Abstract
Introduction
Serum lactate dehydrogenase (LDH) levels are often elevated in cardiovascular diseases. Their prognostic role after subarachnoid hemorrhage (SAH) remains poorly evaluated.
Methods
This is a retrospective single-center study of patients with non-traumatic SAH admitted to the intensive care unit (ICU) of an University Hospital from 2007 to 2022. Exclusion criteria were pregnancy and incomplete medical records or follow-up data. Baseline information, clinical data, radiologic data, the occurrence of neurological complications as well as serum LDH levels during the first 14 days of ICU stay were collected. Unfavorable neurological outcome (UO) at 3 months was defined as a Glasgow Outcome Scale of 1–3.
Results
Five hundred and forty-seven patients were included; median serum LDH values on admission and the highest LDH values during the ICU stay were 192 [160–230] IU/L and 263 [202–351] IU/L, respectively. The highest LDH value was recorded after a median of 4 [2–10] days after ICU admission. LDH levels on admission were significantly higher in patients with UO. When compared with patients with favorable outcome (FO), patients with UO had higher serum LDH values over time. In the multivariate logistic regression model, the highest LDH value over the ICU stay (OR 1.004 [95% CI 1.002 – 1.006]) was independently associated with the occurrence of UO; the area under the receiving operator (AUROC) curve for the highest LDH value over the ICU stay showed a moderate accuracy to predict UO (AUC 0.76 [95% CI 0.72–0.80]; p < 0.001), with an optimal threshold of > 272 IU/L (69% sensitivity and 74% specificity).
Conclusions
The results in this study suggest that high serum LDH levels are associated with the occurrence of UO in SAH patients. As a readily and available biomarker, serum LDH levels should be evaluated to help with the prognostication of SAH patients.
Similar content being viewed by others
Introduction
Rupture of cerebral aneurysm is a frequent cause of subarachnoid hemorrhage (SAH), although it accounts for just 5% of all stroke cases [1]. SAH remains a devastating cause of acute brain injury, as it affects mostly young people with an average age of 55 years and a good life expectancy [2, 3]. Over the past two decades, despite a decrease in overall mortality of SAH [1, 4, 5], morbidity remains still high and long-term neurological outcome is often poor among most of survivors. Indeed, complete recovery has been described in less than one-third while many of the affected patients may suffer from cognitive disfunction that may impair their quality of life and working capacity [2, 3, 6].
Currently, prediction of outcome after SAH is mainly based on the neurological clinical condition on admission, as assessed by the World Federation of Neurological Surgeons (WFNS) score, with a high grade score (i.e. IV-V) being associated with poor prognosis [7]. Nevertheless, different studies have shown that more than 20% of patients with high grade WFNS treated aggressively can recover completely, making initial resuscitation decisions very challenging for clinicians [8, 9]. With the aim of improving the prognostic accuracy of patients affected by SAH, modifications of WFNS scale [10], assessment of the WFNS scale after initial resuscitation [9] as well as different combined scores [11, 12] have been proposed. However, all these scores are mainly based on the clinical information on admission, and do not consider additional factors that may influence the prognosis during hospitalization.
Lactate dehydrogenase (LDH) is a non-specific biomarker expressed in almost all body tissue and it is an important enzyme involved in the anaerobic metabolism [13]. High serum LDH levels may be observed in the presence of tissue damage, hypoxic states and in several well-defined diseases and, in critically ill patients, may also represent a poor prognostic factor [13,14,15,16]. Moreover, high serum LDH values have been associated with the extend of cerebral damage in acute brain injured patients [17]. Recently, in a population of aSAH patients, high serum LDH values before microsurgical clipping were associated with poor neurological outcome at 3 months [18]; in a similar cohort, high serum LDH values on hospital admission were associated with the development of post operative pneumonia (POP) [19]; finally, in another cohort of aSAH patients, high serum LDH values were associated with early mortality [20].
The above-mentioned studies have been conducted almost exclusively in Asian populations, with different selection criteria; therefore, considering some potential variability among different populations, the aim of this study was to evaluate whether LDH might have a prognostic value in SAH patients.
