Abstract
Background
The adequate maternal sleep duration required for favorable obstetric outcomes is unknown. We evaluated the association between maternal sleep duration and low birth weight infants, small for gestational age infants, and macrosomia.
Methods
Participants enrolled in the Japan Environment and Children’s Study, a nationwide birth cohort study, with singleton pregnancies after 22 weeks, who gave birth between 2011 and 2014 were enrolled and categorized into five groups according to maternal sleep duration during pregnancy: < 6.0 h, 6.0–7.9 h, 8.0–8.9 h, 9.0–9.9 h, and 10.0–12.0 h. We evaluated the association between maternal sleep duration and the incidence of low birth weight infants (< 2500 g), very low birth weight infants (< 1500 g), small for gestational age infants, and macrosomia (> 4000 g), with women with maternal sleep duration of 6.0–7.9 h as the reference, using a multiple logistic regression model.
Results
In total, 82,171 participants were analyzed. The adjusted odds ratios (95% confidence intervals) for low birth weight infants in women with maternal sleep duration of 9.0–9.9 h and 10.0–12.0 h and for small for gestational age infants in women with maternal sleep duration of 9.0–9.9 h were 0.90 (0.83–0.99), 0.86 (0.76–0.99), and 0.91 (0.82–0.99), respectively, before adjusting for excessive gestational weight gain. No significant association was observed between maternal sleep duration and these outcomes after adjusting for excessive gestational weight gain. Among women with appropriate gestational weight gain, the adjusted odds ratios (95% confidence intervals) for low birth weight infants and for small for gestational age infants with sleep duration of 9.0–9.9 h were 0.88 (0.80–0.97) and 0.87 (0.78–0.97), respectively.
Conclusions
Maternal sleep duration of 9.0–9.9 h was significantly associated with the decreased incidence of low birth weight infants and small for gestational age infants in pregnant women with appropriate gestational weight gain, compared with that of 6.0–7.9 h. Care providers should provide proper counseling regarding the association between maternal sleep duration and neonatal birth weight and suggest comprehensive maternal lifestyle modifications to prevent low birth weight and small for gestational age infants.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Background
Neonatal birth weight, which is related to perinatal morbidity and mortality [1,2,3,4], is affected by several obstetric complications, including preterm births (PTB), fetal growth restriction (FGR), and preeclampsia [5, 6]. Specifically, infants with birth weight < 1500 g, defined as very low birth weight (VLBW) infants, are severely premature and may experience increased mortality [7]. Additionally, small for gestational age (SGA) infants are also at an increased risk of neonatal and post-neonatal mortality [4, 8, 9]. Further, low birth weight (LBW) and SGA infants are associated with an increased risk of coronary artery disease, diabetes mellitus, and arterial hypertension in adulthood, as described in the Baker hypothesis [10], which has been revised to the concept of developmental origins of health and disease [11]. Conversely, macrosomia, defined as birth weight > 4000 g, is also associated with a risk of morbidity in infants [2, 12].
Several modifiable factors, including maternal pre-pregnancy body weight, gestational weight gain (GWG), and diet, have a major impact on the neonatal birth weight [5, 12,13,14]. Similarly, maternal sleep duration (MSD) during pregnancy also affects obstetric outcomes [15,16,17]. However, the association between MSD and neonatal birth weight remains unclear [15, 18]. Previous studies have reported reduced MSD as a risk factor for the incidence of SGA infants [19, 20], but Morokuma et al. reported no such association in a study of 8631 participants [21]. Moreover, the appropriate MSD required to prevent LBW infants, SGA infants, and macrosomia has not been elucidated. As sleep is often disturbed in pregnant women [15], appropriate MSD could be a great concern.
The present study evaluated the association between MSD and LBW infants, VLBW infants, SGA infants, and macrosomia using the data from a nationwide Japanese birth cohort study.
