Abstract
Background
Myasthenia gravis (MG) is a rare autoimmune disease characterised by muscle weakness, and progression from ocular (oMG) to generalised (gMG) symptoms results in a substantial negative impact on quality of life (QoL). This systematic review aimed to provide an overview of the patient burden experienced by people living with gMG.
Methods
Electronic database searches (conducted March 2022), supplemented by interrogation of grey literature, were conducted to identify studies reporting patient burden outcomes in patients with gMG in Europe, the Middle East and Africa. Results were synthesised narratively due to the heterogeneity across trials.
Results
In total, 39 patient burden publications (representing 38 unique studies) were identified as relevant for inclusion in the systematic review, consisting of 37 publications reporting formal patient-reported outcome measures (PROMs), and two publications describing alternative qualitative assessments of patient experience. The studies included a variety of measures including generic and disease-specific PROMs, as well as symptom-specific PROMs focusing on key comorbidities including depression, anxiety, fatigue and sleep disturbance. The findings showed some variation across studies and PROMs; however, in general there was evidence for worse QoL in patients with gMG than in healthy controls or in patients with oMG, and a trend for worsening QoL with increasing MG severity.
Conclusions
This review highlights the importance of considering patient QoL when developing and assessing treatment and management plans for patients with gMG. However, the heterogeneity identified across studies illustrates the need for further representative and well-powered studies in large cohorts administering consistent, validated questionnaires.
Trial registration
The protocol for this systematic review was registered in PROSPERO: CRD42022328444.
Similar content being viewed by others
Background
Myasthenia gravis (MG) is a rare autoimmune neurological disorder, characterised by the presence of pathogenic antibodies that block and damage post-synaptic receptors in the neuromuscular junction, resulting in impairments in neuromuscular transmission and muscle contraction [12, 20, 21, 34]. As a result, patients develop muscle weakness, which can present as a broad range of symptoms including ocular ptosis, diplopia, dysphagia, dysarthria, limb weakness, and respiratory insufficiency [20, 22]. Recent studies in Europe estimate an MG incidence rate of 4–30 cases per million person-years, with prevalence rates ranging between 150–200 cases per million people [20]. MG affects all ages and racial groups, although women are more commonly affected by early-onset MG (< 50 years) than men, and paediatric MG is very rare [12, 20]. Current treatments for MG constitute supportive care, which focuses on improving and managing the symptoms of the disease. Available therapies include acetylcholinesterase inhibitors, immunosuppressive treatments, thymectomy, intravenous immunoglobulins, and plasmapheresis [21, 34, 35]. Monoclonal antibody treatments are increasingly becoming available for MG, including complement (C5) inhibitors (e.g. eculizumab, ravulizumab), neonatal Fc receptor (FcRn) inhibitors (e.g. efgartigimod, nipocalimab, rozanolixizumab), and B cell depleting agents (e.g. rituximab) [2, 34].
When MG patients are diagnosed they most commonly present with ocular symptoms (oMG), with up to 80% of patients going on to develop generalised MG (gMG); typically within two years of disease onset [20]. Patients with gMG experience a wider range of symptoms than patients with oMG and these can be highly unpredictable, potentially manifesting as recurrent exacerbations requiring intervention [20, 22]. In severe cases, patients experience myasthenic crises where mechanical ventilation is required and, in rare cases, may be fatal [12, 21]. The greater symptom burden and risk of exacerbations experienced by people with gMG compared with oMG suggest that this group have a reduced quality of life (QoL).
To our knowledge, there is no published systematic review that focuses specifically on MG patients experiencing generalised symptoms. The objective of this systematic literature review (SLR) was to identify and summarise evidence relating to patient burden in studies of gMG conducted in Europe, the Middle East and Africa.
Methods
A systematic literature search was performed to identify studies evaluating patient and economic burden in patients with generalised MG in Europe, the Middle East and Africa (EMEA). The study was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [39]. The protocol for the review was registered in International Prospective Register of Systematic Reviews (PROSPERO) on 3rd May 2022 (CRD42022328444).
Electronic searches of the following databases were conducted on 29th March 2022 via the OVID platform: Embase, Medline®, Medline® Daily, Medline® Epub Ahead of Print (In-Process & Other Non-Indexed Citations), Evidence-Based Medicine Reviews, and EconLit. The full search strategy is provided in the Supplementary information. Additional keyword searches were conducted of relevant congress proceedings from the past three years, rare disease and MG-specific advocacy group websites, the University of Sheffield ScHARRHUD utility database, and Google Scholar. The reference lists of eligible studies were also reviewed to identify any further relevant publications that were not already included.
Records were eligible for inclusion if they reported on real-world evidence conducted in patients with gMG. Studies reporting on a mixed MG population were excluded if results for gMG were not reported separately from oMG and the overall proportion of gMG patients in the population was < 80%. Full eligibility criteria are provided in Table 1. Two independent reviewers screened the title and abstract of citations against the pre-defined inclusion/exclusion criteria. This approach is aligned with published guidance [14, 42]. The full texts of citations included at this stage were then obtained to confirm whether the publications met the eligibility criteria. At both the title and abstract and the full publication review stages, any discrepancies between reviewers were resolved through discussion or the intervention of a strategic advisor. Data from eligible studies were summarised in a narrative synthesis.