Methods
Study population
This is a retrospective single-center cohort study including non-traumatic SAH patients admitted to the Intensive Care Unit (ICU) of Erasmus Hospital, Brussels, Belgium between January 2007 and August 2022. Inclusion criteria were: 1) age > 18 years; 2) diagnosis of ruptured aneurysm as the primary cause of SAH on computed tomography (CT) with angiographic confirmation (either computed tomography angiography or cerebral angiography). Exclusion criteria were: 1) pregnancy; 2) patients without 3 months follow up assessment reported in the medical records. This study was approved by Erasme Hospital’s Ethics Committee (P2019/649) that waived the need for informed consent. All methods were carried out in accordance with relevant guidelines and regulations in the declaration of Helsinki.
Data collection
Demographic and clinical data were recorded including age, sex, history of hypertension, chronic obstructive pulmonary disease (COPD), heart disease, liver cirrhosis, chronic renal failure, cancer, immunosuppressive therapy, and previous neurological disease. Neurological status on admission was assessed by the WFNS score and the Glasgow coma scale (GCS). The severity of initial bleeding was evaluated by CT-scan and scored using the modified Fisher scale (mFisher). Severity of the disease was assessed using the Acute Physiology and Chronic Health Evaluation II (APACHE II) score and the Sequential Organ Failure Assessment (SOFA) score.
Aneurysm treatment (i.e. coiling and/or clipping) and use of neuromonitoring, including the need for intracranial pressure (ICP) monitoring, brain tissue oxygenation monitoring (PbtO2), continuous electroencephalogram (cEEG) and external ventricular derivation (EVD), were also recorded. We also collected the development of brain-specific complications, such as rebleeding, intracranial hypertension (ICHT), cerebral vasospasm, delayed cerebral ischemia (DCI), hydrocephalus and seizures, as previously described [21]. Management of such complications (i.e. osmotic therapy, decompressive craniectomy, oral or intra-arterial nimodipine etc.) were also recorded. Daily treatment including use of sedation, vasopressor, inotropes, extracorporeal membrane oxygenation (ECMO), and continuous renal replacement therapy (CRRT) were recorded. Serum LDH values were collected over 14 consecutive days from ICU admission, whenever available.
Neurological status at hospital discharge and the Glasgow outcome scale (GOS) at 3 months (GOS: 1 = dead, 2 = persistent vegetative state, 3 = severe disability, 4 = moderate disability, and 5 = good recovery) [22] were collected for each patient in the follow-up visit or estimated from medical reports; GOS score was dichotomized into unfavorable (UO; GOS 1–3) and favorable (FO; GOS 4 and 5) neurological outcome.
Outcomes
The primary outcome was the prognostic value of admission or the highest LDH value over the ICU stay to predict UO. Secondary outcome was the prognostic value of LDH for in-hospital mortality.
Statistical analysis
JASP 0.16.4 statistical software was used for data processing. Continuous data were expressed as mean (standard deviation [SD]) or median (interquartile ranges) according to data distribution. Differences between groups were performed with Student t-test or Mann–Whitney U-test for normally or non-normally distributed data respectively. Categorical data were presented as numbers (percentage [%]) and comparison between groups was performed by X-square test. Univariate logistic regression was run using UO as the dependent variable and factors associated with poor outcome [23, 24] as independent variables. Multivariate logistic regression was then performed with variables considered significant in the univariate analysis. Results of both univariate and multivariate logistic regression analysis were expressed as odds ratio (OR, with 95% confidence interval [CI]), and significance was taken at p < 0.05. A correlation analysis was also performed to exclude in the multivariate model pairs of variables that were closely related. To compare differences in the variations of LDH values over time in different subgroups, a linear mixed model was performed. The ability of LDH levels to predict UO was assessed using the receiver operating characteristic (ROC) curve and the area under the curve (AUROC) was calculated. Youden’s index was computed to assess the optimal cut-off of the LDH values for sensitivity and specificity to predict UO and in-hospital mortality. A p value below 0.05 was considered as significant.
Results
Study population
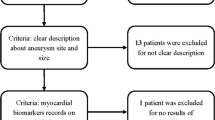
A total of 568 patients with aneurysmal SAH were identified over the study period; of those, 21 patients were excluded due to lost at 3 months follow-up, resulting in a total of 547 patients included in the final analysis. Main characteristics of the study population are shown in Table 1. Patients were predominantly female and had a mean age of 54.0 (± 13) years. The median GCS on admission was 14 [5,6,7,8,9,10,11,12,13,14,15], 246 (45%) patients had a WFNS 4–5 (poor grade), and 495 (90.5%) of the patients presented with a mFisher scale 3–4. The most common comorbidity was arterial hypertension. Intracranial hypertension and cerebral vasospasm were the most common neurological complication; DCI occurred in 134 (24.5%) patients. 172 (31.4%) patients died during hospital stay and 248 (45.3%) had UO.
