Abstract
Background and Objective
Transcranial magnetic stimulation (TMS) is considered as a promising treatment option for post-stroke cognitive impairment (PSCI).Some meta-analyses have indicated that TMS can be effective in treating cognitive decline in stroke patients, but the quality of the studies included and the methodologies employed were less than satisfactory. Thus, this meta-analysis aimed to evaluate the efficacy and safety of TMS for treating post-stroke cognitive impairment.
Methods
We searched online databases like PubMed, Embase, Cochrane Library, and Web of Science to retrieve randomized controlled trials (RCTs) of TMS for the treatment of patients with PSCI. Two independent reviewers identified relevant literature, extracted purpose-specific data, and the Cochrane Risk of Bias Assessment Scale was utilized to assess the potential for bias in the literature included in this study. Stata 17.0 software was used for data analysis.
Results
A total of 10 studies involving 414 patients were included. The results of the meta-analysis showed that TMS was significantly superior to the control group for improving the overall cognitive function of stroke patients (SMD = 1.17, 95% CI [0.59, 1.75], I2 = 86.1%, P < 0.001). Subgroup analyses revealed that high-frequency rTMS (HF-rTMS), low-frequency rTMS (LF-rTMS), and intermittent theta burst stimulation (iTBS) all have a beneficial effect on the overall cognitive function of stroke patients. However, another subgroup analysis failed to demonstrate any significant advantage of TMS over the control group in terms of enhancing scores on the Loewenstein Occupational Therapy Cognitive Assessment (LOTCA) and Rivermead Behavioral Memory Test (RBMT) scales. Nonetheless, TMS demonstrated the potential to enhance the recovery of activities of daily living in stroke patients, as indicated by the Modified Barthel Index (MBI) (SMD = 0.76; 95% CI [0.22, 1.30], I2 = 52.6%, P = 0.121).
Conclusion
This meta-analysis presents evidence supporting the safety and efficacy of TMS as a non-invasive neural modulation tool for improving global cognitive abilities and activities of daily living in stroke patients. However, given the limited number of included studies, further validation of these findings is warranted through large-scale, multi-center, double-blind, high-quality randomized controlled trials.
PROSPERO registration number
CRD42022381034.
Similar content being viewed by others
Introduction
The prevalence of stroke is increasing due to a growing and aging population, making it is the second most common cause of acquired disability worldwide [1]. Post-stroke cognitive impairment (PSCI) is a frequent complication after stroke, and stroke events significantly increase the risk of developing dementia [2, 3], leading to an earlier onset of dementia by up to 10 years [4]. Cognitive decline is strongly associated with a lower quality of life following a stroke [5] and prolongs hospital stays [6]. The pathogenesis of post-stroke cognitive impairment involves various cellular changes such as disrupted redox state, mitochondrial dysfunction, blood-brain barrier disruption, microglia activation, and amyloid-β deposition in the brain parenchyma [7,8,9,10]. Cognitive impairments cannot be solely attributed to the specific locations of stroke but can be caused by damages to anatomically distributed brain networks supporting cognition [11]. Controlling vascular risk factors, drug treatments such as cholinesterase inhibitors and N-methyl-D-aspartate receptor antagonist may improve PSCI [12,13,14]. These drugs have shown effectiveness in enhancing cognitive functioning but are often accompanied by adverse reactions [15,16,17]. Currently, there is no approved pharmacological treatment specifically designed for post-stroke cognitive impairment or dementia [18]. Non-pharmacological therapies, such as lifestyle interventions, cognitive training, physical exercise, and acupuncture, are commonly utilized, but their effectiveness is not significant [19,20,21,22].
Transcranial magnetic stimulation (TMS), a non-invasive and relatively safe form of brain stimulation, has gained popularity for its ability to selectively induce electric currents in specific cortical regions of the brain through electromagnetic induction [23]. This technique, widely used in neurological and psychiatric rehabilitation, can modulate cortical excitability, either by exciting or inhibiting targeted brain regions [24, 25]. Repetitive transcranial magnetic stimulation (rTMS) and theta burst stimulation (TBS) are two primary types of TMS therapies [26]. High-frequency rTMS (HF-rTMS) is known to increase excitability in the target cortical regions, whereas low-frequency rTMS (LF-rTMS) induces the opposite effect [27]. Intermittent theta burst stimulation (iTBS) delivers short bursts of high-frequency pulses intermittently to enhance cortical excitability, while continuous theta burst stimulation (cTBS) applies continuous pulses at a lower frequency to inhibit cortical activity [28]. Both iTBS and cTBS are types of rTMS utilized for neuro-modulation in clinical settings.
rTMS has undergone extensive research in patients with Alzheimer’s disease (AD) and has emerged as an effective treatment for cognitive impairment associated with AD, offering safe and long-lasting effects [29, 30]. Studies have shown that rTMS can mitigate cognitive deficits in AD mice by inhibiting apoptosis through the activation of the cAMP/PKA/CREB signaling pathway [31]. The application of iTBS has demonstrated beneficial effects on depression, executive function, and target engagement of the cognitive control network in older adults [32]. iTBS, acknowledged as a time-saving and cost-effective repetitive transcranial magnetic stimulation regime, has shown promise in animal experiments for improving cognitive decline and alleviating AD-type pathology in APP/PS1 mice [33]. iTBS is regarded as a modified design of rTMS that can serve as a complementary approach to psychotherapy [34].
Previous studies have demonstrated the effectiveness of TMS in patients with post-stroke cognitive impairment [35, 36]. Some researchers have also reported that high-frequency rTMS may not have a discernible impact on cognition in post-stroke patients [37]. Recently, a systematic review and meta-analysis indicated that rTMS is an effective technique for treating post-stroke patients with cognitive impairment [38]. However, it is essential to acknowledge that certain studies included in the meta-analysis were published quite some time ago, potentially compromising the quality of the literature. Furthermore, the meta-analysis relied on comparing final values, which is less efficient and robust than utilizing change scores between baseline and post-intervention measurements [39]. Therefore, our aim is to conduct an updated meta-analysis to assess the effects of TMS on cognitive function in post-stroke patients.