Methods
Study design
In this study, we retrospectively analyzed the data from the Japan Environment and Children’s Study (JECS), which is a nationwide, government-funded, prospective birth cohort study that was started in January 2011 to investigate the effects of environmental factors on children’s health [22, 23]. Briefly, the JECS is funded directly by the Ministry of the Environment, Japan and involves collaboration among the Programme Office (National Institute for Environmental Studies), the Medical Support Centre (National Centre for Child Health and Development), and 15 Regional Centres (Hokkaido, Miyagi, Fukushima, Chiba, Kanagawa, Koshin, Toyama, Aichi, Kyoto, Osaka, Hyogo, Tottori, Kochi, Fukuoka, and South Kyushu/Okinawa) [23]. The eligibility criteria for expectant mothers to participate in the JECS were as follows: (1) residing in the study areas at the time of recruitment and expected to continually reside in Japan for the foreseeable future; (2) an expected delivery date between August 1, 2011 and mid-2014; and (3) capable of participating in the study without difficulty (i.e., able to comprehend the Japanese language and complete the self-administered questionnaires).
We applied either or both of the following recruitment protocols: (1) recruitment at the time of the first prenatal examination at the cooperating obstetric facilities; and (2) recruitment at local government offices issuing a pregnancy journal, called the Maternal and Child Health Handbook, that is given to all expecting mothers in Japan before they receive municipal services for pregnancy, delivery, and childcare. We contacted pregnant women through cooperating health care providers and/or local government offices issuing the Maternal and Child Health Handbooks and registered those willing to participate. Self-administered questionnaires, which were completed by the women during the first and second/third trimesters, were used to collect information on demographic factors, medical and obstetric history, physical and mental health, lifestyle, occupation, environmental exposure at home and at the workplace, housing conditions, and socioeconomic status [23].
In addition to the ethics committees of all participating institutions, the Ministry of the Environment’s Institutional Review Board on Epidemiological Studies reviewed and approved the JECS protocol (No. 100910001). The JECS was conducted in accordance with the principles of the Declaration of Helsinki and other national regulations and guidelines. Written informed consent was obtained from all participants.
Data collection
The current analysis used the data set released in June 2016 (data set: jecs-ag-20160424). Specifically, we used three types of data: (1) M-T1, obtained from a self-reported questionnaire that was collected during the first trimester (the first questionnaire) and included questions regarding maternal medical background; (2) M-T2, obtained from a self-reported questionnaire that was collected during the second or third trimester (second questionnaire) and included questions regarding lifestyle and socioeconomic status; and (3) Dr-0m, collected from the medical record transcripts provided by each participant’s institution that included data for obstetrical outcomes such as gestational age, birth weight, neonatal sex, and maternal body weight.
The participants with singleton pregnancies after 22 weeks were included in the present study. Women with abortion, stillbirths, and missing information were excluded from the analysis. There were no significant differences in characteristics between those included in and excluded from the analysis (data not shown).
Exposure variables
We calculated MSD using the data of the questionnaire in M-T2 [24]. The participants submitted data about their bedtime and waking up time and were categorized into five groups according to MSD during pregnancy: MSD < 6.0 h, MSD 6.0–7.9 h, MSD 8.0–8.9 h, MSD 9.0–9.9 h, and MSD 10.0–12.0 h. These categories were based on the report of the Ministry of Health, Labour and Welfare, Japan [25], which suggests 6.0–7.9 h of sleep as the appropriate sleep duration for general adults to prevent several health problems. Participants who reported MSD > 12.0 h were excluded from the present study because they were considered to have reported inaccurate MSDs.
Obstetric outcomes and confounding factors
We classified LBW infants into two categories: LBW infants < 2500 g and VLBW infants < 1500 g [1]. SGA infants were defined as a birth weight < 1.5 standard deviations, corrected for parity, gestational age, and sex, according to the “New Japanese neonatal anthropometric charts for gestational age at birth” [8]. Macrosomia was defined as neonatal birth weight > 4000 g [12].