LDH values and neurological outcome
Median serum LDH values on admission and the highest LDH values during the ICU stay were 192 [160–230] IU/L and 263 [202–351] IU/L, respectively. Serum LDH values on admission were significantly higher in patients with UO when compared to others (215 [179.8–260] vs. 176 [152–202] IU/L; p < 0.001), as well as the highest LDH value (323 [257–429] vs. 226 [159–279] IU/L; p < 0.001). Figure 1 and Supplemental Table 1 summarize LDH values over time, according to the neurological outcome; median serum LDH were significantly higher over time in patients with UO when compared to others.
Evolution of median daily lactate dehydrogenase (LDH) levels over time in the first 14 days of hospitalization according to neurological outcome at 3 months. Unfavorable neurological outcome was defined as Glasgow outcome scale (GOS) of 1–3 and favorable neurological outcome was defined as a GOS of 4–5. P- value was calculated using a linear mixed model approach
Patients with UO were older, had lower GCS score on admission and had more frequently an initial poor WFNS grade and higher mFisher scale than others. Also, patients with UO had higher APACHE II and SOFA score on admission and developed more frequently cerebral complications, including DCI, rebleeding, intracranial hypertension, epilepsy, and hydrocephalus than others (Table 1). In the multivariate logistic regression model, the highest LDH value over the ICU stay (OR 1.004 [95% CI 1.002 – 1.006]) was independently associated with the occurrence of UO, together with older age, WFNS score, the occurrence of DCI, ICHT and rebleeding (Table 2). The AUROC curve (Fig. 2) for the highest LDH value over the ICU stay showed a moderate accuracy to predict UO (AUC 0.76 [95% CI 0.72–0.80]; p < 0.001). The Youden’s index identified the threshold of the highest LDH value > 272 IU/L for the best combination of sensitivity (69%) and specificity (74%) to predict UO.
LDH values and in-hospital mortality
Non-survivors at hospital discharge (n = 172) had higher serum LDH values on admission when compared with survivors (n = 375) (221 [187–270] vs. 180 [154–211] IU/L; p < 0.001), as well as the highest LDH value (310 [246–404] vs. 247 [190–320] IU/L; p < 0.001). Supplemental Table 2 summarize the main characteristics of the studied population, according to hospital survival; Supplemental Fig. 1 and Supplemental Table 3 summarize LDH values over time, according to hospital survival; median serum LDH were significantly higher over time in non-survivors, when compared to survivors.
In the multivariate logistic regression model, the highest LDH value over the ICU stay (OR 1.001 [95% CI 1.000 – 1.002]) was independently associated with in-hospital mortality, together with older age, WFNS score, the occurrence of DCI, rebleeding and hydrocephalus (Suppl Table 4). The AUROC curve (Fig. 2) for the highest LDH value over the ICU stay showed poor accuracy to predict in-hospital mortality (AUC 0.67 [95% CI 0.63–0.72]; p < 0.001).
Discussion
In this retrospective study on a population of SAH patients, higher LDH values over the ICU stay and on admission were significantly higher in patients with poor outcome, when compared to others. Also, high LDH values over the ICU stay were independently associated with UO. The highest LDH showed a moderate accuracy to predict unfavorable outcome.
LDH is cytoplasmatic enzyme involved in the anaerobic metabolism pathway and it is present in almost all body tissue in different isomers. As such, serum LDH levels may increase in case of high tissue turnover [13]. For this reason, it is a useful and widely used biomarker in some malignancies, such as breast and lung cancer, as it could predict survival [25, 26] and response to specific therapy [27, 28]. Serum LDH levels may also increase in case of some infectious diseases, such as sepsis [29] or pneumonia [30, 31], as well as in many other pathological conditions such as liver disease, hemolytic anemia, myocardial infarction, trauma, and infections such as encephalitis, meningitis, encephalitis, and HIV [13]. Nevertheless, in SAH patients the role of serum LDH level has not been widely described and few data are available. Rupture of cerebral aneurysm results in primary brain damage due to the leakage of blood in the subarachnoid space which increases ICP and decreases cerebral blood flow [32]. This phenomenon triggers inflammatory brain reactions, pro-apoptotic and necrotic pathways that contribute to the disruption of the blood–brain barrier (BBB) and secondary brain injury [32,33,34]. Cell death by apoptosis or necrosis begins early after SAH and causes a release of cytoplasmic contents in the cerebrospinal fluids (CSF) [34, 35], including LDH.