Formal quality assessment using a validated checklist was not undertaken due to the anticipated heterogeneity in study design between relevant studies. However, key study characteristics that may impact the validity of the results (e.g. patient sample size, patient withdrawal and study perspective) were summarised to assist with establishing the robustness of the results reported in individual studies.
Results
The process of study selection is documented in the PRISMA flow diagram (Fig. 1). The electronic database search identified a total of 7,720 articles. After the removal of 2,026 duplicates, 5,694 articles were screened by title and abstract. In total, 5,558 articles were excluded. The remaining 136 articles were deemed potentially relevant and subsequently screened based on the full publication. Hand searching of conference proceedings, additional sources, and reference lists of included studies yielded five additional relevant publications. Upon review of the full publications, a further 100 articles were excluded. This resulted in a total of 41 publications that met the inclusion criteria for the SLR. A list of the included studies and a summary of their key characteristics is provided in Table 2.
PRISMA diagram. A Including studies tagged on the basis of country and systematic reviews. B To ensure the most relevant data was being considered for inclusion, a post-hoc amendment to the protocol was included to exclude studies during title/abstract screening that did not indicate relevant outcome data
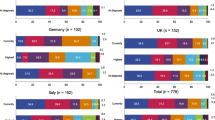
Of the 41 included publications, 39 (representing 38 unique studies) reported on patient burden, consisting of formal patient-reported outcome measures (PROMs) (n = 37) [1, 3,4,5,6,7,8,9,10,11, 13, 15,16,17,18, 23, 24, 26, 28, 29, 31, 33, 38, 40, 41, 43,44,45,46,47,48,49,50,51,52, 54, 55] (Fig. 2), or alternative assessments of patient experience (n = 2) [32, 36]. The remaining two publications reported only on outcomes related to economic burden and are not the focus of this article [30, 37]. The majority of the patient burden studies were conducted in Europe (n = 32) [1, 4,5,6,7, 11, 13, 15,16,17,18, 23, 24, 26, 28, 29, 31,32,33, 36, 38, 40, 41, 43,44,45,46,47,48, 51, 52, 54, 55] with five studies conducted in Middle Eastern countries [3, 8, 10, 49, 50], and one study conducted in South Africa [9].
Overall, 12 patient burden studies recruited entirely gMG populations [4, 5, 7, 11, 16, 28, 29, 32, 40, 43,44,45, 52], and 15 studies recruited a mixed MG population but reported subgroup data for gMG [1, 3, 6, 10, 15, 17, 18, 24, 33, 41, 46, 47, 50, 51, 55]. In the remaining 11 studies a mixed MG population was reported with no gMG subgroup data [8, 9, 13, 23, 26, 31, 36, 38, 48, 49, 54]. However, the proportion of gMG patients in the population exceeded the pre-specified proportion of 80% in all 11 studies and data were therefore extracted for this combined population and considered equivalent to gMG. Only one study reported both gMG subgroup data and outcomes for an overall population that was > 80% gMG [6]. In this case data were extracted for the gMG subgroup where available, with the remaining outcomes extracted based on the full study population. The total sample size of the included studies (including non-gMG patients) ranged from 6–1,660 patients [33, 40], with approximately half of the studies including less than 50 patients with gMG [1, 3, 6, 8, 9, 11, 15,16,17, 23, 28, 29, 31, 38, 40, 41, 44, 47, 48, 52, 55]. An overview of trends identified in the extracted data is presented in Table 3, and summarised descriptively in the subsequent sections.
Generic PROMs
In total, 20 publications (representing 19 unique studies) reported the results of non-symptom-specific generic QoL measures in patients with gMG (Fig. 2). The most common measures were the 36-Item Short Form Survey (SF-36) (n = 9) [4, 10, 16, 31, 38, 41, 49,50,51], EuroQoL Five Dimension Questionnaire 3 Levels (EQ-5D-3L) or 5 Levels (EQ-5D-5L) (n = 3) [5, 18, 40], and European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ) (n = 2) [7, 13]. Other PROMs that were not symptom- or MG-specific included measures related to social support [31, 33, 49]; acceptance of illness [31, 49]; post-traumatic growth [31]; patient mood [9]; and general QoL [23, 28, 29, 44].
Impact of gMG on QoL
Three of the studies investigating non-symptom-specific generic PROMs reported significantly lower scores in patients with gMG compared with healthy controls [9, 16, 28, 29]. SF-36 scores were found to be significantly lower in gMG patients compared with healthy reference values for physical functioning, role limitation due to physical problems, and general health perceptions [16]. A study assessing patients using the Profile of Mood States (POMS) found that gMG patients had significantly higher scores for tension, anger, fatigue and confusion [9]. Another study found that patients’ perceived level of physical and cognitive performance on a 10-point visual analogue scale (VAS) was significantly lower in gMG patients versus controls [28, 29]. In contrast to these findings, one study reported that the difference in quality of life index (QLI) score between gMG patients and healthy controls was narrowly non-significant [23].
