [25]. However, supervised analytical models may produce the phenomenon of overfitting [26]. There are two parameters, R2Y and Q2 in the model, of which R2Y measures the goodness of fit and Q2 measures the predictive ability of the model. The closer these two values are to 1, the more exemplary the model, with Q2 > 0.5 indicating good predictability [27]. A negative value of Q2 indicatesthe overfitting of the model, suggesting no significant difference between the groups [28]. Univariate statistical analysis was performed using SPSS 22.0 software (IBM, USA), and P < 0.05 was considered statistically significant.
Results
Association between fetal gender and AF metabolic profile
The demographics including the number of AF samples, maternal age range, and gestational week range based on gender were outlined in Table 1. A total of 64 maternal AF samples of female fetuses and 50 male fetuses were included. For the female and male fetal groups, the medians of the gestational week were both at 20 (17–24) weeks, while the maternal age was at 31.92 ± 5.20 and 31.00 ± 5.65 years, respectively. When comparing the 2 groups, there were no statistically significant differences in the gestational week (P = 0.80) and maternal age (P = 0.25).
In an unsupervised PCA analysis model, there was no significant trend of separation of the metabolic profiles between the female and male fetal groups (Fig. 1A). The OPLS-DA model showed a trend of separation between the two groups, but the parameter Q2 value was -0.224, indicating that this trend of separation was attributed to the overfitting of the models rather than the difference in the gender groups (Fig. 1B). These findings suggested no significant association between the difference in the fetal gender and the total metabolic profile of AF.
A total of 265 metabolites were detected in the amniotic fluid, of which 178 were successfully identified. Of these, the proportions of amino acids, organic acids, carbohydrates, nucleotides, lipids, indoles, fatty acids and others were 30%, 25%, 19%, 7%, 3%, 3%, 3%, and 10%, respectively.
Univariate analysis revealed 7 differently expressed metabolites between the two groups, including isoleucine, 3-aminoisobutanoic acid, ribonolactone, fructose 6-phosphate, citric acid, phosphoenolpyruvic acid, and hypoxanthine (Table 2).
Association between maternal age and AF metabolic profile
The demographics including the number of AF samples, fetal gender composition, and gestational week range based on the maternal age were outlined in Table 3. AF samples were categorized into 4 groups of maternal ages, including 21–25 years, 26–30 years, 31–35 years and 36–40 years, with the numbers of AF samples in each group were 16, 37, 33 and 19, respectively, while the numbers of female/male fetuses were 8/8, 19/18, 20/13 and 11/8, respectively, with no significant difference between the groups. Similarly, no significant difference was demonstrated in the medians of the gestational week between the groups, which were 19.5 (17–24) weeks, 20 (18–24) weeks, 20 (17–23) weeks and 20 (18–23) weeks, respectively.
PCA was performed to explore the differences in the metabolic profile between the four groups, which showed no significant trend of separation (Fig. 2A). Similar findings were observed when the PLS-DA model was performed (Fig. 2B). The model parameter Q2 value of the first three components was-0.22 and the P-value of the permutation test of the model was 0.54. These findings indicated that maternal age had no significant influence on the AF metabolic profile. Among the 178 metabolites successfully identified, only 2-hydroxybutyric acid, guanidinosuccinic acid, erythrose and putrescine were significantly different among these groups (Table 4).
Association between gestational week and AF metabolic profile
A total of 109 samples were selected to explore the association between the gestational week and the metabolic profile of AF, as shown in Table 5. There were 3 gestational week groups, which were 17–18 weeks (12 cases), 19–20 weeks (67 cases), and 21–22 weeks (30 cases), with the numbers of female/male fetuses in each group were 5/7, 39/28, and 18/12, respectively, the difference of which between the groups was not statistically significant. Also, no significant difference was demonstrated between the groups in the medians of the maternal age, which were 28.5 (24–42) years, 31 (21- 42) years, and 31 (24–42) years, respectively.