We considered the following parameters as potential confounding factors: maternal age, body mass index (BMI) before pregnancy, parity, maternal smoking status, maternal alcohol consumption status, maternal educational status, annual household income, PTB before 37 weeks, and GWG. Maternal age was categorized into three groups: < 20 years, 20–29 years, and ≥ 30 years, based on a previous study that showed that maternal age was related to certain obstetric outcomes, such as PTB, LBW infants, and SGA infants [26]. BMI before pregnancy was categorized into three groups: < 18.5 kg/m2, 18.5–24.9 kg/m2, and ≥ 25.0 kg/m2 [27, 28]. Parity was categorized into two groups: nulliparous and multiparous. We requested maternal participants to provide information about their smoking status by choosing one of the following: “Currently smoking,” “Never,” “Previously did but quit before realizing current pregnancy,” and “Previously did but quit after realizing current pregnancy.” The maternal participants who chose “Currently smoking” comprised the smoking category, whereas the other participants comprised the non-smoking category. We also asked the maternal participants to provide information about their alcohol consumption status by choosing one of the following: “Never drank,” “Quit drinking before pregnancy,” “Quit drinking during early pregnancy,” and “Kept drinking during pregnancy” [29]. The maternal participants who chose “Kept drinking during pregnancy” comprised the drinking category, whereas the other participants comprised the non-drinking category. Maternal educational status was categorized into four groups based on the number of years of education: junior high school, < 10 years; high school, 10–12 years; professional school or university, 13–16 years; and graduate school, ≥17 years. Annual household income was categorized into four groups: < 2,000,000 JPY, 2,000,000–5,999,999 JPY, 6,000,000–9,999,999 JPY, and ≥ 10,000,000 JPY. PTB was defined as birth before 37 weeks of gestation, the record of which was collected from Dr-0m data. The data for GWG were obtained from Dr-0m data, which included information about body weight before pregnancy (kg) and body weight just before delivery (kg). GWG was defined as body weight just before delivery minus body weight before pregnancy (kg). We defined appropriate GWG as < 12 kg and excessive GWG as ≥ 12 kg, according to the criteria aimed at appropriate birth weight, described by the Ministry of Health, Labour and Welfare, Japan [27, 28], where the appropriate GWG is defined as 9–12 kg for women with pre-pregnancy BMI below 18.8 kg/m2 and 7–12 kg for women with pre-pregnancy BMI between 18.5–24.9 kg/m2, and individually assigned for women with pre-pregnancy BMI over 25.0 kg/m2. These confounding factors were chosen on the basis of their clinical importance [5, 13, 14]. Since GWG in particular is indicative of lifestyle, it was thought to be a significant confounding factor.
Statistical analyses
Participant characteristics were summarized according to the maternal sleeping status. One-way analysis of variance and the Kruskal-Wallis test were used to compare continuous variables among different MSD groups, according to the difference in the distribution of data. The chi-square test was used to compare categorical variables.
Initially, crude odds ratios (cORs), adjusted odds ratios (aORs), and 95% confidence intervals (CI) for LBW infants, VLBW infants, SGA infants, and macrosomia were calculated using a multiple logistic regression model, with women with MSD of 6.0–7.9 h as the reference. In Model 1, the odds ratios for LBW infants and VLBW infants were adjusted for maternal age, BMI before pregnancy, parity, maternal smoking status, maternal alcohol consumption status, maternal educational status, annual household income, and PTB before 37 weeks. The odds ratio for SGA infants was adjusted for maternal age, BMI before pregnancy, maternal smoking status, maternal alcohol consumption status, maternal educational status, and annual household income. The odds ratio for macrosomia was adjusted for maternal age, BMI before pregnancy, parity, maternal smoking status, maternal alcohol consumption status, maternal educational status, and annual household income. In Model 2, excessive GWG was added as a confounding factor (in addition to those of Model 1) to calculate the aORs for these outcomes.
Further, we stratified the participants based on GWG, and cORs and aORs for LBW infants and SGA infants were calculated using a multiple logistic regression model, with women with MSD of 6.0–7.9 h as the reference, using the same confounding factors as Model 1.
SPSS version 26 (IBM Corp., Armonk, NY) was used to perform the statistical analyses. Differences with p-values < 0.05 were considered statistically significant.
Results
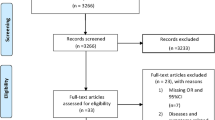
The total number of fetal records during 2011–2014 was 104,102. After applying our inclusion criteria, 82,171 participants were eligible for this study (Fig. 1). There were missing data regarding maternal age in 5 cases, height before pregnancy in 69, weight before pregnancy in 57, parity in 2310, maternal smoking status in 1888, maternal alcohol consumption status in 1625, maternal educational status in 489, annual household income in 6103, GWG in 1719, neonatal birth weight in 21, sex of the newborn in 2, and inaccurate MSD (≤0 or > 12 h) in 1800. Among the 82,171 participants, 3958 (4.8%) had MSD of < 6.0 h, 37,944 (46.2%) had MSD of 6.0–7.9 h, 23,769 (28.9%) had MSD of 8.0–8.9 h, 11,976 (14.6%) had MSD of 9.0–9.9 h, and 4524 (5.5%) had MSD of 10.0–12.0 h.