Severity of gMG
A number of studies assessed the relationship between general QoL measures and MG classification, as assessed by the Osserman and Myasthenia Gravis Foundation of America (MGFA) classification systems, which range from stage I (oMG) to stage IV/V, respectively [27, 53]. The majority of these studies did not provide statistical analysis of the impact of increasing severity within gMG (stage II +); however, there was typically a trend for worsening QoL between stage II and higher stages [7, 10, 16, 18, 41, 51]. One study reported significantly lower EORTC QLQ scores for social, cognitive, emotional and vegetative scales for patients in stage III/IV versus stage II [7], while a second study reported a significantly lower SF-36 physical functioning score for patients in MGFA stage III or more compared with stage II [51]. A final study found that SF-36 scores differed significantly between patients with gMG, oMG and bulbar MG (bMG), which impacts the jaw and throat muscles [50]. QoL scores were highest in patients with oMG and lowest in patients with bMG [50].
Treatment
Six of the included studies evaluated non-symptom-specific general QoL in patients after receiving specific treatments for gMG, including thymectomy (n = 5) [4, 7, 13, 38, 44] and rituximab (n = 1) [40]. The rituximab study found a positive tendency towards an improvement in EQ-5D-3L overall score and VAS following treatment [40]. Findings related to the impact of thymectomy on QoL were inconclusive, with one study reporting a significant improvement in SF-36 over time following thymectomy up to a maximum of 10 years [4], while a second study reported no difference in SF-36 between patients with and without thymectomies [38]. One study found no significant differences in EORTC QLQ scores between gMG patients undergoing open thymectomy versus minimally invasive thoracoscopic thymectomy [7]. A further study reported improvements in EORTC QLQ score following thymectomy with a mean follow-up of over 7 years [13]. The final study reported patient’s subjective evaluation of their QoL following thymectomy, finding that the majority of patients considered themselves to be in good or very good condition after an average of 13 years of follow-up [44].
Other factors affecting QoL
One study evaluated differences in gMG patients’ quality of life based on their Patient Acceptable Symptom State (PASS), a single-item assessment in which patients indicate whether they are satisfied with their current symptom state (PASS-positive) or dissatisfied with their current symptom state (PASS-negative) [5]. PASS-negative gMG patients had significantly lower EQ-5D-3L and EQ-5D-VAS scores than PASS-positive gMG patients [5].
Three studies reported on PROMs assessing social support, including the Multidimensional Scale of Perceived Social Support (MSPSS) (n = 2) [31, 49], and the ENRICHD Social Support Inventory (ESSI) (n = 1) [33]. One study found that MSPSS score correlated with total SF-36 score, and that MSPSS was higher in MG patients with autoantibodies against muscle-specific tyrosine kinase (MuSK + MG) than in MG patients with autoantibodies to acetylcholine receptor (AChR + MG) [49]. This may be due to the more severe symptoms associated with MuSK + MG versus AChR + MG, resulting in greater support from friends or family members [49]. Overall, SF-36 score was better in MuSK + MG patients, particularly in mental domains, despite these patients tending to have a more severe form of the disease [49]. The second MSPSS study assessed QoL in patients with gMG with and without a psychiatric diagnosis, finding that MSPSS scores were significantly higher in patients without a psychiatric diagnosis, and that MSPSS score correlated with SF-36 general health score; with patients with a psychiatric diagnosis having worse SF-36 scores than patients without a psychiatric diagnosis [31].
Two studies reported PROMs related to patients’ acceptance of or adjustment to living with gMG: the Psychosocial Adjustment to Illness Scale – Self Report (PAIS-SR) [31], and the Acceptance of Illness Scale (AIS) [49]. The AIS study found that patients’ scores did not differ between MuSK + MG and AChR + MG [49]. The second study found that PAIS-SR score was significantly lower in gMG patients without a psychiatric diagnosis than those with a psychiatric diagnosis, and that PAIS-SR score correlated with Hospital Anxiety and Depression Scale (HADS) scores [31]. In the same study, patients were also asked to complete the Post-Traumatic Growth Inventory (PTGI) assessing whether they had experienced positive changes after trauma; no significant difference in PTGI scores was identified between the two groups [31].
Symptom-specific PROMS
In total, 18 publications (representing 17 unique studies) reported on symptom-specific PROMs (Fig. 2), including measures of depression (n = 14) [1, 3, 5, 6, 23, 24, 28, 29, 31, 33, 48,49,50, 52], anxiety (n = 9) [6, 9, 23, 24, 31, 33, 49, 50, 52], fatigue (n = 9) [1, 5, 24, 28, 29, 33, 46, 47, 52], and sleep disturbance (n = 7) [1, 17, 23, 24, 28, 29, 52].
Depression
The most frequently reported depression-related PROMs were the Hamilton Depression Rating Scale (HAM-D) (n = 4), [6, 49, 50, 52], Beck's Depression Inventory (BDI) (n = 3) [1, 6, 48], and HADS depression subscale (n = 3) [24, 31, 33], with the remaining measures reported in one study each: the Center for Epidemiologic Studies Depression Scale (CES-D) [28, 29], Major Depression Inventory (MDI) [5], Patient Health Questionnaire (PHQ)-9 [3], and the Zung Self-Rating Depression Scale (SDS) [23]. Four studies reported significantly higher scores for gMG patients compared with healthy controls on the HAM-D [52], BDI [48], CES-D, and SDS [23] scales. Findings on MG severity were mixed, one study found that HAM-D score increased with more severe Osserman stage, while there was no significant difference in BDI across stages [6]. A further study found that HAM-D score varied significantly between oMG, gMG and bMG, with oMG patients having the worst scores [50]. In contrast, three studies reported no significant differences between gMG and oMG on the HADS-D [24], BDI [1] and PHQ-9 [3] scales. Other findings included a significantly higher HADS-D score in gMG patients with psychiatric disorders versus gMG patients without such disorders [31], and similar HAM-D scores in patients with MuSK + MG and AChR + MG [49].