The PCA revealed no significant separation in the AF metabolic profiles among the three gestational week groups (Fig. 3A), which were further validated by the PLS-DA model with a negative Q2 value (Fig. 3B). These findings indicated that gestational week had no significant influence on the AF metabolic profile at 17 to 22 weeks, but had an impact on the level of 23 metabolites, as shown in Table 6.
Discussion
The innovations of chromatography, mass spectrometry, as well as bio-information technology have enabled metabolomics to develop rapidly. Metabolomics is a growing field of research especially in liver disease, cancer, cardiovascular disease, traditional Chinese medicine, and intestinal microbiota. Loomba et al. [29] have used metabolomics to evaluate the relationship between dose and therapeutic effect of GS-0976 in patients with nonalcoholic steatohepatitis (NASH). Also, Huang et al. [30] have carried out a large prospective serum metabolomic research involving 523 cases of lethal prostate cancer and an equal number of matched controls. In the study, 34 metabolites were associated with lethal prostate cancer, in which dipeptide leucylglycine and three gamma-glutamyl amino acids were associated with an increased risk of lethal prostate cancer. The study by Chen et al. [31] has applied the untargeted metabolomics method to examine and compare serum samples of untreated black hypertensives treated with slow sodium tablets or placebo tablets, and identified β-hydroxyisovalerate and methionine sulfone were significantly increased in the treatment group, indicating that low sodium diet reduces blood pressure. In traditional Chinese medicine, studies have applied metabolomics to explore the components and pharmacokinetics of ginseng [32]. In recent years, research on intestinal microorganisms has been popularized. The study by Hagan et al. [33] has demonstrated the important role of intestinal microorganisms in regulating the body's immunity. All in all, metabolomics is a valuable tool for exploring drug efficacy, screening of tumor markers, studying of pathological mechanisms, drug metabolism and many other aspects.
Metabolomics also has important applications in the studies of abnormalities in pregnant women or fetuses during pregnancy. Bahado-Singh et al. [34] have used NMR to explore the serum profile of pregnant women at 11–13 weeks to predict pre-eclampsia, and the results demonstrated that a combination of citrate, hydroxyisovalerate, glycerol, and methionine produced a better predictive effect (75%). Also, the study by Ciborowski et al. [35] has revealed that serum metabolites in early pregnancy could predict the risk of macrosomia. AF is crucial to the normal development of the fetus. It surrounds the fetus, which acts as a mechanical buffer to balance the external pressure. It also contains a variety of nutrients and growth factors that are needed for the growth of the fetus [36]. The diversity in the composition of the AF provides a good source of research materials, providing opportunities for the assessment of fetal maturity, disease diagnosis, the discovery of biomarkers, etc. [37,38,39]. Therefore, scientific research on the composition of AF is greatly warranted by applying untargeted metabolomics. It is recognized that the composition of the AF changes with the growth of the fetus, but at present, little is known regarding the factors that affect the composition of the AF. In this study, we have applied an untargeted metabolomics method to identify as many minute molecular metabolites as possible in a selected cohort of AFs. Our findings revealed that the main metabolites in AF were amino acids, followed by organic acids, carbohydrates and fatty acids, which provided further insight into the composition of the AF. Nevertheless, the effects of several factors such as the gestational week, maternal age and fetal gender on the metabolic composition of AF remained inadequately understood.
Following the analysis of AF metabolic profile using an untargeted metabolomics approach, the findings were correlated with factors including fetal gender, maternal age, and gestational week. We found that there was no significant correlation between the AF metabolic profile and differences in the fetal gender, maternal age and gestational week within a small range of maternal age (21 to 40 years) and gestational week (17 to 22 weeks). However, several metabolite levels were affected. Our findings were consistent with the study by Graca et al. [40] that examining AF metabolism using Nuclear Magnetic Resonance (NMR) spectroscopy on 51 AF samples. Additionally, our findings revealed the metabolites that may be influenced by these three factors. Furthermore, the study by Orczyk-Pawilowicz et al. [21] has demonstrated the differences in the AF metabolic profile between the second and third trimester of pregnancy, suggesting that the gestational week may affect the constitutions of the AF if gestational week varies greatly. All in all, the influence of gestational week on the metabolic profile of AF is dependent on the range of gestational week.