Table 1 summarizes the maternal background and obstetric outcomes based on the MSD groups. All maternal background characteristics were significantly affected by MSD. The incidence of LBW infants was significantly lower in women with MSD of 9.0–9.9 and 10.0–12.0 h than in the other groups. No significant differences were observed in the incidence of PTB, VLBW infants, SGA infants, and macrosomia among the groups.
Table 2 shows the cORs and aORs for LBW infants, VLBW infants, SGA infants, and macrosomia among all groups, with women with MSD of 6.0–7.9 h as a reference. The aORs (95% CIs) for LBW infants in women with MSD of 9.0–9.9 and 10.0–12.0 h and for SGA infants in women with MSD of 9.0–9.9 h were 0.90 (0.83–0.99), 0.86 (0.76–0.99), and 0.91 (0.82–0.99), respectively, before adjusting for excessive GWG in Model 1. However, no significant association was observed between MSD and these outcomes after adjusting for excessive GWG in Model 2. Additionally, there was no significant association between MSD and VLBW infants and macrosomia in Models 1 and 2.
Table 3 shows the cORs and aORs for LBW infants and SGA infants among all groups, with women with MSD of 6.0–7.9 h as a reference, after stratification by GWG. Among women with appropriate GWG, the aORs (95% CIs) for LBW infants and SGA infants in women with MSD of 9.0–9.9 h were 0.88 (0.80–0.97) and 0.87 (0.78–0.97), respectively. However, no significant association was observed between MSD and these outcomes in women with excessive GWG.
Discussion
The present study showed an association between MSD over 9.0 h and the decreased incidence of LBW and SGA infants. However, this association was not sustained after adjusting for excessive GWG, which implies that both long MSD and excessive GWG are associated with a decreased incidence of LBW and SGA infants. Additionally, MSD of 9.0–9.9 h was significantly associated with the decreased incidence of LBW and SGA infants in women with appropriate GWG.
The present study, using a large birth cohort, showed that both MSD and GWG had an association with neonatal birth weight. Previous studies with relatively small sample sizes have shown a conflicting relationship between MSD and neonatal birth weight [15, 19,20,21]. Based on the findings of the present study, we speculate that long MSD, along with GWG, may be associated with the decreased incidence of LBW and SGA infants [14]. Although the effect of MSD on GWG is not clearly defined [30, 31], recent studies have reported that excessive sleep duration increases obesity in non-pregnant adults [32, 33]. The fetuses in mothers with excessive GWG may receive more nutrients and experience greater growth through increased plasma volume, which may increase cardiac output and utero-placental blood flow compared to those in mothers with insufficient GWG [34, 35]. Therefore, sufficient GWG by proper diet and sufficient MSD are required to prevent the incidence of LBW and SGA infants; however, the disadvantages of maternal obesity should be considered [36, 37].
The direct effects of MSD alone on obstetric outcomes in the appropriate GWG group have not been clarified yet. Maternal inflammatory stress has been reported to be related to several obstetric outcomes such as PTB, FGR, and preeclampsia [38,39,40]. Previous studies have also reported that disturbed maternal sleep may cause adverse obstetric outcomes, with augmentation of maternal inflammatory response [15, 41]. Increased inflammation may interfere with the remodeling of spiral arteries in the placenta, thereby leading to PTB, FGR, and preeclampsia [42,43,44]. Thus, preventing reduced MSD may reduce maternal inflammation and prevent these adverse obstetric outcomes. Further, maternal inflammatory stress is affected by lifestyle, including dietary habits and exercise [45, 46], and comprehensive lifestyle modification may help in reducing inflammatory stress.
However, MSD of 10.0–12.0 h was not associated with a decreased incidence of LBW and SGA infants in women with appropriate GWG. This may be because MSD of 10.0–12.0 h might be affected by maternal diseases, conditions, and behaviors, such as depression, excessive mental stress, and use of sleeping pills [24, 47, 48], which may potentially decrease neonatal birth weight [49,50,51]. Moreover, because excessively long MSD may have other unfavorable effects, including excessive GWG [32, 33], we do not suggest that pregnant women should have MSDs of over 10.0 h.