The predictive value of serum LDH values is not a new finding. In 1978, Rao et al. [17] described that serum LDH levels were directly proportional to the clinical (level of consciousness) and radiological severity of brain injury, possibly representing the extent of brain damage in head injury patients. More recently, Zan et al. [20] in a retrospective study, have shown that LDH level on admission is an independent predictor of all-cause mortality in patients with SAH. In their analysis, the authors showed that for each 1-point increase in LDH, the chance of 90-days of mortality increase of 1.98 (95% CI 1.30- 3.20). Our study found an association between the highest LDH in the first 14 days of hospitalization and in hospital mortality. Another study including 647 aSAH patients reported that development of post operative pneumonia occurred more frequently in patients with serum LDH level greater than 250 U/L than in others [19]. Interestingly, in patients who underwent microsurgical clipping, pre operative LDH levels were associated with poor neurological outcome at 3 months [18]; the AUROC curve of serum LDH level on admission to predict unfavorable outcome was 0.70 (95% CI = 0.65–0.75), and the optimal cutoff value for serum LDH levels as a predictor of poor-outcome was 202 IU/L. Similarly, we also found an association between the highest LDH level in the first 14 days of hospitalization and poor neurological outcome at 3 months with an AUROC curve of AUC 0.76 [95% CI 0.72–0.80]; p < 0.001 with a higher LDH cutoff (272 U/L) compared to previous study [18]. Moreover, unlike the previous studies, we also considered the evolution of LDH values in the first 14 days of hospitalization, showing consistently higher LDH levels in patients with poor outcome/in-hospital mortality during early brain injury and delayed cerebral injury periods.
A retrospective study by Anan et al. [36] showed that LDH levels in CSF were higher in patients with DCI rather than in non-DCI patients but no differences in serum LDH levels were observed between the two groups. However, these results were obtained from a very small sample of only 19 patients of whom only 6 developed DCI. On the contrary, a prospective study on a population of cardiac arrest survivors [37] has shown that compared with patients with favorable outcome, both serum and CSF LDH levels were higher in patients with poor neurological outcome at 3 months evaluated by the cerebral performance category (CPC) scale. It can be assumed that serum LDH levels may be influenced by the global state of hypoperfusion as it occurs during cardiac arrest; however, both CSF and serum LDH levels follow the same trend in patients with unfavorable outcome. Even though we have studied a different population of brain injured patients and we did not collect LDH in CSF, these results are consistent with our findings.
As a readily and available biomarker, monitoring serum LDH values would be helpful for clinicians to identify patients at a higher risk of poor prognosis. In our study, the highest LDH value was observed after a median of 4 days which is before the period in which DCI can usually develop for example [38]. Thus, it would be interesting in future studies to investigate in a possible association between LDH values and neurological complications such as DCI, and whether the serum LDH level increases before such complications become clinically evident.
Our study has some limitations. First, we did not consider concurrent factors that may lead to an increase in LDH, such as the occurrence of pneumonia or sepsis, and we did not exclude patients with underlying diseases that may already present an increase in serum LDH levels, such as patients with oncological or severe liver failure. However, in our studied population, patients with oncological or severe liver disease were a negligible minority of 4,8% and 1,1% respectively. Regarding infectious pulmonary complications, the median values of MV in patients with UO were 4 days that may be too short to be consistent with the onset and resolution of a ventilator associated pneumonia for example [39, 40]. So, although we have not considered all possible confounding factors, we can speculate that since the increase in serum LDH levels are observed in patients with more severe neurological disease, that is mainly due to the SAH that promotes both neurological and systemic deterioration [41]. Second, this is a retrospective study with potential biases due to date derived from clinical records; third this is a single center study and this results may reflect local characteristics only, although our results are consistent with previous studies in different centers. Fourth, we did not measure CSF LDH levels which would be interesting to help investigate the effects of local neurological inflammation on outcome. Fifth we focused only on short term outcomes.