There were several limitations to our study. Firstly, maternal BMI may influence the metabolic profile of AF but this was not explored. Secondly, sample sizes were small for several subgroup analyses. Nevertheless, our findings provide a basis for further research, which will shed light on how these factors may influence the AF metabolic profile and will generate opportunities for clinical application.
In summary, this study correlated the AF metabolic profile with several factors including detailed classifications of the gestational week, maternal age, and fetal gender. A small range of maternal age or gestational week did not significantly affect the AF metabolic profile but impacted several metabolite expressions. Gestational week, maternal age and fetal gender may affect the expression of some metabolites and thus, correlation of these factors in clinical studies is paramount. This study demonstrates a new approach in analyzing the metabolites in AF that guides the further study of biomarkers in pregnancy-related diseases.
Availability of data and materials
All authors had full access to the data and materials. Data is available from the authors upon reasonable request.
References
Kacerovsky M, Vlkova B, Musilova I, Andrys C, Pliskova L, Zemlickova H, et al. Amniotic fluid cell-free DNA in preterm prelabor rupture of membranes. Prenat Diagn. 2018;38(13):1086–95.
Jang JH, Jung YW, Shim SH, Sin YJ, Lee KJ, Shim SS, et al. Global gene expression changes of amniotic fluid cell free RNA according to fetal development. Eur J Obstet Gynecol Reprod Biol. 2017;216:104–10.
Bardanzellu F, Fanos V. The choice of amniotic fluid in metabolomics for the monitoring of fetus health – update. Expert Rev Proteomics. 2019;16(6):487–99.
Rinschen MM, Ivanisevic J, Giera M, Siuzdak G. Identification of Bioactive Metabolites Using Activity Metabolomics. Nat Rev Mol Cell Biol. 2019;20(6):353–67.
Nicholson JK, Lindon JC, Holmes E. 'Metabonomics': understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica. 1999;29:1181–9.
Troisi J, Cavallo P, Colucci A, Pierri L, Scala G, Symes S, et al. Metabolomics in genetic testing. Adv Clin Chem. 2020;94:85–153.
Ros-Mazurczyk M, Jelonek K, Marczyk M, Binczyk F, Pietrowska M, Polanska J, et al. Serum lipid profile discriminates patients with early lung cancer from healthy controls. Lung Cancer. 2017;112:69–74.
Pinu FR, Beale DJ, Paten AM, Kouremenos K, Swarup S, Schirra HJ, et al. Systems biology and multi-omics integration: viewpoints from the metabolomics research community. Metabolites. 2019;9(4):76.
Kim S, Jang WJ, Yu H, Ryu IS, Jeong CH, Lee S. Integrated Non-targeted and targeted metabolomics uncovers dynamic metabolic effects during short-term abstinence in methamphetamine self-administering rats. J Proteome Res. 2019;18(11):3913–25.
Ghatak A, Chaturvedi P, Weckwerth W. Metabolomics in plant stress physiology. Adv Biochem Eng Biotechnol. 2018;164:187–236.
Sands BE. Biomarkers of inflammation in inflammatory bowel disease. Gastroenterology. 2015;149(5):1275–85.
Johnson CH, Ivanisevic J, Siuzdak G. Metabolomics: beyond biomarkers and towards mechanisms. Nat Rev Mol Cell Biol. 2016;17(7):451–9.
Palmer EA, Cooper HJ, Dunn WB. Investigation of the 12-month stability of dried blood and urine spots applying untargeted UHPLC-MS metabolomic assays. Anal Chem. 2019;91(22):14306–13.