Anxiety
The most frequently reported anxiety-related PROMs were the Hamilton Anxiety Rating Scale (HAM-A) (n = 4) [6, 49, 50, 52] and HADS-A (n = 3) [24, 31, 33], with the remaining measures reported in one study each: Beck Anxiety Inventory (BAI) [6], Institute for Personality & Ability Testing (IPAT) Anxiety Scale [9], and the Zung Self-Rating Anxiety Scale (SAS) [23]. Three studies found significantly higher anxiety scores in patients with gMG versus healthy controls, using the IPAT B-score [9], SAS [23], and HAM-A [52] scales. Two studies reported higher HAM-A scores at higher MGFA [50] and Osserman stages [6], and one study found that HAM-A was higher in patients with gMG versus oMG [50]. In contrast, a further study found no significant difference in HADS-A score between patients with gMG and oMG [24]. Other findings included a significantly higher HADS-A score in gMG patients with psychiatric disorders versus gMG patients without such disorders [31], and similar HAM-A scores in patients with MuSK + MG and AChR + MG [49].
Fatigue
The measures used to assess fatigue were highly variable. The Chalder Fatigue Questionnaire (CFQ) [24, 33] and Fatigue Severity Scale (FSS) [47, 52] were each reported in two studies, with the remaining measures reported in one study each: Checklist Individual Strength fatigue (CIS-f) [46], Fatigue Assessment Scale (FAS) [1], Fatigue Impact Scale (FIS) [1], Fatigue Scale for Motor and Cognitive Functions (FSMC) [28, 29], Multidimensional Fatigue Inventory (MFI)-20 [5]. Two studies assessed fatigue in patients with gMG versus healthy controls, finding that fatigue scores were significantly higher for gMG patients on the FSS [52], and FSMC scales (23,24). Two further studies found a significant difference between gMG and oMg patients, with gMG patients having higher scores on the CFQ [24] and FIS scales [1]. Other findings included significantly higher CIS-f scores in women with gMG than in men with gMG [46], and significantly higher MFI-20 scores in PASS-negative gMG patients than in PASS-positive gMG patients [5].
Sleep disturbance
The most frequently reported sleep-related PROMs were the Epworth Sleepiness Scale (ESS) [1, 17, 23, 52] and Pittsburgh Sleep Quality Index (PSQI) [17, 23, 28, 29, 52], reported in four studies each, with the remaining measures reported in one study each: Insomnia Severity Index (ISI) [24], Self-Rating Questionnaire for Sleep and Awakening Quality (SSA) [23]. Sleep-related findings in gMG patients were mixed. Two studies found no difference in ESS total score between gMG patients and healthy controls [23, 52]; whereas, PSQI score was higher in gMG patients than controls in three studies [23, 28, 29, 52], as was SSA score in one study [23]. A further study reported that ISI score was higher in patients with gMG versus oMG [24], with a final study reporting a significant relationship between QoL and subjective sleep duration, as well as finding that sleep disorders were more prevalent in the gMG population than in healthy controls [52].
Disease-specific PROMs
In total, 16 publications (representing 15 unique studies) reported disease-specific PROMs in patients with gMG (Fig. 2). The most common were Myasthenia Gravis Quality of Life (MG QoL)-15 (n = 10 studies [5, 11, 17, 28, 29, 33, 40, 50, 52, 54, 55], one of which employed the revised version, MG QoL-15r [50]), and Myasthenia Gravis Activities of Daily Living (MG-ADL) (n = 9) [5, 8, 11, 26, 28, 29, 33, 43, 45, 54]. Two other measures were reported in one study each: the Italian Myasthenia Gravis Questionnaire (IMGQ) [15] and the Myasthenia Gravis Fatigue Scale (MGFS) [15]. One study assessed MGFS, MG-ADL and MG-QoL 15 in patients with gMG versus healthy controls, finding significantly worse values in gMG patients [28, 29]. Two studies reported significantly lower scores in patients with gMG compared with oMG on the MG QoL-15 [55] and MG QoL-15r [50], whereas one study reported a numerically lower MG QoL-15 score in patients with gMG vs oMG or patients in remission [17]. A final study reported an increase in MG QoL-15r score with increasing MGFA stage [50].
Five studies reported on disease-specific QoL in patients before and after receiving various treatments for MG, including thymectomy (n = 3) [8, 26, 45], rituximab (n = 1) [40], methotrexate (n = 1) [43], and standard care (n = 1) [54]. Four of these studies reported an improvement in disease-specific QoL following treatment [8, 40, 43, 54], one study found no difference in MG-ADL score across different thymectomy approaches [45], and the remaining study assessed MG-ADL in patients who had undergone thymectomy but the researchers were unable to evaluate the change in QoL as the data were incomplete [26]. Other findings included a significantly higher MG-ADL score in PASS-negative compared with PASS-positive patients [5].
Patient experience
Two publications did not use formal tools to assess quality of life, instead conducting qualitative evaluations of patient experience [32, 36]. One publication focused on family planning decision-making in women with gMG, finding that gMG influenced family planning in the majority of patients [36]. The second publication reported summary statements describing the lived experience of patients with gMG, which were generated in an analysis led by a panel of patient advocates and informed by patient insights [32]. Five key themes were identified encompassing fluctuating and unpredictable symptoms; trade-offs in all aspects of life; treatment inertia; disconnection from healthcare professionals; and feelings of anxiety, frustration, guilt, anger, loneliness and depression [32].