The strength of the present study is that the aORs for LBW and SGA infants provide clear information for perinatal counselling. Owing to the large study population of > 80,000 participants, our results should be considered reliable. Because pregnant women have more sleep problems, affected by gestational age and hormonal changes [24], than their non-pregnant counterparts, the association between MSD and fetal and neonatal health may be a great concern for pregnant women. There is no consensus on the appropriate MSD required to prevent adverse obstetric outcomes. Therefore, the present study would be helpful in suggesting the appropriate MSD required to prevent the incidence of LBW and SGA infants. Furthermore, because the JECS is a prospective cohort study, elucidation of long-term childhood outcomes based on MSD and neonatal birth weight in the future would strengthen the conclusions of this study.
The present study has some limitations. First, MSD in the present study was based on self-reported questionnaire data, which might have resulted in an inaccurate calculation of actual MSD. Nevertheless, several studies have shown a moderate correlation between self-reported and objectively-evaluated sleep duration measurements [52, 53]. Moreover, MSD is a volatile index because it varies daily in individuals and may vary with gestational age [24]. Careful interpretation is needed regarding these instabilities of MSD. Further study with polysomnography and unified gestational age may address this limitation. Second, we did not account for the quality of sleep by evaluating factors such as time zone, division, sleep location, and next to whom the participants are sleeping. Sleeping habits, including night and midday sleep durations, may also affect the infants’ weights. We evaluated the MSD as a simple quantitative measurement of maternal sleep to easily counsel pregnant women. Careful interpretation of the results is needed because the quality of maternal sleep, in addition to MSD, may also affect the obstetric outcomes. Finally, as this was a retrospective observational study, we could not clarify a cause-and-effect relationship. Although there was a significant association between MSD and the incidence of LBW and SGA infants in a certain setting, careful interpretation of the results is needed.
Conclusions
This study revealed that both MSD over 9.0 h and excessive GWG were significantly associated with the decreased incidence of LBW and SGA infants, and that MSD of 9.0–9.9 h was significantly associated with the decreased incidence of LBW and SGA infants in women with appropriate GWG. It is important for care providers to provide the latest data regarding the association between MSD and neonatal birth weight for proper counselling, and to suggest comprehensive modifications in the lifestyles of pregnant women, including sufficient MSD, to prevent the incidence of LBW and SGA infants. The present study may shed some light on the appropriate MSD required to prevent the incidence of LBW and SGA infants.
Availability of data and materials
Data are unsuitable for public deposition due to ethical restrictions and legal framework of Japan. It is prohibited by the Act on the Protection of Personal Information (Act No. 57 of 30 May 2003, amendment on 9 September 2015) to publicly deposit the data containing personal information. Ethical Guidelines for Epidemiological Research enforced by the Japan Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labour and Welfare also restricts the open sharing of the epidemiologic data. All inquiries about access to data should be sent to: jecs-en@nies.go.jp. The person responsible for handling enquiries sent to this e-mail address is Dr. Shoji F. Nakayama, JECS Programme Office, National Institute for Environmental Studies.
Abbreviations
- aOR:
-
Adjusted odds ratio
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- cOR:
-
Crude odds ratio
- FGR:
-
Fetal growth restriction
- GWG:
-
Gestational weight gain
- JECS:
-
Japan Environment and Children’s Study
- LBW:
-
Low birth weight
- MSD:
-
Maternal sleep duration
- PTB:
-
Preterm births
- SGA:
-
Small for gestational age
- VLBW:
-
Very low birth weight
References
Hughes MM, Black RE, Katz J. 2500-g low birth weight cutoff: history and implications for future research and policy. Matern Child Health J. 2017;21:283–9.
Wilcox AJ. On the importance--and the unimportance--of birthweight. Int J Epidemiol. 2001;30:1233–41.
Lawn JE, Cousens S, Zupan J. Lancet neonatal survival steering team. 4 million neonatal deaths: when? Where? Why? Lancet. 2005;365:891–900.
Katz J, Lee AC, Kozuki N, Lawn JE, Cousens S, Blencowe H, et al. Mortality risk in preterm and small-for-gestational-age infants in low-income and middle-income countries: A pooled country analysis. Lancet. 2013;382:417–25.
Kramer MS. Determinants of low birth weight: methodological assessment and meta-analysis. Bull World Health Organ. 1987;65:663–737.
Nafday SM. Abnormalities of fetal growth. In: Campbell DE, editor. Neonatology for Primary Care. Am Acad Pediatr; 2015. p. 323–344.
