Abstract
Objective
Few studies correlated n-terminal pro-brain natriuretic peptide (NT-proBNP) with early neurological deterioration (END) and prognosis of acute ischaemic stroke (AIS) patients with rt-PA intravenous thrombolysis. Therefore this study aimed to investigate the relationship between NT-proBNP and END, and prognosis after intravenous thrombolysis in patients with AIS.
Methods
A total of 325 patients with AIS were enrolled. We performed the natural logarithm transformation on the NT-proBNP [ln(NT-proBNP)]. Univariate and multivariate logistic regression analyses were performed to assess the relationship between ln(NT-proBNP) and END, and prognosis and receiver operating characteristic (ROC) curves were used to show the sensitivity and specificity of NT-proBNP.
Results
After thrombolysis, among 325 patients with AIS, 43 patients (13.2%) developed END. In addition, three months follow-up showed a poor prognosis in 98 cases (30.2%) and a good prognosis in 227 cases (69.8%). Multivariate logistic regression analysis showed that ln(NT-proBNP) was an independent risk factor for END (OR = 1.450,95%CI:1.072 ~ 1.963, P = 0.016) and poor prognosis at three months follow-up (OR = 1.767, 95%CI: 1.347 ~ 2.317, P < 0.001) respectively. According to ROC curve analysis, ln(NT-proBNP) (AUC 0.735, 95%CI: 0.674 ~0.796, P < 0.001) had a good predictive value for poor prognosis, with a predictive value of 5.12 and sensitivity and specificity of 79.59% and 60.35% respectively. When combined with NIHSS to predict END(AUC 0.718, 95%CI: 0.631 ~ 0.805, P < 0.001) and poor prognosis(AUC 0.780, 95%CI: 0.724 ~ 0.836, P < 0.001), the predictive value of the model is further improved.
Conclusion
NT-proBNP is independently associated with END and poor prognosis in patients with AIS following intravenous thrombolysis and has a particular predictive value for END and poor prognosis.
Similar content being viewed by others
Introduction
Stroke is the second leading cause of disability and death worldwide, with a recent study showing that in 2020 it is estimated that 3.4 million stroke events, 17.8 million stroke patients, and 3.4 million stroke deaths will occur in people over 40 years of age in China [1, 2]. Intravenous thrombolysis, such as recombinant tissue plasminogen activator (rt-PA) within 4.5 h is an effective treatment method for AIS [3]. However, it has been reported [4,5,6] that within 24–72 h after intravenous thrombolysis, 3.0%-32.8% of AIS patients experienced early neurological deterioration (END). Additionally, research has demonstrated a clear correlation between END and a higher risk of functional disability, death, and a worse clinical result in AIS after three months [5, 7, 8]. Current approaches to identifying high-risk individuals in AIS patients rely heavily on assessing clinical features and neuroimaging present at onset, the patient's initial response to treatment, and relative biomarkers. Therefore, identifying new biomarkers to forecast AIS patients' prognoses is crucial for clinical treatment planning and intervention.
NT-proBNP is an inert fragment broken down by brain natriuretic peptide (BNP) and then released from the stretched ventricular myocardium. Because of its longer half-life, higher plasma concentration, and superior diagnostic resolution, NT-proBNP is the preferable biomarker over BNP. Stretch stimulation, such as volume overload and hemodynamic stress, raises NT-proBNP levels [9]. The prognosis of patients with cardioembolic stroke and AIS is correlated with NT-proBNP, according to earlier studies [5, 10]. However, little is known about the predictive ability of NT-proBNP in AIS treated with intravenous thrombolysis. Therefore, this study aimed to investigate NT-proBNP levels' predictive power in AIS patients following intravenous thrombolysis.