Discussion
The objective of this systematic review was to identify and summarise the existing body of evidence for patient burden in MG, with a specific focus on patients experiencing generalised symptoms (gMG) in Europe, the Middle East and Africa. A total of 38 unique studies were identified as relevant for inclusion, encompassing 36 studies reporting the results of general, symptom-specific or disease-specific PROMs. Many of the included studies reported a substantial impact of gMG on patient QoL, with this impact increasing with increasing MG severity. This finding is in line with a recent paper showing that MGFA grade is a strong predictor of all aspects of health-related quality of life (HRQoL) in MG patients [19]. A systematic review of the humanistic burden of MG (Gelinas 2022) also drew similar conclusions, finding that patients with MG experience worse HRQoL than the general population [22]. The Gelinas 2022 SLR covered a broader data set than the present review; not being limited in geography, study design, and MG subtype. Our review also includes more recent data and draws attention to some key data gaps regarding patient burden in gMG. Furthermore, the majority of the studies identified in the review were conducted in Europe, illustrating that further studies from a broader range of countries are required to provide greater insight into the patient experience of gMG in the EMEA region.
Our review was conducted according to robust methodology, and a comprehensive data-set was obtained; however, there was substantial variation in sample sizes, patient populations and study design across the included studies. A total of 40 different tools were used across 38 studies, with a high level of heterogeneity in the comparisons analysed (Fig. 2). Some PROMs were only reported in a limited number of studies, and the differences in the tools limit our ability to summarise and compare across studies. There is therefore a need for further representative and well-powered studies in large cohorts administering consistent, validated questionnaires.
Our review also included searches for data relating to the economic burden of patients with gMG. Substantial data gaps were identified, with measures of economic burden of gMG primarily limited to impact of MG on work capability and healthcare resource use outcomes, such as hospitalisations or length of hospital stay [4, 5, 7, 8, 26, 30, 31, 36, 37, 43, 44]. Direct economic data were limited to a single cost-utility analysis, which reported reduced overall healthcare costs in six patients with gMG treated with rituximab [40]. The lack of available comparative data and the heterogeneity of the reported outcomes make it difficult to draw any conclusions regarding the economic burden of gMG, and these data are therefore not presented in this article.
In contrast to this uncertainty, the patient-led analysis of MG patient burden identified in the review clearly describes the impact of MG on QoL and emphasised the need for greater understanding of the reality of living with MG [32]. A limitation of this study is the geographical restriction to the EMEA region, which may reduce the generalisability of the findings. However, a recent study of gMG patient experience in the US found that patients report similar difficulties, including unpredictable symptoms that impact many aspects of life including social functioning, work capacity and finances [25]. Only two studies identified in this review assessed qualitative aspects of gMG patient burden [32, 36]. This limited focus on qualitative assessments of patient burden versus formal PROMs points to the need for further analyses in this particular area to better reflect patient’s lived experience of MG.
Conclusions
Despite the limitations of the published literature, the patient burden of gMG remains clear, with this review identifying a range of studies that report a substantial impact of gMG on patient QoL. Key findings from the analysis of patient lived experience included concerns around treatment-inertia and undertreatment of MG, as well as a disconnect between patients and healthcare professionals in both the perception of disease burden and treatment goals [32]. This review therefore emphasises the importance of considering patient QoL when developing treatment and management plans for patients with gMG, thus ensuring that optimal support is provided to these patients.
Availability of data and materials
All MG-related data extraction tables generated during this study are included in this published article or its Supplementary information files.