Conclusion
Lactate dehydrogenase is an easily available non-specific biomarker associated with in hospital mortality and short term unfavorable neurological outcomes in non-traumatic subarachnoid hemorrhage studies. Future large multi-center studies adjusting for other causes of LDH increase such as infection are needed to better define the use of LDH as part of neuro prognostication.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Etminan N, et al. Worldwide Incidence of Aneurysmal Subarachnoid Hemorrhage According to Region, Time Period, Blood Pressure, and Smoking Prevalence in the Population: A Systematic Review and Meta-analysis. JAMA Neurol. 2019;76(5):588–97. https://doi.org/10.1001/jamaneurol.2019.0006.
English SW. Long-Term Outcome and Economic Burden of Aneurysmal Subarachnoid Hemorrhage: Are we Only Seeing the Tip of the Iceberg? Neurocrit Care. 2020;33(1):37–8. https://doi.org/10.1007/s12028-020-00943-1.
Rinkel GJE, Algra A. Long-term outcomes of patients with aneurysmal subarachnoid haemorrhage. Lancet Neurol. 2011;10(4):349–56. https://doi.org/10.1016/S1474-4422(11)70017-5.
Chan V, Lindsay P, McQuiggan J, Zagorski B, Hill MD, O’Kelly C. Declining Admission and Mortality Rates for Subarachnoid Hemorrhage in Canada between 2004 and 2015. Stroke. 2019;50(1):181–4. https://doi.org/10.1161/STROKEAHA.118.022332.
Gouvêa Bogossian E, et al. “Time course of outcome in poor grade subarachnoid hemorrhage patients: a longitudinal retrospective study,.” BMC Neurol. 2021;21(1):1–10.
Macdonald RL, Schweizer TA. Spontaneous subarachnoid haemorrhage. Lancet. 2017;389(10069):655–66. https://doi.org/10.1016/S0140-6736(16)30668-7.
Teasdale GM, Drake CG, Hunt W, Kassell N, Sano K, Pertuiset B, De Villiers JC. A universal subarachnoid hemorrhage scale: report of a committee of the World Federation of Neurosurgical Societies. J Neurol Neurosurg Psychiatry. 1988;51(11):1457. https://doi.org/10.1136/jnnp.51.11.1457.
de Winkel J, et al. Early predictors of functional outcome in poor-grade aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. BMC Neurol. 2022;22(1):1–10. https://doi.org/10.1186/s12883-022-02734-x.
Van Donkelaar CE, et al. Timing of Clinical Assessment. J Neurosurg. 2017;126(1):52–9. https://doi.org/10.3171/2016.1.JNS152136.52.
Fung C, et al. Reconsidering the logic of World Federation of Neurosurgical Societies grading in patients with severe subarachnoid hemorrhage. J Neurosurg. 2016;124(2):299–304. https://doi.org/10.3171/2015.2.JNS14614.
Van Donkelaar CE, et al. Prediction of Outcome After Aneurysmal Subarachnoid Hemorrhage: Development and Validation of the SAFIRE Grading Scale. Stroke. 2019;50(4):837–44. https://doi.org/10.1161/STROKEAHA.118.023902.
Jaja BNR, et al. Development and validation of outcome prediction models for aneurysmal subarachnoid haemorrhage: The SAHIT multinational cohort study. BMJ. 2018;360(January):1–17. https://doi.org/10.1136/bmj.j5745.
Farhana A, Lappin SL. “Biochemistry , Lactate Dehydrogenase,.” 2022. p. 1–9 no. M.
Wu Y, et al. Serum lactate dehydrogenase activities as systems biomarkers for 48 types of human diseases. Sci Rep. 2021;11(1):1–8. https://doi.org/10.1038/s41598-021-92430-6.
Martha JW, Wibowo A, Pranata R. Prognostic value of elevated lactate dehydrogenase in patients with COVID-19: a systematic review and meta-analysis. Postgrad Med J. 2022;98(1160):422–7. https://doi.org/10.1136/postgradmedj-2020-139542.
Su D, et al. The relationship between serum lactate dehydrogenase level and mortality in critically ill patients. Biomark Med. 2021;15:551–9. https://doi.org/10.2217/bmm-2020-0671.