McLeod JS, Menon A, Matusko N, Weiner GM, Gadepalli SK, Barks J, et al. Comparing mortality risk models in VLBW and preterm infants: systematic review and meta-analysis. J Perinatol. 2020;40:695–703.
Itabashi K, Miura F, Uehara R, Nakamura Y. New Japanese neonatal anthropometric charts for gestational age at birth. Pediatr Int. 2014;56:702–8.
Pulver LS, Guest-Warnick G, Stoddard GJ, Byington CL, Young PC. Weight for gestational age affects the mortality of late preterm infants. Pediatrics. 2009;123:e1072–7.
Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1:1077–81.
Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305:1733–6.
Committee on Practice Bulletins—Obstetrics. Macrosomia: ACOG Practice Bulletin, Number 216. Obstet Gynecol. 2020;135:e18–35.
Kramer MS. The epidemiology of adverse pregnancy outcomes: an overview. J Nutr. 2003;133:1592S–6S.
Goldstein RF, Abell SK, Ranasinha S, Misso M, Boyle JA, Black MH, et al. Association of gestational weight gain with maternal and infant outcomes: A systematic review and meta-analysis. JAMA. 2017;317:2207–25.
Okun ML, Roberts JM, Marsland AL, Hall M. How disturbed sleep may be a risk factor for adverse pregnancy outcomes. Obstet Gynecol Surv. 2009;64:273–80.
Heazell A, Li M, Budd J, Thompson J, Stacey T, Cronin RS, et al. Association between maternal sleep practices and late stillbirth - findings from a stillbirth case-control study. BJOG. 2018;125:254–62.
Myoga M, Tsuji M, Tanaka R, Shibata E, Askew DJ, Aiko Y, et al. Impact of sleep duration during pregnancy on the risk of gestational diabetes in the Japan environmental and Children's study (JECS). BMC Pregnancy Childbirth. 2019;19:483.
Warland J, Dorrian J, Morrison JL, O'Brien LM. Maternal sleep during pregnancy and poor fetal outcomes: A scoping review of the literature with meta-analysis. Sleep Med Rev. 2018;41:197–219.
Abeysena C, Jayawardana P, DE A Seneviratne R. Maternal sleep deprivation is a risk factor for small for gestational age: a cohort study. Aust N Z J Obstet Gynaecol. 2009;49:382–7.
Franco-Sena AB, Kahn LG, Farias DR, Ferreira AA, Eshriqui I, Figueiredo ACC, et al. Sleep duration of 24 h is associated with birth weight in nulli- but not multiparous women. Nutrition. 2018;55–56:91–8.
Morokuma S, Shimokawa M, Kato K, Sanefuji M, Shibata E, Tsuji M, et al. Maternal sleep and small for gestational age infants in the Japan environment and Children's study: A cohort study. BMC Res Notes. 2017;10:394.
Kawamoto T, Nitta H, Murata K, Toda E, Tsukamoto N, Hasegawa M, et al. Rationale and study design of the Japan environment and Children’s study (JECS). BMC Public Health. 2014;14:25.
Michikawa T, Nitta H, Nakayama SF, Yamazaki S, Isobe T, Tamura K, et al. Baseline profile of participants in the Japan environment and Children’s study (JECS). J Epidemiol. 2018;28:99–104.
Konishi M, Tomotaki A, Yamamoto-Hanada K, Mezawa H, Ayabe T, Ishitsuka K, et al. Sleep status varies by age among Japanese women during preconception and pregnancy in a nationwide birth cohort study [the Japan environment and Children’s study (JECS)]. Sleep Biol Rhythms. 2018;17:161–72.
Ministry of Health, Labour and Welfare, Japan. Sleep guidelines for health promotion (in Japanese). Available at: https://www.mhlw.go.jp/file/06-Seisakujouhou-10900000-Kenkoukyoku/0000047221.pdf. Accessed 15 Aug 2020.
Kyozuka H, Fujimori K, Hosoya M, Yasumura S, Yokoyama T, Sato A, et al. The effect of maternal age at the first childbirth on gestational age and birth weight: the Japan environment and Children’s study (JECS). J Epidemiol. 2019;29:187–91.
Minakami H, Maeda T, Fujii T, Hamada H, Iitsuka Y, Itakura A, et al. Guidelines for obstetrical practice in Japan: Japan Society of Obstetrics and Gynecology (JSOG) and Japan Association of Obstetricians and Gynecologists (JAOG) 2014 edition. J Obstet Gynaecol Res. 2014;40:1469–99.