Materials and methods
This meta-analysis was conducted according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [40]. This study was prospectively registered with the PROSPERO database of systematic reviews (CRD42022381034): https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022381034.
Literature search strategy
We searched four databases (Pubmed, Embase, Cochrane Library, and Web of Science) for published randomized controlled trials (RCTs) from database inception up to May 2024. The search strategy used the following terms: ((((((cerebral OR cerebellar OR intracerebral OR intracranial OR brain OR cerebrovascular) AND (bleed* OR haemorrhage* OR hemorrhage* OR infarction* OR occlusion* OR emboli* OR embolus OR thrombus OR thrombosis OR thrombi)) OR (stroke OR apoplexy OR post stroke OR post-stroke))) AND (cognitive OR cognition OR dementia OR processing OR attention OR language OR visuospatial OR memory OR executive function OR intelligence)) AND (transcranial magnetic stimulation OR TMS OR rTMS OR theta burst stimulation OR TBS OR iTBS OR cTBS)) AND (randomized controlled trial)).
Inclusion and exclusion criteria
We formulated the literature inclusion criteria by the principles of PICOS (Population, Intervention, Comparison, Outcomes and Study): (1) participants diagnosed with stroke, (2) cognitive impairment at least one domain in attention and executive, memory, language and visuospatial function caused by stroke, (3) including intervention group (rTMS or iTBS) and control group (Sham or no stimulation), (4) outcomes including cognitive function assessment, and complete clinical data was provided in the literature, (5) randomized controlled trial, (6) participants were adults (≥ 18 years), (7) articles published in English language, (8) studies in recent 10 years. The exclusion criteria were: (1) severe cognitive decline that impedes cooperation, (2) cognitive impairment or dementia before stroke, (3) data sets that were incomplete and unable to be analyzed, even after attempting to contact the authors via email, and in cases where there were articles from the same study, previous and incomplete data will be excluded.
Data extraction
Two reviewers (S.H. and W.C.) working independently examined and extracted data from each included study. The extracted information included (1) general characteristics: author, year of publication, study design, number of participants, mean age, stroke duration; (2) intervention: type of stimulation, location of stimulation, intensity, frequency, total pulses per treatment, sham stimulation method, intervention time and adverse effects; (3) statistical data of the score of cognitive performance. Any discrepancies in data obtained by the two reviewers were resolved by discussing with another professional researcher to reach a consensus.
Quality of studies and risk of bias assessment
The quality of included studies was assessed using the Cochrane Handbook for Systematic Reviews of Interventions [41]. The following characteristics were assessed: (1) random sequence generation; (2) allocation concealment; (3) blinding; (4) handling of incomplete outcome data; (5) evidence of selective outcome reporting; (6) other potential risks that could impact the validity of the study. The risk of bias for each criterion was categorized as low, high, or unclear.
Statistical analysis
Stata 17.0 software was used for Meta-analysis. Cochrane Rev-Man 5.4 software was used for quality assessment. Homogeneity test (Q test) and I2 value was used to test the heterogeneity of the included research. The effect of TMS on cognitive function in post-stroke patients was defined as the mean difference (MD) in the change of cognitive indicators relative to baseline (before stimulus treatment) in the experimental and control groups. Given the diversity of cognitive indicators applied in the included studies, standardized mean difference (SMD) and 95% confidence intervals were used to summarize eligible trial pooled effect sizes. SMD is often used in meta-analysis to compare mean differences between groups with outcome variables measured on different scales. Because two studies [42, 43] did not show a net change of cognitive scores between baseline and post intervention, the following formulas were used:
Mean changes = Mean post − Mean baseline;
If this correlation coefficient is unknown, it may be estimated as 0.5. If there is a similar study that reports summary statistics for change from baseline, baseline and final values, a better estimate (Chap. 6.5.2.8, Cochrane Handbook) of the correlation coefficient is:
In some studies, the standard errors of the mean or standard deviations were not given but figures, which had to be recalculated. In the case of one included study [44], the approximate data were extracted from figures in this paper using the online version of the web-based WebPlotDigitizer (https://apps.automeris.io/wpd/index.zh_CN.html, Copyright 2010–2022 Ankit Rohatgi) software.
Heterogeneity was quantified using the I2 statistic, and I2 ≤ 50% was considered low heterogeneity, then the meta-analysis was conducted with fixed effects model. I2 > 50% indicated substantial heterogeneity, and the random effects model was adopted for meta-analysis. In addition, high statistical heterogeneity was analyzed by subgroup analysis.
Sensitivity analysis was also used to explore the source of heterogeneity, and funnel plot, Begg’s and Egger’s tests were performed to evaluate publication bias. Statistical significance was considered for p-values less than 0.05.
Result
Search results
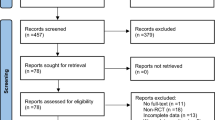
The initial search identified a total of 646 records, and 435 studies remained after excluding 211 duplicate records. Of these, 435 studies were excluded after reading titles and abstracts (including studies published more than 10 years ago). Two reviewers (S.H. and W.C.) independently read the full-text articles of the 21 studies, and 11 studies were excluded. Eventually, 10 randomized controlled trials were included in this meta-analysis [43,44,45,46,47,48,49,50,51,52]. Figure 1 shows a flowchart of screening and selection process.