Rao CJ, Shukla PK, Mohanty S, Reddy YJV. Predictive value of serum lactate dehydrogenase in Predictive value of serum lactate dehydrogenase in head injury. J Neurol Neurosurg Psychiatry. 1978;41(10):948–53. https://doi.org/10.1136/jnnp.41.10.948.
Zheng S, et al. Higher Serum Levels of Lactate Dehydrogenase Before Microsurgery Predict Poor Outcome of Aneurysmal Subarachnoid Hemorrhage. Front Neurol. 2021;12(August):1–9. https://doi.org/10.3389/fneur.2021.720574.
Ding CY, Peng L, Lin YX, Yu LH, Wang DL, Kang DZ. Elevated Lactate Dehydrogenase Level Predicts Postoperative Pneumonia in Patients with Aneurysmal Subarachnoid Hemorrhage. World Neurosurg. 2019;129:e821–30. https://doi.org/10.1016/j.wneu.2019.06.041.
Zan X, et al. Lactate dehydrogenase predicting mortality in patients with aneurysmal subarachnoid hemorrhage. Ann Clin Transl Neurol. 2022;9(10):1565–73. https://doi.org/10.1002/acn3.51650.
Gouvea Bogossian, E., Diaferia, D., Ndieugnou Djangang, N. et al. Brain tissue oxygenation guided therapy and outcome in non-traumatic subarachnoid hemorrhage. Sci Rep. 2021;11:16235. https://doi.org/10.1038/s41598-021-95602-6.
Wilson JTL, Pettigrew LEL, Teasdale GM. Structured interviews for the glasgow outcome scale and the extended glasgow outcome scale: Guidelines for their use. J Neurotrauma. 1998;15(8):573–80. https://doi.org/10.1089/neu.1998.15.573.
Lantigua H, et al. Subarachnoid hemorrhage: Who dies, and why? Crit Care. 2015;19(1):1–10. https://doi.org/10.1186/s13054-015-1036-0.
Jaja BNR, et al. Clinical prediction models for aneurysmal subarachnoid hemorrhage: A systematic review. Neurocrit Care. 2013;18(1):143–53. https://doi.org/10.1007/s12028-012-9792-z.
Pelizzari G, et al. Lactate Dehydrogenase (LDH) response to first-line treatment predicts survival in metastatic breast cancer: First clues for a cost-effective and dynamic biomarker. Cancers (Basel). 2019;11(9):1–13. https://doi.org/10.3390/cancers11091243.
Tjokrowidjaja A, et al. Pre- and on-treatment lactate dehydrogenase as a prognostic and predictive biomarker in advanced non–small cell lung cancer. Cancer. 2022;128(8):1574–83. https://doi.org/10.1002/cncr.34113.
Cona MS, Lecchi M, Cresta S, Damian S, Del Vecchio M, Necchi A, Poggi MM, Raggi D, Randon G, Ratta R, et al. Combination of Baseline LDH, Performance Status and Age as Integrated Algorithm to Identify Solid Tumor Patients with Higher Probability of Response to Anti PD-1 and PD-L1 Monoclonal Antibodies. Cancers. 2019;11(2):223. https://doi.org/10.3390/cancers11020223.
Forkasiewicz A, Dorociak M, Stach K, et al. The usefulness of lactate dehydrogenase measurements in current oncological practice. Cell Mol Biol Lett. 2020;25:35. https://doi.org/10.1186/s11658-020-00228-7.
Zein JG, Lee GL, Tawk M, Dabaja M, Kinasewitz GT. Prognostic Significance of Elevated Serum Lactate Dehydrogenase (LDH) in Patients with Severe Sepsis. Chest. 2004;126(4):873S. https://doi.org/10.1378/chest.126.4_meetingabstracts.873s.
Figueira Gonçalves, J.M., Hernández Pérez, J.M., Acosta Sorensen, M. et al. Biomarkers of acute respiratory distress syndrome in adults hospitalised for severe SARS-CoV-2 infection in Tenerife Island, Spain. BMC Res Notes. 2020;13:555. https://doi.org/10.1186/s13104-020-05402-w.
Lu A, Wang C, Zhang X, Wang L, Qian L. Lactate dehydrogenase as a biomarker for prediction of refractory mycoplasma pneumoniae pneumonia in children. Respir Care. 2015;60(10):1469–75. https://doi.org/10.4187/respcare.03920.