Abbreviations
- AIS:
-
Acceptance of Illness Scale
- AChR:
-
Acetylcholine receptor
- BAI:
-
Beck Anxiety Inventory
- BDI:
-
Beck's Depression Inventory
- bMG:
-
Bulbar myasthenia gravis
- CES-D:
-
Center for Epidemiologic Studies Depression Scale
- CFQ:
-
Chalder Fatigue Questionnaire
- CIS-f:
-
Checklist Individual Strength fatigue
- EBMR:
-
Evidence-Based Medicine Reviews
- EMEA:
-
Europe, the Middle East and Africa
- ENRICHD:
-
Enhancing Recovery in Coronary Heart Disease
- EORTC QLQ:
-
European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire
- EQ-5D:
-
EuroQoL Five Dimension Questionnaire
- EQ-5D-3L:
-
EuroQoL Five Dimension Questionnaire 3 Levels
- EQ-5D-5L:
-
EuroQoL Five Dimension Questionnaire 5 Levels
- ESS:
-
Epworth Sleepiness Scale
- ESSI:
-
ENRICHD Social Support Inventory
- FACIT:
-
Functional Assessment of Chronic Illness Therapy
- FAS:
-
Fatigue Assessment Scale
- FcRn:
-
Neonatal Fc receptor
- FIS:
-
Fatigue Impact Scale
- FSMC:
-
Fatigue Scale for Motor and Cognitive Functions
- gMG:
-
Generalised myasthenia gravis
- HADS-A:
-
Hospital Anxiety and Depression Scale Anxiety
- HADS-D:
-
Hospital Anxiety and Depression Scale Depression
- HAM-A:
-
Hamilton Anxiety Rating Scale
- HAM-D:
-
Hamilton Depression Rating Scale
- HRQoL:
-
Health-related quality of life
- ICU:
-
Intensive care unit
- IMGQ:
-
Italian Myasthenia Gravis Questionnaire
- IPAT:
-
Institute for Personality & Ability Testing
- ISI:
-
Insomnia Severity Index
- LPR:
-
Low density lipoprotein receptor-related protein
- MDI:
-
Major Depression Inventory
- MFI:
-
Multidimensional Fatigue Inventory
- MG:
-
Myasthenia gravis
- MG-ADL:
-
Myasthenia Gravis Activities of Daily Living
- MGFA:
-
Myasthenia Gravis Foundation of America
- MGFS:
-
Myasthenia Gravis Fatigue Scale
- MG-QoL:
-
Myasthenia Gravis Quality of Life
- MSPSS:
-
Multidimensional Scale of Perceived Social Support
- MuSK:
-
Muscle-specific tyrosine kinase
- NA:
-
Not applicable
- oMG:
-
Ocular myasthenia gravis
- PAIS-SR:
-
Psychosocial Adjustment to Illness Scale – Self Report
- PASS:
-
Patient Acceptable Symptom State
- PGIC:
-
Patients' Global Impression of Change
- PGIS:
-
Patient Global Impression of Symptom Severity
- PHQ:
-
Patient Health Questionnaire
- POMS:
-
Profile of Mood States
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROM:
-
Patient-reported outcome measure
- PSQI:
-
Pittsburgh Sleep Quality Index
- PTGI:
-
Post-Traumatic Growth Inventory
- PTSD:
-
Post-traumatic stress disorder
- QLI:
-
Quality of life index
- QoL:
-
Quality of life
- SAS:
-
Self-Rating Anxiety Scale
- SDS:
-
Self-Rating Depression Scale
- SLR:
-
Systematic literature review
- SF8:
-
Short Form-8 Health Survey
- SF-36:
-
36-Item Short Form Survey
- STAI:
-
State-Trait Anxiety Inventory
- SSA:
-
Self-Rating Questionnaire for Sleep and Awakening Quality
- VAS:
-
Visual analogue scale
- WPAI:
-
Work productivity and activity impairment
References
Akkan Suzan A, Kahraman Koytak P, Uluc K, Tanridag T. Physical and mental fatigue in myasthenia gravis and its correlation with other symptoms. Acta Neurol Belg. 2022;25:25.
Alabbad S, AlGaeed M, Sikorski P, Kaminski HJ. Monoclonal antibody-based therapies for myasthenia gravis. BioDrugs. 2020;34:557–66.
Alanazy MH. Prevalence and associated factors of depressive symptoms in patients with myasthenia gravis: A cross-sectional study of two tertiary hospitals in Riyadh. Saudi Arabia Behav Neurol. 2019;2019:9367453.
Ambrogi V, Mineo TC. Active ectopic thymus predicts poor outcome after thymectomy in class III myasthenia gravis. J Thorac Cardiovasc Surg. 2012;143:601–6.
Andersen LK, Jakobsson AS, Revsbech KL, Vissing J. Causes of symptom dissatisfaction in patients with generalized myasthenia gravis. J Neurol. 2022;269(6):3086–93.
Aysal F, Karamustafalioglu O, Ozcelik B, Yilmaz M, Karamustafalioglu N, Yumrukcal H, Tankaya O. The relationship of symptoms of anxiety and depression with disease severity and treatment modality in Myasthenia gravis: A cross-sectional study. Noropsikiyatri Arsivi. 2013;50(4):295–300.
Bachmann K, Burkhardt D, Schreiter I, Kaifi J, Busch C, Thayssen G, Izbicki JR, Strate T. Long-term outcome and quality of life after open and thoracoscopic thymectomy for myasthenia gravis: Analysis of 131 patients. Surg Endosc Other Interv Tech. 2008;22(11):2470–7.
Baram A, Salih KAH, Saqat BH. Thymectomy for non-thymomatous myasthenia gravis: Short and long term outcomes, a single-center 10 years’ experience. International Journal of Surgery Open. 2021;35:100381.
Bartel PR, Lotz BP. Neuropsychological test performance and affect in myasthenia gravis. Acta Neurol Scand. 1995;91:266–70.
Basta IZ, Pekmezovic TD, Peric SZ, Kisic-Tepavcevic DB, Rakocevic-Stojanovic VM, Stevic ZD, Lavrnic DV. Assessment of health-related quality of life in patients with myasthenia gravis in Belgrade (Serbia). Neurol Sci. 2012;33(6):1375–81.
Birnbaum S, Bachasson D, Sharshar T, Porcher R, Hogrel JY, Portero P. Free-Living Physical Activity and Sedentary Behaviour in Autoimmune Myasthenia Gravis: A Cross-Sectional Study. Journal of neuromuscular diseases. 2021;8:689–97.
Bubuioc AM, Kudebayeva A, Turuspekova S, Lisnic V, Leone MA. The epidemiology of myasthenia gravis. J Med Life. 2021;14:7–16.
Busch C, Machens A, Pichlmeier U, Emskotter T, Izbicki JR. Long-term outcome and quality of life after thymectomy for myasthenia gravis. Ann Surg. 1996;224(2):225–32.