Study characteristics
Ten studies were included in this meta-analysis, comprising a total of 414 participants. The characteristics of the included studies are presented in Table 1.All patients included in this review had a diagnosis of stroke, and their cognitive function was assessed. The experimental group in seven studies received rTMS treatment, and three studies received iTBS. One trial included 2 intervention arms and one control group (rTMS vs. iTBS vs. control) [50]. The TMS stimulation location in eight studies [43,44,45, 47,48,49,50, 52] was the left dorsal lateral prefrontal cortex (DLPFC) with high-frequency rTMS (HF-rTMS) stimulation (≥ 5 Hz) or iTBS, and the remaining two studies were right DLPFC and contralateral DLPFC with low-frequency rTMS (LF-rTMS) stimulation (1 Hz). The details of each study are provided in Table 1.
Study quality
Risk of bias in the included studies was evaluated using Cochrane’s risk of bias tool [53]. The results were as illustrated in Fig. 2.All studies in this review were RCTs. Four studies [48,49,50, 52] were double blinded and the other four were single blinded [43, 44, 46, 49]. Two studies did not mention if blinded [45, 47]. The control group in 6 studies included sham stimulation [46, 48,49,50,51,52]. Eight studies described random sequences generated using random number tables or computer programs [44, 46,47,48,49,50,51,52]. Two studies reported allocation procedures with concealment [50, 52]. Therefore, all included studies were considered to have a mild risk of bias (Fig. 3).
(A) Risk of bias summary: review authors’ judgments about each risk of bias item for each included study. (B) Risk of bias graph: review authors’ judgements about each risk of bias item presented as percentages across all included studies.
Effect of TMS on cognition in stroke patients
Global cognition was measured by the Mini-Mental Status Examination (MMSE), Montreal Cognitive Assessment (MoCA) scale in this study, Loewenstein Occupational Therapy Cognitive Assessment (LOTCA), Repeatable Battery for the Assessment of Neuropsychological Status (RBANS). MMSE and MoCA are both valid cognitive tools in stroke patients [54]. LOTCA is a relatively systematic assessment method in evaluating cognitive function in patients with stroke, and is slightly better than the MMSE [55]. The RBANS is a widely used brief test for detecting cognitive impairment in various neuropsychiatric conditions, which has also been applied to assess cognitive function in stroke patients [56].
A total of 10 RCTs (NTMS=216, Ncontrol=213) were included in the pooled meta-analysis to access the effects of TMS vs. sham/no stimulation on global cognition in stroke patients. The improvement in global cognition among stroke patients was significantly greater in TMS group compared to the control group (SMD = 1.17, 95% CI [0.59, 1.75], I2 = 86.1%, P < 0.001) (Fig. 3), and random effects model was used because of substantial heterogeneity.
Subgroup analysis of the effect of HF-rTMS, LF-rTMS and iTBS
Subgroup analyses were performed based on stimulation types (HF-rTMS, LF-rTMS, and iTBS). The results revealed that LF-rTMS had an SMD of 1.82(95%CI [0.34, 3.30], I2 = 88.8%, P = 0.003) and HF-rTMS had an SMD of 1.36(95%CI [0.27, 2.44], I2 = 88.8%, P < 0.001). The SMD between trials in iTBS group was 0.58 (95%CI [0.17, 0.99], I2 = 39.4%, P = 0.175). Subgroup analyses revealed that all forms of TMS yielded a positive effect on the global cognitive function of stroke patients (Fig. 4).
Subgroup analysis of LF-rTMS, HF-rTMS and iTBS on cognitive function in stroke patients. Abbreviations: SMD Standardized mean differences, CI confidence intervals, LF-rTMS low-frequency repetitive transcranial magnetic stimulation, HF-rTMS High-frequency repetitive transcranial magnetic stimulation, iTBS intermittent theta burst stimulation
Subgroup analysis of the effect of TMS on LOTCA, RBMT, MBI
We also performed subgroup analysis of selected outcomes (LOTCA, RBMT, and MBI) and compared the influence of TMS treatment on the recovery of executive capacity, memory, and activity of daily living in patients with stroke. LOTCA is a series of tests designed for occupational therapists, to assess a person’s cognitive processing ability and to determine whether a person is able to carry out everyday functional tasks [57]. The Rivermead Behavioural Memory Test (RBMT) was designed specifically to evaluate memory abilities for the performance of daily tasks [58]. The Modified Barthel Index (MBI) is a commonly used scale that measure disability or dependence in activities of daily living in stroke patients [59].Three studies reported LOTCA scores and two studies reported RBMT. The pooled results did not reveal that TMS was better than control group on the improvement of LOTCA and RBMT scores (SMD = 0.51; 95% CI [-0.08, 1.10], I2 = 64.6%, P = 0.037, and SMD = 0.35, 95% CI [-1.60, 2.31], I2 = 93.7%, P < 0.001) with random-effect model (Fig. 5). However, when it came to the changes of MBI scores, the difference between the two groups was statistically significant according to the analyses (SMD = 0.76; 95% CI [0.22, 1.30], I2 = 52.6%, P = 0.121), which indicated that TMS improved the MBI scores more efficiently (Fig. 6).
Publication bias and sensitivity analysis
Egger’s regression tests were conducted to evaluate the influence of small studies as publication bias. The P-value of Egger’s test do not support the existence of publication bias for all interventions (t = 1.18, P = 0.269). The shape of Begg’s funnel plot seems to be asymmetric (Pr >|z|=0.213) (Fig. 7). To identify the influence of individual studies on the overall meta-analysis, a sensitivity analysis was conducted. This involved systematically omitting each study to evaluate its impact on the collective results. The analysis revealed that no single study significantly affected the overall effect sizes (Fig. 8). Thus, our meta-analysis was relatively stable.