Ministry of Health, Labour and Welfare, Japan. Guidelines for health promotion in pregnant women (in Japanese). Available at: https://www.mhlw.go.jp/houdou/2006/02/h0201-3a.html. Accessed 15 Aug 2020.
Yokoyama Y, Takachi R, Ishihara J, Ishii Y, Sasazuki S, Sawada N, et al. Validity of short and long self-administered food frequency questionnaires in ranking dietary intake in middle-aged and elderly Japanese in the Japan public health center-based prospective study for the next generation (JPHC-NEXT) protocol area. J Epidemiol. 2016;26:420–32.
Gay CL, Richoux SE, Beebe KR, Lee KA. Sleep disruption and duration in late pregnancy is associated with excess gestational weight gain among overweight and obese women. Birth. 2017;44:173–80.
Balieiro LCT, Gontijo CA, Fahmy WM, Maia YCP, Crispim CA. Does sleep influence weight gain during pregnancy? A prospective study. Sleep Sci. 2019;12:156–64.
Jike M, Itani O, Watanabe N, Buysse DJ, Kaneita Y. Long sleep duration and health outcomes: A systematic review, meta-analysis and meta-regression. Sleep Med Rev. 2018;39:25–36.
Tan X, Chapman CD, Cedernaes J, Benedict C. Association between long sleep duration and increased risk of obesity and type 2 diabetes: A review of possible mechanisms. Sleep Med Rev. 2018;40:127–34.
Neggers Y, Goldenberg RL. Some thoughts on body mass index, micronutrient intakes and pregnancy outcome. J Nutr. 2003;133:1737S–40S.
Rosso P, Salas SP. Mechanism of fetal growth retardation in the underweight mother. In: Allen L, King J, Lonnerdal B, editors. Nutrient regulation during pregnancy, lactation, and infant growth. New York: Plenum Press; 1994. p. 1–9.
Bogaerts A, Van den Bergh BR, Ameye L, Witters I, Martens E, Timmerman D, et al. Interpregnancy weight change and risk for adverse perinatal outcome. Obstet Gynecol. 2013;122:999–1009.
Villamor E, Cnattingius S. Interpregnancy weight change and risk of adverse pregnancy outcomes: A population-based study. Lancet. 2006;368:1164–70.
Romero R, Espinoza J, Gonçalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med. 2006;11:317–26.
Bartha JL, Romero-Carmona R, Comino-Delgado R. Inflammatory cytokines in intrauterine growth retardation. Acta Obstet Gynecol Scand. 2003;82:1099–102.
Freeman DJ, McManus F, Brown EA, Cherry L, Norrie J, Ramsay JE, et al. Short- and long-term changes in plasma inflammatory markers associated with preeclampsia. Hypertension. 2004;44:708–14.
Chang JJ, Pien GW, Duntley SP, Macones GA. Sleep deprivation during pregnancy and maternal and fetal outcomes: is there a relationship? Sleep Med Rev. 2010;14:107–14.
Fluhr H, Krenzer S, Stein GM, Stork B, Deperschmidt M, Wallwiener D, et al. Interferon-gamma and tumor necrosis factor-alpha sensitize primarily resistant human endometrial stromal cells to Fas-mediated apoptosis. J Cell Sci. 2007;120:4126–33.
Salamonsen LA, Hannan NJ, Dimitriadis E. Cytokines and chemokines during human embryo implantation: roles in implantation and early placentation. Semin Reprod Med. 2007;25:437–44.
Arias F, Rodriquez L, Rayne SC, Kraus FT. Maternal placental vasculopathy and infection: two distinct subgroups among patients with preterm labor and preterm ruptured membranes. Am J Obstet Gynecol. 1993;168:585–91.
Ishibashi M, Kyozuka H, Yamaguchi A, Fujimori K, Hosoya M, Yasumura S, et al. Effect of proinflammatory diet before pregnancy on gestational age and birthweight: the Japan environment and Children's study. Matern Child Nutr. 2020;16:e12899.
Stigger FS, Zago Marcolino MA, Portela KM, Plentz RDM. Effects of exercise on inflammatory, oxidative, and neurotrophic biomarkers on cognitively impaired individuals diagnosed with dementia or mild cognitive impairment: A systematic review and meta-analysis. J Gerontol A Biol Sci Med Sci. 2019;74:616–24.