Cahill WJ, Calvert JH, Zhang JH. Mechanisms of early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2006;26(11):1341–53. https://doi.org/10.1038/sj.jcbfm.9600283.
Werner C, Engelhard K, Salehi A, Zhang JH, Obenaus A. Pathophysiology of traumatic brain injury. J Cereb Blood Flow Metab. 2007;99(7):2320–39. https://doi.org/10.1093/bja/aem131.
Friedrich V, Flores R, Sehba FA. Cell death starts early after subarachnoid hemorrhage. Neurosci Lett. 2012;512(1):6–11. https://doi.org/10.1016/j.neulet.2012.01.036.
JA Vázquez, M del Carmen Adducci, Monzón DG, Iserson KV. Lactic Dehydrogenase in Cerebrospinal Fluid May Differentiate Between Structural and Non-structural Central Nervous System Lesions in Patients with Diminished Levels of Consciousness. J Emerg Med. 2009;37(1):93–7. https://doi.org/10.1016/j.jemermed.2008.04.032.
Anan M, Nagai Y, Fudaba H, Fujiki M. Lactate and Lactate Dehydrogenase in Cistern as Biomarkers of Early Brain Injury and Delayed Cerebral Ischemia of Subarachnoid Hemorrhage. J Stroke Cerebrovasc Dis. 2020;29(5).
Park JS, et al. Cerebrospinal fluid lactate dehydrogenase as a potential predictor of neurologic outcomes in cardiac arrest survivors who underwent target temperature management. J Crit Care. 2020;57:49–54. https://doi.org/10.1016/j.jcrc.2020.02.001.
Dodd WS, et al. Pathophysiology of delayed cerebral ischemia after subarachnoid hemorrhage: A review. J Am Heart Assoc. 2021;10(15):1–18. https://doi.org/10.1161/JAHA.121.021845.
Pelosi P, et al. Management and outcome of mechanically ventilated neurologic patients. Crit Care Med. 2011;39(6):1482–92. https://doi.org/10.1097/CCM.0b013e31821209a8.
Kalanuria AA, Zai W, Mirski M. Ventilator-associated pneumonia in the ICU. Crit Care. 2014;18(2):1–8. https://doi.org/10.1186/cc13775.
Stevens RD, Nyquist PA. The systemic implications of aneurysmal subarachnoid hemorrhage. J Neurol Sci. 2007;261(1–2):143–56. https://doi.org/10.1016/j.jns.2007.04.047.
Acknowledgements
Not applicable.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
IC, EGB and FST contributed to conception and design of the study. IC, CS, TS, MS, GC, AD, MM, DD, NND and FO collected data and performed data curation. EGB, IC and CS performed the statistical analysis. IC, EGB and FST wrote the first draft of the manuscript. SS and LM revised the manuscript for intellectual content and English editing. All authors contributed to manuscript revision, read, and approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by Erasme Hospital Ethics Committee under the protocol number P2019/649 approved 23/05/2019. Due to the retrospective nature of the study the need for informed written consent was waived by the Erasme Ethics Committee under the protocol number P2019/649 approved 23/05/2019. All methods were carried out in accordance with relevant guidelines and regulations in the declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Supplemental Table S1. Serum lactate dehydrogenase (LDH) values during the first 14 days in the studied population. Data are presented as median (IQRs). Unfavorable outcome (UO) was defined as Glasgow outcome scale (GOS) of 1-3 ate 3 months. Favorable outcome was defined as GOS of 4-5 at 3 months. Supplemental Table S2. Characteristics of the patient population, according to hospital mortality. Supplemental Table S3. Serum lactate dehydrogenase (LDH) values during the first 14 days in the studied population. Data are presented as median (IQRs). Supplemental Table S4. Logistic regression of factors associated with in hospital death. Supplemental Figure 1. Evolution of lactate dehydrogenase (LDH) levels over time according to hospital survival in the first 14 days of hospitalizations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cavalli, I., Stella, C., Stoll, T. et al. Serum LDH levels may predict poor neurological outcome after aneurysmal subarachnoid hemorrhage. BMC Neurol 23, 228 (2023). https://doi.org/10.1186/s12883-023-03282-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-023-03282-8