Adverse reaction
TMS is a noninvasive form of brain stimulation. Generally, TMS is considered safe and well-tolerated. In this systematic review, only three included studies reported adverse effects [43, 45, 46, 48]. Those patients in TMS groups felt discomfort because of the unique sounds that occur during stimulation and facial muscle contractions [45]. Three studies showed that several patients experienced transient headaches or dizziness in the TMS group, and patients in sham or control group complained light dizziness or headache [43, 46, 48]. However, these symptoms disappeared quickly without any specific interventions. These symptoms disappeared quickly without any specific interventions.
Discussion
This comprehensive meta-analysis involved an in-depth review of ten randomized controlled trials, showing the significant superiority of TMS over the control group in enhancing the overall cognitive function of stroke patients, while presenting minimal adverse reactions. These findings not only align with but also reinforce previous meta-analytic results in this field. Moreover, TMS demonstrated potential in improving the recovery of activities of daily living in stroke patients. However, subgroup analysis did not reveal a clear advantage of TMS over the control group in terms of enhancing scores on the LOTCA and RBMT scales, indicating the necessity for further research in this area.
Many stroke patients in the process of recovery often encounter various cognitive deficits, such as difficulties in attention, memory, executive functioning, and information processing [60]. The occurrence of cognitive impairment is closely related to the damage of specific brain regions such as the frontal lobe, anterior temporal lobe, cingulate gyrus, and hippocampus [61]. Cerebral ischemic injury-induced cognitive impairment involves numerous signaling pathways. Various transcription factors, intracellular adhesion molecules, and endogenous growth factors play a role in the pathogenesis of stroke-related cognitive impairment, offering potential therapeutic targets for treatment [62]. Preclinical mechanisms for cognitive function improvement after stroke include neuroplasticity, angiogenesis, inflammatory response modulation, and neurotrophic factor activity. These processes contribute to brain repair, synaptic rewiring, and functional recovery [63, 64]. TMS is a non-invasive technique that targets specific areas of the cerebral cortex. TMS studies have provided valuable insights into the pathophysiology of neurodegenerative disorders and stroke, further enhancing our understanding of post-stroke brain reorganization [65].
The results of our study indicated a significant improvement in global cognitive function with the use of TMS in stroke patients. The global cognitive assessment tools used in this study included MMSE, MoCA, and RBANS. Although MMSE and MOCA are two cognitive screening tools, many studies use these two scales to assess patient’s cognitive status before and after treatment for clinical trial or practice [66]. In this study, the intervention types of TMS included HF-rTMS, LF-rTMS, and iTBS. Subgroup analyses revealed that all three TMS treatments had a positive impact on global cognitive function in stroke patients. According to the theory of imbalanced interhemispheric interactions induced by stroke [67], HF-rTMS and iTBS protocols were considered “excitatory”, while LF-rTMS was considered ‘‘inhibitory” [68]. The dorsolateral prefrontal cortex (DLPFC) plays a critical role in cognitive control, and applying TMS to the DLPFC can enhance cognitive processing [69]. The left DLPFC has been linked to the regulation of stress-related cognitive processes and physiological responses [61, 70]. Among the selected studies, eight chose the left DLPFC as the stimulation site, while the remaining studies focused on the right or contralateral DLPFC. The excitatory stimulation of the left DLPFC and inhibitory stimulation of the right DLPFC may enhance cognitive function in stroke patients, with potential dependence on handedness. Research confirms that the dominant DLPFC hemisphere is typically located in the left hemisphere for the majority of right-handed individuals, while 16.7% of left-handed individuals also exhibit left-sided dominance in their DLPFC hemisphere [71]. Subgroup analysis indicated that there was no statistically significant difference between the two groups when using the LOTCA and RBMT scales. The LOTCA test involves multiple cognitive tasks and typically takes around 45 minutes to complete. LOTCA is considered to be a time-consuming and demanding tool, offering a more comprehensive assessment compared to other cognitive evaluations like the MMSE or MoCA [72]. In the subgroup analysis, it was not conclusively demonstrated that TMS intervention led to a superior improvement in patients’ LOTCA scores, thus further research is needed to confirm this. The RBMT, on the other hand, is specifically designed to detect impairment in everyday memory function, which includes various domains of memory function such as immediate memory, delayed memory, recognition memory, prospective memory, visual memory, verbal memory, spatial memory, and orientation [73]. Some researchers have found that TMS has a limited effect on working memory in patients with brain disorders [74]. Another study did not observe a significant effect of TMS on working memory in patients with Alzheimer’s disease [75]. In our study, we observed that the effect of TMS on memory improvement was not superior to that of the control group. TMS may primarily improve cognitive function in stroke patients by enhancing their executive function [52, 76]. Research has shown that TMS can enhance mental flexibility and task-switching abilities in the executive function of patients with mild cognitive impairment [77]. In terms of the daily living abilities of stroke patients, our study found that TMS can significantly improve their Barthel Index scores compared to the control group, indicating that TMS can enhance their ability to perform daily task. Although the MBI does not directly measure cognitive function, it does reflect a patient’s level of independence in performing these tasks, which can be influenced by cognitive impairment. TMS may potentially reduce the risk of depression in post-stroke patients, thereby further enhancing their daily life capabilities [78]. When it comes to adverse reactions, TMS therapy generally demonstrates good safety. The most common side effects were headache, fatigue, and pain/discomfort at the stimulation site [79], which are typically mild and easily manageable. A rare but serious adverse event of TMS treatment is seizure [80]. In this study, we observed that adverse reactions to TMS involved headache and dizziness, both of which promptly resolved without the need for specific interventions.
Limitation
Our meta-analysis applied strict inclusion and exclusion criteria. However, this study does have several limitations: (a) Variations in stimulation frequency and intervention duration existed among the included studies, and most of the studies had relatively small sample sizes. (b) The randomized controlled trials lacked standardization. Some studies did not include sham stimulation as negative controls, and there were instances where allocation concealment or blinding was not properly implemented. (c) Differences in participant age and variations in the severity of their illnesses may have influenced the rehabilitation outcomes. (d) The effectiveness of TMS administration in these studies may not be definitively confirmed due to the limited number of available studies. Given these limitations, it is important to note that the conclusions drawn from this meta-analysis may be affected.