Centre for Reviews and Dissemination. Systematic Reviews: CRD's guidence for undertaking reviews in health care. York: University of York; 2009. ISBN 978-1-900640-47-3.
Cioncoloni D, Casali S, Ginanneschi F, Carone M, Veronica B, Rossi A, Giannini F. Major motor-functional determinants associated with poor self-reported health-related quality of life in myasthenia gravis patients. Neurol Sci. 2016;37(5):717–23.
De Freitas Fregonezi GA, Regiane-Resqueti V, Pradas J, Vigil L, Casan P. The relationship between lung function and health-related quality of life in patients with generalized myasthenia gravis. Archivos de Bronconeumologia. 2006;42(5):218–24.
De Lapiscina EHM, Aguirre MEE, Blanco TA, Pascual IJ. Myasthenia gravis: Sleep quality, quality of life, and disease severity. Muscle Nerve. 2012;46(2):174–80.
Dewilde S, Kousoulakou H, Janssen M, Claeys K, Friconneau M, Jacob S, Meisel A, Day L, Quinn C, Larkin M, Leighton T, Phillips G, Paci S. Digital Data Collection to Measure the Impact of Myasthenia Gravis on Patients’ Quality of Life in the Real World: Report at Baseline. Value in Health. 2022;25(1 Supplement):S246.
Dewilde S, Philips G, Paci S, Beauchamp J, Chiroli S, Quinn C, Day L, Larkin M, Palace J, Berrih-Aknin S. Patient-reported burden of myasthenia gravis: baseline results of the international prospective, observational, longitudinal real-world digital study MyRealWorld-MG. BMJ Open. 2023;13: e066445.
Dresser L, Wlodarski R, Rezania K, Soliven B. Myasthenia Gravis: Epidemiology, Pathophysiology and Clinical Manifestations. J Clin Med. 2021;10(11):2235.
Farmakidis C, Pasnoor M, Dimachkie MM, Barohn RJ. Treatment of myasthenia gravis. Neurol Clin. 2018;36:311–37.
Gelinas D, Parvin-Nejad S, Phillips G, Cole C, Hughes T, Silvestri N, Govindarajan R, Jefferson M, Campbell J, Burnett H. The humanistic burden of myasthenia gravis: A systematic literature review. J Neurol Sci. 2022;437: 120268.
Happe S, Klosch G, Zeitlhofer J. Perception of dreams and subjective sleep quality in patients with myasthenia gravis. Neuropsychobiology. 2004;50(1):21–7.
Hoffmann S, Ramm J, Grittner U, Kohler S, Siedler J, Meisel A. Fatigue in myasthenia gravis: risk factors and impact on quality of life. Brain Behav. 2016;6(10):e00538.
Jackson K, Parthan A, Lauher-Charest M, Broderick L, Law N, Barnett C. Understanding the Symptom Burden and Impact of Myasthenia Gravis from the Patient’s Perspective: A Qualitative Study. Neurology and Therapy. 2023;12:107–28.
Jastrzebska A, Jastrzebski M, Ryniewicz B, Kostera-Pruszczyk A. Treatment outcome in juvenile-onset myasthenia gravis. Muscle Nerve. 2019;59(5):549–54.
Jayam Trouth A, Dabi A, Solieman N, Kurukumbi M, Kalyanam J. Myasthenia gravis: a review Autoimmune diseases. 2012;2012: 874680.
Jordan B, Mehl T, Schweden TLK, Menge U, Zierz S. Assessment of physical fatigability and fatigue perception in myasthenia gravis. Muscle Nerve. 2017;55:657–63.
Jordan B, Schweden TLK, Mehl T, Menge U, Zierz S. Cognitive fatigue in patients with myasthenia gravis. Muscle Nerve. 2017;56:449–57.
Kaukiainen L, Pirskanen R, Hokkanen E. Social aspects in myasthenia gravis. Acta Neurol Scand. 1977;55(5):377–84.
Kotan VO, Kotan Z, Aydin B, Taskapilioglu O, Karli HN, Yalvac HD, Ozkaya G, Sarandol A, Turan OF, Kirli S. Psychopathology, psychosocial factors and quality of life in patients with myasthenia gravis. Journal of Neurological Sciences. 2016;33(3):482–93.
Law N, Davio K, Blunck M, Lobban D, Seddik K. The Lived Experience of Myasthenia Gravis: A Patient-Led Analysis. Neurology and therapy. 2021;10:1103–25.
Lehnerer S, Jacobi J, Schilling R, Grittner U, Marbin D, Gerischer L, Stascheit F, Krause M, Hoffmann S, Meisel A. Burden of disease in myasthenia gravis: taking the patient’s perspective. J Neurol. 2022;269(6):3050–63.
Menon D, Bril V. Pharmacotherapy of Generalized Myasthenia Gravis with Special Emphasis on Newer Biologicals. Drugs. 2022;82:865–87.
Morren JA, Li Y. Myasthenia gravis: Frequently asked questions. Clevel Clin J Med. 2023;90:103–13.
Ohlraun S, Hoffmann S, Klehmet J, Kohler S, Grittner U, Schneider A, Heuschmann PU, Meisel A. Impact of myasthenia gravis on family planning: How do women with myasthenia gravis decide and why? Muscle Nerve. 2015;52(3):371–9.