Li Y, Sun Y, Yang L, Huang M, Zhang X, Wang X, et al. Analysis of biomarkers for congenital heart disease based on maternal amniotic fluid metabolomics. Front Cardiovasc Med. 2021;8:671191.
Liu E, Perl A. Pathogenesis and treatment of autoimmune rheumatic diseases. Curr Opin Rheumatol. 2019;31(3):307–15.
Sinha R, Sharma B, Dangi AK, Shukla P. Recent metabolomics and gene editing approaches for synthesis of microbial secondary metabolites for drug discovery and development. World J Microbiol Biotechnol. 2019;35(11):166.
Ruoppolo M, Scolamiero E, Caterino M, Mirisola V, Franconi F, Campesi I. Female and male human babies have distinct blood metabolomic patterns. Mol Biosyst. 2015;11(9):2483–92.
Chaleckis R, Murakami I, Takada J, Kondoh H, Yanagida M. Individual variability in human blood metabolites identifies age-related differences. Proc Natl Acad Sci U S A. 2016;113(16):4252–9.
Beuchel C, Becker S, Dittrich J, Kirsten H, Toenjes A, Stumvoll M, et al. Clinical and lifestyle related factors influencing whole blood metabolite levels - A comparative analysis of three large cohorts. Mol Metab. 2019;29:76–85.
Dhungana S, Carlson JE, Pathmasiri W, McRitchie S, Davis M, Sumner S, et al. Impact of a western diet on the ovarian and serum metabolome. Maturitas. 2016;92:134–42.
Orczyk-Pawilowicz M, Jawien E, Deja S, Hirnle L, Zabek A, Mlynarz P. Metabolomics of Human Amniotic Fluid and Maternal Plasma during Normal Pregnancy. PLoS One. 2016;11(4):e0152740.
Ni Y, Qiu Y, Jiang W, Suttlemyre K, Su M, Zhang W, et al. ADAP-GC 2.0: deconvolution of coeluting metabolites from GC/TOF-MS data for metabolomics studies. Anal Chem. 2012;84:6619–29.
Chen WL, Wang YY, Zhao A, Xia L, Xie G, Su M, et al. Enhanced fructose utilization mediated by SLC2A5 is a unique metabolic feature of acute myeloid leukemia with therapeutic potential. Cancer Cell. 2016;30:779–91.
Kellogg JJ, Kvalheim OM, Cech NB. Composite score analysis for unsupervised comparison and network visualization of metabolomics data. Anal Chim Acta. 2020;1095:38–47.
Kandasamy S, Yoo J, Yun J, Kang HB, Seol KH, Ham JS. 1H HRMAS-NMR based metabolic fingerprints for discrimination of cheeses based on sensory qualities. Saudi J Biol Sci. 2020;27(6):1446–61.
Podlecka-Piętowska A, Kacka A, Zakrzewska-Pniewska B, Nojszewska M, Zieminska E, Chalimoniuk M, et al. Altered cerebrospinal fluid concentrations of hydrophobic and hydrophilic compounds in early stages of multiple sclerosis-metabolic profile analyses. J Mol Neurosci. 2019;69(1):94–105.
Triba MN, Le Moyec L, Amathieu R, Goossens C, Bouchemal N, Nahon P, et al. PLS/OPLS models in metabolomics: the impact of permutation of dataset rows on the K-fold cross-validation quality parameters. Mol Biosyst. 2015;11(1):13–9.
Goïta Y, Chao de la Barca JM, Keïta A, Diarra MB, Dembélé KC, Chabrun F, et al. Sexual dimorphism of metabolomic profile in arterial hypertension. Sci Rep. 2020;10(1):7517.
Loomba R, Kayali Z, Noureddin M, Ruane P, Lawitz EJ, Bennett M, et al. GS-0976 reduces hepatic steatosis and fibrosis markers in patients with nonalcoholic fatty liver disease. Gastroenterology. 2018;155(5):1463–73.