Conclusion
Overall, this meta-analysis has shown that TMS is a safe and effective non-invasive neural modulation tool in the treatment of post-stroke cognitive impairment. TMS has shown significant improvements not only in global cognitive abilities but also in activities of daily living for stroke patients. However, it is worth noting that TMS has been linked to certain adverse effects, such as headaches or dizziness. Further research involving larger sample sizes and improved experimental design is still required to determine the optimal therapeutic protocol and validate the benefits of TMS in treating post-stroke cognitive impairment.
Data availability
All the data analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Abbreviations
- TMS:
-
Transcranial magnetic stimulation
- rTMS:
-
Repetitive transcranial magnetic stimulation
- iTBS:
-
Intermittent theta burst stimulation
- cTBS:
-
Continuous theta burst stimulation
- RCT:
-
Randomized controlled trial
- PSCI:
-
Post-stroke cognitive impairment
- AD:
-
Alzheimer’s disease
- MMSE:
-
Mini-mental state examination
- MoCA:
-
Montreal cognitive assessment
- LOTCA:
-
Loewenstein occupational therapy cognitive assessment
- MBI:
-
Modified Barthel index
- SMD:
-
Standardized mean differences
- CI:
-
Confidence intervals
- RBANS:
-
Repeatable battery for the assessment of neuropsychological status
- RBMT:
-
Rivermead behavioral memory test
- DLPFC:
-
Dorsolateral prefrontal cortex
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analyses
- NC:
-
Not clear
References
Fleury L, Koch PJ, Wessel MJ, Bonvin C, San Millan D, Constantin C, Vuadens P, Adolphsen J, Cadic Melchior A, Brügger J, et al. Toward individualized medicine in stroke-the TiMeS project: protocol of longitudinal, multi-modal, multi-domain study in stroke. Front Neurol. 2022;13:939640.
Delgado J, Masoli J, Hase Y, Akinyemi R, Ballard C, Kalaria RN, Allan LM. Trajectories of cognitive change following stroke: stepwise decline towards dementia in the elderly. Brain Commun. 2022;4(3):fcac129.
Goulay R, Mena Romo L, Hol EM, Dijkhuizen RM. From stroke to Dementia: a Comprehensive Review exposing tight interactions between stroke and Amyloid-β formation. Translational Stroke Res. 2020;11(4):601–14.
Mijajlović MD, Pavlović A, Brainin M, Heiss WD, Quinn TJ, Ihle-Hansen HB, Hermann DM, Assayag EB, Richard E, Thiel A, et al. Post-stroke dementia - a comprehensive review. BMC Med. 2017;15(1):11.
Cumming TB, Brodtmann A, Darby D, Bernhardt J. The importance of cognition to quality of life after stroke. J Psychosom Res. 2014;77(5):374–9.
Ten Brink AF, Hajos TR, van Bennekom C, Nachtegaal J, Meulenbelt HE, Fleuren JF, Kouwenhoven M, Luijkx MM, Wijffels MP, Post MW. Predictors of physical independence at discharge after stroke rehabilitation in a Dutch population. Int J Rehabilitation Res Int Z fur Rehabilitationsforschung Revue Int De recherches de Readaptation. 2017;40(1):37–45.
Jurcau A, Simion A. Oxidative stress in the pathogenesis of Alzheimer’s Disease and Cerebrovascular Disease with therapeutic implications. CNS Neurol Disord Drug Target. 2020;19(2):94–108.
Hosseini L, Karimipour M, Seyedaghamiri F, Abolhasanpour N, Sadigh-Eteghad S, Mahmoudi J, Farhoudi M. Intranasal administration of mitochondria alleviated cognitive impairments and mitochondrial dysfunction in the photothrombotic model of mPFC stroke in mice. J Stroke Cerebrovasc Diseases: Official J Natl Stroke Association. 2022;31(12):106801.
MacKenzie JL, Ivanova N, Nell HJ, Giordano CR, Terlecky SR, Agca C, Agca Y, Walton PA, Whitehead SN, Cechetto DF. Microglial inflammation and cognitive dysfunction in Comorbid Rat models of Striatal ischemic stroke and Alzheimer’s Disease: effects of antioxidant Catalase-SKL on behavioral and Cellular Pathology. Neuroscience. 2022;487:47–65.
Ouyang F, Jiang Z, Chen X, Chen Y, Wei J, Xing S, Zhang J, Fan Y, Zeng J. Is cerebral Amyloid-β deposition related to post-stroke cognitive impairment? Translational Stroke Res. 2021;12(6):946–57.
Kolskår KK, Ulrichsen KM, Richard G, Dørum ES, de Schotten MT, Rokicki J, Monereo-Sánchez J, Engvig A, Hansen HI, Nordvik JE, et al. Structural disconnectome mapping of cognitive function in poststroke patients. Brain Behav. 2022;12(8):e2707.
Wu H, Ren Z, Gan J, Lü Y, Niu J, Meng X, Cai P, Li Y, Gang B, You Y, et al. Blood pressure control and risk of post-stroke dementia among the elderly: a population-based screening study. Front Neurol. 2022;13:956734.
Battle CE, Abdul-Rahim AH, Shenkin SD, Hewitt J, Quinn TJ. Cholinesterase inhibitors for vascular dementia and other vascular cognitive impairments: a network meta-analysis. Cochrane Database Syst Rev. 2021;2(2):Cd013306.
Ma HM, Zafonte RD. Amantadine and memantine: a comprehensive review for acquired brain injury. Brain Injury. 2020;34(3):299–315.