Onyekwulu FA, Onwuekwe IO. Critical care of myasthenia gravis in a resource poor setting: A study of South East Nigeria. Neurologist. 2010;16(6):368–70.
Padua L, Evoli A, Aprile I, Caliandro P, Mazza S, Padua R, Tonali P. Health-related quality of life in patients with myasthenia gravis and the relationship between patient-oriented assessment and conventional measurements. Neurol Sci. 2001;22:363–9.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Peres J, Martins R, Alves JD, Valverde A. Rituximab in generalized myasthenia gravis: Clinical, quality of life and cost-utility analysis. Porto Biomedical Journal. 2017;2(3):81–5.
Raggi A, Leonardi M, Antozzi C, Confalonieri P, Maggi L, Cornelio F, Mantegazza R. Concordance between severity of disease, disability and health-related quality of life in Myasthenia gravis. Neurol Sci. 2010;31(1):41–5.
Rethlefsen ML, Kirtley S, Waffenschmidt S, Ayala AP, Moher D, Page MJ, Koffel JB. PRISMA-S: an extension to the PRISMA statement for reporting literature searches in systematic reviews. Syst Rev. 2021;10:1–19.
Rodolico C, Bonanno C, Brizzi T, Nicocia G, Trimarchi G, Lupica A, Pugliese A, Musumeci O, Toscano A. Methotrexate as a steroid-sparing agent in myasthenia gravis: A preliminary retrospective study. J Clin Neuromuscul Dis. 2021;23(2):61–5.
Roth T, Ackermann R, Stein R, Inderbitzi R, Rosler K, Schmid RA. Thirteen years follow-up after radical transsternal thymectomy for myasthenia gravis. Do short-term results predict long-term outcome? Eur J Cardiothorac Surg. 2002;21(4):664–70.
Ruckert JC, Sobel HK, Gohring S, Einhaupl KM, Muller JM. Matched-pair comparison of three different approaches for thymectomy in myasthenia gravis. Surg Endosc. 2003;17:711–5.
Ruiter AM, Verschuuren JJGM, Tannemaat MR. Prevalence and associated factors of fatigue in autoimmune myasthenia gravis. Neuromuscul Disord. 2021;31(7):612–21.
Sabre L, Westerberg E, Liik M, Punga AR. Diversity in mental fatigue and social profile of patients with myasthenia gravis in two different Northern European countries. Brain Behav. 2017;7(4):e00653.
Sitek EJ, Bilińska MM, Wieczorek D, Nyka WM. Neuropsychological assessment in myasthenia gravis. Neurol Sci. 2009;30:9–14.
Stankovic M, Peric S, Stojiljkovic Tamas O, Stankovic T, Nikolic A, Lavrnic D, Basta I. Quality of life in patients with MuSK positive myasthenia gravis. Acta Neurol Belg. 2018;118(3):423–7.
Stojanov A, Milosevic V, Dordevic G, Stojanov J. Quality of Life of Myasthenia Gravis Patients in Regard to Epidemiological and Clinical Characteristics of the Disease. Neurologist. 2019;24(4):115–20.
Szczudlik P, Sobieszczuk E, Szyluk B, Lipowska M, Kubiszewska J, Kostera-Pruszczyk A. Determinants of Quality of Life in Myasthenia Gravis Patients. Front Neurol. 2020;11:553626.
Tascilar NF, Saracli O, Kurcer MA, Ankarali H, Emre U. Is there any relationship between quality of life and polysomnographically detected sleep parameters/disorders in stable myasthenia gravis? Acta Neurol Belg. 2018;118(1):29–37.
Thanvi BR, Lo TCN. Update on myasthenia gravis. Postgrad Med J. 2004;80:690–700.
Thomsen JLS, Vinge L, Harbo T, Andersen H. Gender differences in clinical outcomes in myasthenia gravis: A prospective cohort study. Muscle Nerve. 2021;64(5):538–44.
Westerberg E, Landtblom AM, Punga AR. Lifestyle factors and disease-specific differences in subgroups of Swedish Myasthenia Gravis. Acta Neurol Scand. 2018;138(6):557–65.
Acknowledgements
We thank Rachel Glenister (Mtech Access) who provided medical writing services in the preparation of the manuscript, funded by consultancy payments from Janssen Pharmaceuticals.
Funding
Open access funding provided by University of Bergen. This study was funded by Janssen Pharmaceuticals.
Author information
Authors and Affiliations
Contributions
JM, AB, WN, JL, WK, SS and GMB were involved in the conception and design of the study and contributed to the revision of the manuscript. EH, CRM and SAM contributed to the design of the study, conducted the systematic review, and contributed to the revision of the manuscript. NEG contributed substantively to the interpretation of the data, and the drafting and revision of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
JM, AB, WN, JL, WK, SS and GMB are employees of Janssen Pharmaceuticals. EH, CRM and SAM are employees of Mtech Access and received payment from Janssen Pharmaceuticals to carry out this review. NEG hhas received financial support from UCB, Argenx, Janssen, Merck, Roche, Alexion, Immunovant, Huma, Dianthus, Denka, and Grifols.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
McCallion, J., Borsi, A., Noel, W. et al. Systematic review of the patient burden of generalised myasthenia gravis in Europe, the Middle East, and Africa. BMC Neurol 24, 61 (2024). https://doi.org/10.1186/s12883-024-03553-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-024-03553-y