Huang J, Mondul AM, Weinstein SJ, Derkach A, Moore SC, Sampson JN, et al. Prospective serum metabolomic profiling of lethal prostate cancer. Int J Cancer. 2019;145(12):3231–43.
Chen L, He FJ, Dong Y, Huang Y, Harshfield GA, Zhu H. Sodium reduction, metabolomic profiling, and cardiovascular disease risk in untreated black hypertensives. Hypertension. 2019;74(1):194–200.
Wu W, Jiao C, Li H, Ma Y, Jiao L, Liu S. LC-MS based metabolic and metabonomic studies of Panax ginseng. Phytochem Anal. 2018;29(4):331–40.
Hagan T, Cortese M, Rouphael N, Boudreau C, Linde C, Maddur MS, et al. Antibiotics-driven gut microbiome perturbation alters immunity to vaccines in humans. Cell. 2019;178(6):1313-1328.e13.
Bahado-Singh RO, Akolekar R, Mandal R, Dong E, Xia J, Kruger M, et al. Metabolomics and first-trimester prediction of early-onset preeclampsia. J Matern Fetal Neonatal Med. 2012;25(10):1840–7.
Ciborowski M, Zbucka-Kretowska M, Bomba-Opon D, Wielgos M, Brawura-Biskupski-Samaha R, Pierzynski P, et al. Potential first trimester metabolomic biomarkers of abnormal birth weight in healthy pregnancies. Prenat Diagn. 2014;34(9):870–7.
Velika B, Birkova A, Dudic R, Urdzik P, Marekova M. Selected physicochemical properties of amniotic fluid according to week of pregnancy. Bratisl Lek Listy. 2018;119(3):175–9.
Kamath-Rayne BD, Du Y, Hughes M, Wagner EA, Muglia LJ, DeFranco EA, et al. Systems biology evaluation of cell-free amniotic fluid transcriptome of term and preterm infants to detect fetal maturity. BMC Med Genomics. 2015;8:67.
Zhang J, Wang Y, Ma D, Sun Y, Li Y, Yang P, et al. Carrier Screening and prenatal diagnosis for spinal muscular atrophy in 13,069 chinese pregnant women. J Mol Diagn. 2020;22(6):817–22.
Novakovic TR, Dolicanin ZC, Djordjevic NZ. Oxidative stress biomarkers in amniotic fluid of pregnant women with hypothyroidism. J Matern Fetal Neonatal Med. 2019;32(7):1105–10.
Graça G, Duarte IF, Barros AS, Goodfellow BJ, Diaz S, Carreira IM, et al. (1)H NMR based metabonomics of human amniotic fluid for the metabolic characterization of fetus malformations. J Proteome Res. 2009;8(8):4144–50.
Acknowledgements
We thank all the participants for their contributions.
Funding
This work was supported by the National Natural Science Foundation of China (81770236), the National Key Research and Development Program of China (2018YFC1002402) and Jiangsu Natural Science Foundation (BK20181121).
Author information
Authors and Affiliations
Contributions
YL and YS were responsible for comprehensive sample collection, data collection, experimental operation, data analysis and paper writing. XZ, XW, PY, XG and XZ were responsible for sample and information collection. ZX, TJ and PH were responsible for the funding, research design, and paper writing. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by Medical Ethics Committee of Nanjing Maternity and Child Health Care Hospital(Code number: [2018] NO.91). All participants signed informed consent before receiving amniocentesis, agreeing to use the remaining samples from clinical tests for scientific research. Therefore, this study did not re-sign the informed consent before the subsequent retrospective analysis, and informed consent was waived given the retrospective nature of our study. All methods were carried out in accordance with relevant guidelines and regulations under Ethics approval.
Consent for publication
Not applicable because there is no details, images, or videos related to an individual person.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, Y., Sun, Y., Zhang, X. et al. Relationship between amniotic fluid metabolic profile with fetal gender, maternal age, and gestational week. BMC Pregnancy Childbirth 21, 638 (2021). https://doi.org/10.1186/s12884-021-04116-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12884-021-04116-6