Farooq MU, Min J, Goshgarian C, Gorelick PB. Pharmacotherapy for vascular cognitive impairment. CNS Drugs. 2017;31(9):759–76.
Shi X, Ren G, Cui Y, Xu Z. Comparative efficacy and acceptability of cholinesterase inhibitors and Memantine based on dosage in patients with vascular cognitive impairment: a Network Meta-analysis. Curr Alzheimer Res. 2022;19(2):133–45.
Quinn TJ, Richard E, Teuschl Y, Gattringer T, Hafdi M, O’Brien JT, Merriman N, Gillebert C, Huyglier H, Verdelho A, et al. European Stroke Organisation and European Academy of Neurology joint guidelines on post-stroke cognitive impairment. Eur Stroke J. 2021;6(3):I–xxxviii.
Rost NS, Brodtmann A, Pase MP, van Veluw SJ, Biffi A, Duering M, Hinman JD, Dichgans M. Post-stroke Cognitive Impairment and Dementia. Circul Res. 2022;130(8):1252–71.
Teuschl Y, Matz K, Brainin M. Prevention of post-stroke cognitive decline: a review focusing on lifestyle interventions. Eur J Neurol. 2013;20(1):35–49.
Shin M, Lee A, Cho AY, Son M, Kim YH. Effects of process-based cognitive training on memory in the healthy Elderly and patients with mild cognitive impairment: a Randomized Controlled Trial. Psychiatry Invest. 2020;17(8):751–61.
Bo W, Lei M, Tao S, Jie LT, Qian L, Lin FQ, Ping WX. Effects of combined intervention of physical exercise and cognitive training on cognitive function in stroke survivors with vascular cognitive impairment: a randomized controlled trial. Clin Rehabil. 2019;33(1):54–63.
Li L, Yang L, Luo B, Deng L, Zhong Y, Gan D, Wu X, Feng P, Zhu F. Acupuncture for Post-stroke Cognitive Impairment: an overview of systematic reviews. Int J Gen Med. 2022;15:7249–64.
Valero-Cabré A, Amengual JL, Stengel C, Pascual-Leone A, Coubard OA. Transcranial magnetic stimulation in basic and clinical neuroscience: a comprehensive review of fundamental principles and novel insights. Neurosci Biobehav Rev. 2017;83:381–404.
Ziemann U. Thirty years of transcranial magnetic stimulation: where do we stand? Exp Brain Res. 2017;235(4):973–84.
Gutierrez MI, Poblete-Naredo I, Mercado-Gutierrez JA, Toledo-Peral CL, Quinzaños-Fresnedo J, Yanez-Suarez O, Gutierrez-Martinez J. Devices and Technology in Transcranial magnetic stimulation: a systematic review. Brain Sci. 2022;12(9):1218.
Jannati A, Oberman LM, Rotenberg A, Pascual-Leone A. Assessing the mechanisms of brain plasticity by transcranial magnetic stimulation. Neuropsychopharmacology: Official Publication Am Coll Neuropsychopharmacol. 2023;48(1):191–208.
Conforto AB, Anjos SM, Saposnik G, Mello EA, Nagaya EM, Santos W Jr., Ferreiro KN, Melo ES, Reis FI, Scaff M, et al. Transcranial magnetic stimulation in mild to severe hemiparesis early after stroke: a proof of principle and novel approach to improve motor function. J Neurol. 2012;259(7):1399–405.
Moffa AH, Boonstra TW, Wang A, Martin D, Loo C, Nikolin S. Neuromodulatory effects of theta burst stimulation to the prefrontal cortex. Sci data. 2022;9(1):717.
Zhang T, Sui Y, Lu Q, Xu X, Zhu Y, Dai W, Shen Y, Wang T. Effects of rTMS treatment on global cognitive function in Alzheimer’s disease: a systematic review and meta-analysis. Front Aging Neurosci. 2022;14:984708.
Budak M, Bayraktaroglu Z, Hanoglu L. The effects of repetitive transcranial magnetic stimulation and aerobic exercise on cognition, balance and functional brain networks in patients with Alzheimer’s disease. Cogn Neurodyn. 2023;17(1):39–61.
Bao Z, Bao L, Han N, Hou Y, Feng F. rTMS alleviates AD-induced cognitive impairment by inhibitng apoptosis in SAMP8 mouse. Aging. 2021;13(24):26034–45.
Cristancho P, Arora J, Nishino T, Berger J, Carter A, Blumberger D, Miller P, Snyder A, Barch D, Lenze EJ. A pilot randomized sham controlled trial of bilateral iTBS for depression and executive function in older adults. Int J Geriatr Psychiatry. 2023;38(1):e5851.
Zhu Y, Huang H, Chen Z, Tao Y, Liao LY, Gao SH, Wang YJ, Gao CY. Intermittent Theta Burst Stimulation Attenuates Cognitive Deficits and Alzheimer’s Disease-Type Pathologies via ISCA1-Mediated Mitochondrial Modulation in APP/PS1 Mice. Neurosci Bull 2023:182–200.
Kujovic M, Benz D, Riesbeck M, Bahr C, Kriegs C, Reinermann D, Jänner M, Neufang S, Margittai Z, Kamp D, et al. Theta burst stimulation add on to dialectical behavioral therapy in borderline-personality-disorder: methods and design of a randomized, single-blind, placebo-controlled pilot trial. Eur Arch Psychiatry Clin NeuroSci. 2024;274(1):87–96.
Starosta M, Cichoń N, Saluk-Bijak J, Miller E. Benefits from Repetitive Transcranial Magnetic Stimulation in Post-stroke Rehabilitation. J Clin Med. 2022;11(8):2149.
Hernandez-Pavon JC, Harvey RL. Noninvasive Transcranial magnetic brain stimulation in stroke. Phys Med Rehabil Clin North Am. 2019;30(2):319–35.