Lopez R, Barateau L, Evangelista E, Dauvilliers Y. Depression and hypersomnia: A complex association. Sleep Med Clin. 2017;12:395–405.
Grigolon RB, Trevizol AP, Cerqueira RO, Lee Y, Mansur RB, McIntyre RS, et al. Hypersomnia and bipolar disorder: A systematic review and meta-analysis of proportion. J Affect Disord. 2019;246:659–66.
Grote NK, Bridge JA, Gavin AR, Melville JL, Iyengar S, Katon WJ. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67:1012–24.
Li X, Gao R, Dai X, Liu H, Zhang J, Liu X, et al. The association between symptoms of depression during pregnancy and low birth weight: A prospective study. BMC Pregnancy Childbirth. 2020;20:147.
Wang LH, Lin HC, Lin CC, Chen YH, Lin HC. Increased risk of adverse pregnancy outcomes in women receiving zolpidem during pregnancy. Clin Pharmacol Ther. 2010;88:369–74.
Lauderdale DS, Knutson KL, Yan LL, Liu K, Rathouz PJ. Self-reported and measured sleep duration: how similar are they? Epidemiology. 2008;19:838–45.
Cespedes EM, Hu FB, Redline S, Rosner B, Alcantara C, Cai J, et al. Comparison of self-reported sleep duration with actigraphy: results from the Hispanic community health study/study of Latinos Sueño ancillary study. Am J Epidemiol. 2016;183:561–73.
Acknowledgements
The authors are grateful to all the participants of the study and to the Ministry of the Environment, Japan. Members of the JECS group as of 2020 are as follows: Michihiro Kamijima (principal investigator, Nagoya City University, Nagoya, Japan), Shin Yamazaki (National Institute for Environmental Studies, Tsukuba, Japan), Yukihiro Ohya (National Center for Child Health and Development, Tokyo, Japan), Reiko Kishi (Hokkaido University, Sapporo, Japan), Nobuo Yaegashi (Tohoku University, Sendai, Japan), Koichi Hashimoto (Fukushima Medical University, Fukushima, Japan), Chisato Mori (Chiba University, Chiba, Japan), Shuichi Ito (Yokohama City University, Yokohama, Japan), Zentaro Yamagata (University of Yamanashi, Chuo, Japan), Hidekuni Inadera (University of Toyama, Toyama, Japan), Takeo Nakayama (Kyoto University, Kyoto, Japan), Hiroyasu Iso (Osaka University, Suita, Japan), Masayuki Shima (Hyogo College of Medicine, Nishinomiya, Japan), Youichi Kurozawa (Tottori University, Yonago, Japan), Narufumi Suganuma (Kochi University, Nankoku, Japan), Koichi Kusuhara (University of Occupational and Environmental Health, Kitakyushu, Japan), and Takahiko Katoh (Kumamoto University, Kumamoto, Japan).
Funding
The Japan Environment and Children’s Study was funded by the Ministry of the Environment, Japan. The findings and conclusions of this article are solely the responsibility of the authors and do not represent the official views of the Ministry of the Environment, Japan.
Author information
Authors and Affiliations
Consortia
Contributions
T.M. conceptualized and designed the study. T.M. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. T.M., H.K., T.F., S.Y.1, A.Y., S.M., K.H., H.N., and K.F. contributed to the study design. T.M. wrote the manuscript. M.H., S.Y.2, K.H., K.S., A.S., Y.O., H.N., K.F., and the JECS group reviewed the manuscript and provided critical advice. All authors approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The JECS protocol was reviewed and approved by the Ministry of the Environment Institutional Review Board on Epidemiological Studies and by the Ethics Committees of all participating institutions (Supplementary file). The JECS was conducted in accordance with the Helsinki Declaration and other national regulations and guidelines. Written informed consent was obtained from all participants.
Consent for publication
Written informed consent was obtained from all participants.
Competing interests
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and had no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; and no other relationships or activities that could appear to have influenced the submitted work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Murata, T., Kyozuka, H., Fukuda, T. et al. Maternal sleep duration and neonatal birth weight: the Japan Environment and Children’s Study. BMC Pregnancy Childbirth 21, 295 (2021). https://doi.org/10.1186/s12884-021-03670-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12884-021-03670-3