Kim BR, Kim DY, Chun MH, Yi JH, Kwon JS. Effect of repetitive transcranial magnetic stimulation on cognition and mood in stroke patients: a double-blind, sham-controlled trial. Am J Phys Med Rehabil. 2010;89(5):362–8.
Li KP, Sun J, Wu CQ, An XF, Wu JJ, Zheng MX, Hua XY, Xu JG. Effects of repetitive transcranial magnetic stimulation on post-stroke patients with cognitive impairment: a systematic review and meta-analysis. Behav Brain Res. 2022;439:114229.
Fu R, Holmer HK. Change score or follow-up score? Choice of mean difference estimates could impact meta-analysis conclusions. J Clin Epidemiol. 2016;76:108–17.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clinical Res ed). 2021;372:n71.
Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, Thomas J. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:Ed000142.
Murad MH, Wang Z, Chu H, Lin L. When continuous outcomes are measured using different scales: guide for meta-analysis and interpretation. BMJ (Clinical Res ed). 2019;364:k4817.
Zhang Y, Chu M, Zheng Y, Zhang F, Yu H, Ye X, Xie H, Chen J, Qian Z, Zeng C, et al. Effects of Combined Use of Intermittent Theta Burst Stimulation and Cognitive Training on Poststroke Cognitive Impairment: a single-blind randomized controlled trial. Am J Phys Med Rehabil. 2024;103(4):318–24.
Chu M, Zhang Y, Chen J, Chen W, Hong Z, Zhang Y, Yu H, Zhang F, Ye X, Li J, et al. Efficacy of intermittent Theta-Burst Stimulation and Transcranial Direct current stimulation in treatment of Post-stroke Cognitive Impairment. J Integr Neurosci. 2022;21(5):130.
Park IS, Yoon JG. The effect of computer-assisted cognitive rehabilitation and repetitive transcranial magnetic stimulation on cognitive function for stroke patients. J Phys Ther Sci. 2015;27(3):773–6.
Lu H, Zhang T, Wen M, Sun L. Impact of repetitive transcranial magnetic stimulation on post-stroke dysmnesia and the role of BDNF Val66Met SNP. Med Sci Monitor: Int Med J Experimental Clin Res. 2015;21:761–8.
Yin M, Liu Y, Zhang L, Zheng H, Peng L, Ai Y, Luo J, Hu X. Effects of rTMS treatment on cognitive impairment and resting-state brain activity in Stroke patients: a Randomized Clinical Trial. Front Neural Circuits. 2020;14:563777.
Li Y, Luo H, Yu Q, Yin L, Li K, Li Y, Fu J. Cerebral functional manipulation of Repetitive Transcranial Magnetic Stimulation in cognitive impairment patients after stroke: an fMRI study. Front Neurol. 2020;11:977.
Liu Y, Yin M, Luo J, Huang L, Zhang S, Pan C, Hu X. Effects of transcranial magnetic stimulation on the performance of the activities of daily living and attention function after stroke: a randomized controlled trial. Clin Rehabil. 2020;34(12):1465–73.
Tsai PY, Lin WS, Tsai KT, Kuo CY, Lin PH. High-frequency versus theta burst transcranial magnetic stimulation for the treatment of poststroke cognitive impairment in humans. J Psychiatry Neuroscience: JPN. 2020;45(4):262–70.
Li H, Ma J, Zhang J, Shi WY, Mei HN, Xing Y. Repetitive Transcranial Magnetic Stimulation (rTMS) modulates thyroid hormones Level and Cognition in the Recovery Stage of Stroke patients with cognitive dysfunction. Med Sci Monitor: Int Med J Experimental Clin Res. 2021;27:e931914.
Li W, Wen Q, Xie YH, Hu AL, Wu Q, Wang YX. Improvement of poststroke cognitive impairment by intermittent theta bursts: a double-blind randomized controlled trial. Brain Behav. 2022;12(6):e2569.
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Clinical Res ed). 2011;343:d5928.
Cumming TB, Churilov L, Linden T, Bernhardt J. Montreal Cognitive Assessment and Mini-mental State examination are both valid cognitive tools in stroke. Acta Neurol Scand. 2013;128(2):122–9.
Wang SY, Gong ZK, Sen J, Han L, Zhang M, Chen W. The usefulness of the Loewenstein Occupational Therapy Cognition Assessment in evaluating cognitive function in patients with stroke. Eur Rev Med Pharmacol Sci. 2014;18(23):3665–72.
Larson E, Kirschner K, Bode R, Heinemann A, Goodman R. Construct and predictive validity of the repeatable battery for the assessment of neuropsychological status in the evaluation of stroke patients. J Clin Exp Neuropsychol. 2005;27(1):16–32.
Katz N, Itzkovich M, Averbuch S, Elazar B. Loewenstein Occupational Therapy Cognitive Assessment (LOTCA) battery for brain-injured patients: reliability and validity. Am J Occup Therapy: Official Publication Am Occup Therapy Association. 1989;43(3):184–92.
Man DW, Chung JC, Mak MK. Development and validation of the online rivermead behavioral memory test (OL-RBMT) for people with stroke. NeuroRehabilitation. 2009;24(3):231–6.
Ohura T, Hase K, Nakajima Y, Nakayama T. Validity and reliability of a performance evaluation tool based on the modified Barthel Index for stroke patients. BMC Med Res Methodol. 2017;17(1):131.
Cramer SC, Richards LG, Bernhardt J, Duncan P. Cognitive deficits after stroke. Stroke. 2023;54(1):5–9.
Xu T, Zhang S, Zhou F, Feng T. Stimulation of left dorsolateral prefrontal cortex enhances willingness for task completion by amplifying task outcome value. J Experimental Psychol Gen. 2022;1122:1133.
Kaur M, Sharma S. Molecular mechanisms of cognitive impairment associated with stroke. Metab Brain Dis. 2022;37(2):279–87.
Kalaria RN, Akinyemi R, Ihara M. Stroke injury, cognitive impairment and vascular dementia. Biochim Biophys Acta. 2016;1862(5):915–25.
Hannan J, Wilmskoetter J, Fridriksson J, Hillis AE, Bonilha L, Busby N. Brain health imaging markers, post-stroke aphasia and cognition: a scoping review. NeuroImage Clin. 2023;39:103480.
Agarwal S, Koch G, Hillis AE, Huynh W, Ward NS, Vucic S, Kiernan MC. Interrogating cortical function with transcranial magnetic stimulation: insights from neurodegenerative disease and stroke. J Neurol Neurosurg Psychiatry. 2019;90(1):47–57.
Cohen S, Cummings J, Knox S, Potashman M, Harrison J. Clinical trial endpoints and their clinical meaningfulness in early stages of Alzheimer’s Disease. J Prev Alzheimer’s Disease. 2022;9(3):507–22.
Volz LJ, Sarfeld AS, Diekhoff S, Rehme AK, Pool EM, Eickhoff SB, Fink GR, Grefkes C. Motor cortex excitability and connectivity in chronic stroke: a multimodal model of functional reorganization. Brain Struct Function. 2015;220(2):1093–107.
Lefaucheur JP, Aleman A, Baeken C, Benninger DH, Brunelin J, Di Lazzaro V, Filipović SR, Grefkes C, Hasan A, Hummel FC, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014–2018). Clin Neurophysiology: Official J Int Federation Clin Neurophysiol. 2020;131(2):474–528.
Webler RD, Fox J, McTeague LM, Burton PC, Dowdle L, Short EB, Borckardt JJ, Li X, George MS, Nahas Z. DLPFC stimulation alters working memory related activations and performance: an interleaved TMS-fMRI study. Brain Stimul. 2022;15(3):823–32.
Era V, Carnevali L, Thayer JF, Candidi M, Ottaviani C. Dissociating cognitive, behavioral and physiological stress-related responses through dorsolateral prefrontal cortex inhibition. Psychoneuroendocrinology. 2021;124:105070.
Surya JR, Habelhah B, Haroon J, Mahdavi K, Jordan K, Becerra S, Venkatraman V, Deveney C, Bystritsky A, Kuhn T, et al. Functional MRI lateralization [M1] of dlPFC and implications for Transcranial Magnetic Stimulation (TMS) targeting. Diagnostics (Basel Switzerland). 2023;13(16):2690.
Zwecker M, Levenkrohn S, Fleisig Y, Zeilig G, Ohry A, Adunsky A. Mini-mental state examination, cognitive FIM instrument, and the Loewenstein Occupational Therapy Cognitive Assessment: relation to functional outcome of stroke patients. Arch Phys Med Rehabil. 2002;83(3):342–5.
Steibel NM, Olchik MR, Yassuda MS, Finger G, Gomes I. Influence of age and education on the rivermead behavioral memory test (RBMT) among healthy elderly. Dement Neuropsychologia. 2016;10(1):26–30.
Begemann MJ, Brand BA, Ćurčić-Blake B, Aleman A, Sommer IE. Efficacy of non-invasive brain stimulation on cognitive functioning in brain disorders: a meta-analysis. Psychol Med. 2020;50(15):2465–86.
Wei Z, Fu J, Liang H, Liu M, Ye X, Zhong P. The therapeutic efficacy of transcranial magnetic stimulation in managing Alzheimer’s disease: a systemic review and meta-analysis. Front Aging Neurosci. 2022;14:980998.
Yu H, Liu S, Dai P, Wang Z, Liu C, Zhang H. Effects of Repetitive Transcranial Magnetic Stimulation on Gait and Postural Control Ability of Patients with executive dysfunction after stroke. Brain Sci. 2022;12(9):1185.
Sacco L, Ceroni M, Pacifico D, Zerboni G, Rossi S, Galati S, Caverzasio S, Kaelin-Lang A, Riccitelli GC. Transcranial Magnetic Stimulation Improves Executive Functioning through modulation of Social Cognitive networks in patients with mild cognitive impairment: preliminary results. Diagnostics (Basel Switzerland). 2023;13(3):415.
Hordacre B, Comacchio K, Williams L, Hillier S. Repetitive transcranial magnetic stimulation for post-stroke depression: a randomised trial with neurophysiological insight. J Neurol. 2021;268(4):1474–84.
Caulfield KA, Fleischmann HH, George MS, McTeague LM. A transdiagnostic review of safety, efficacy, and parameter space in accelerated transcranial magnetic stimulation. J Psychiatr Res. 2022;152:384–96.
Stultz DJ, Osburn S, Burns T, Pawlowska-Wajswol S, Walton R. Transcranial magnetic stimulation (TMS) safety with respect to seizures: a Literature Review. Neuropsychiatr Dis Treat. 2020;16:2989–3000.
Acknowledgements
Not applicable.
Funding
This work was funded by the Zhejiang Basic Public Welfare Research Project (LGF19H270004), Zhejiang Scientific Research Foundation of Traditional Chinese Medicine (2021ZB056), Zhejiang Provincial Medical and Health Science and Technology Program Project (2023RC139).
Author information
Authors and Affiliations
Contributions
M.J. and Y.Z. were responsible for the study design. M.J. analyzed the data and wrote the initial draft. W.C. and S.H. conducted literature searches and data extraction. G.P. provided assistance with writing and edited the paper. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhu, M., Huang, S., Chen, W. et al. The effect of transcranial magnetic stimulation on cognitive function in post-stroke patients: a systematic review and meta-analysis. BMC Neurol 24, 234 (2024). https://doi.org/10.1186/s12883-024-03726-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-024-03726-9