Methods
Subjects
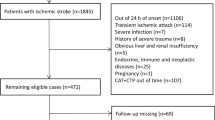
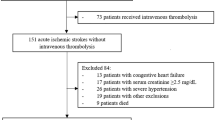
This is a single-center retrospective observational study. We gathered information on AIS patients who had rt-PA intravenous thrombolysis at the Affiliated Hospital of Xuzhou Medical University from December 2020 to July 2022. Inclusion standards: (1) Acute ischemic stroke confirmed by CT or MRI; (2) Receive standard rt-PA intravenous thrombolytic doses within 4.5 h of AIS diagnosis (0.9 mg/Kg, the maximum dose does not exceed 90 mg) and standard usage (Within 1 min, 10% of the dose is injected intravenously, and within 60 min, the remaining 90% of the dose is pumped continuously by the intravenous route). (3) With complete data and the patients sign the informed consent. Exclusion criteria: (1) bridging artery thrombolysis or endovascular interventional therapy; (2) complicated with acute myocardial infarction, myocardial or valvular disease, myocarditis complications; (3) with a background of chronic renal failure and chronic cardiac failure; (4) A malignant tumor, an endocrine disorder, or a severe autoimmune condition; (5) The patient cannot cooperate with follow-up or other clinical data are incomplete. Informed consent was obtained from all participating patients or their legally authorized representatives. The study was approved by The Ethics Committee of the Affiliated Hospital of Xuzhou Medical University and conducted according to the Declaration of Helsinki. The enrolment flow chart is shown in Fig. 1.
Baseline data collection
Age, gender, BMI, blood pressure (systolic and diastolic) at admission, smoking, and alcohol history, time from onset to intravenous thrombolysis(OTT), medical history (hypertension, diabetes, atrial fibrillation, coronary heart disease, history of stroke or transient ischemic attack), baseline National Institutes of Health Stroke Scale (NIHSS) score, infarct distribution (anterior circulation, posterior circulation, anterior and posterior circulation distribution), symptomatic intracranial hemorrhage (sICH) and other demographic and medical data are all included. Smoking is defined as consuming one or more cigarettes daily for a while longer than a year. Alcohol use is defined as the consumption of 100 g or more of alcohol per day for at least a year [11]. Stroke etiology was classified into four categories by the Trial of Org 10,172 in Acute Stroke Treatment (TOAST) classification [12]. Laboratory data, including NT-proBNP, low-density lipoprotein cholesterol, triglycerides, etc.
Outcome evaluation
According to the European Cooperative Acute Stroke Study (ECASS)-III criteria [13], sICH is defined as intracranial bleeding that results in an increase in NIHSS score of ≥ 4 points after thrombolysis or death. END was characterized as an NIHSS total score rise of ≥ 4 points or the occurrence of new neurological impairments within 24 h following thrombolysis [14]. Depending on whether END happened during hospitalization following intravenous thrombolysis, patients were separated into END and non-END groups. An expert stroke center neurologist kept track of patients three months after discharge through outpatient visits or phone calls to patients and loved ones. A score of ≥ 3 on the modified Rankin Scale (mRS) indicated a bad prognosis, whereas a score of ≤ 2 indicated a good prognosis.
Statistical analysis
The data were analyzed using SPSS 25.0 statistical software. The Shapiro–Wilk test was used to determine whether the measurement data were normal. Measurement data that fit the normal distribution were reported as mean ± standard deviation, and the groups were compared using two independent sample t-tests. While measurement data that did not fit the normal distribution were represented by the median (quartiles), and the non-parametric Mann–Whitney U test was employed to compare groups; frequency and percentage (%) were used to express count data, and Pearson χ2 or Fisher's exact test was employed to compare groups. On NT-proBNP, a natural log transformation [ln(NT-proBNP)] was carried out. Considering the effect of age on patients with AIS, we included age and variables with a P value < 0.05 in univariate analysis in a multifactorial logistic regression model to investigate potential risk factors for END and poor prognosis. Besides, we drew the ROC curve to explore the value of ln(NT-proBNP) and related factors in predicting END and poor prognosis, and the good cut-off value was calculated. P < 0.05 was used to denote a statistically significant difference.
Results
Primary results
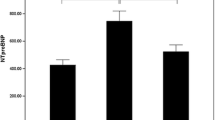
This research comprised 325 AIS patients, with a median age of 68 years (59–76 years) and 208 men (64%), and 117 females (36%). Preliminary findings following intravenous thrombolytic therapy showed that some patients experienced END and poor prognosis. NT-proBNP levels were higher in patients with END and poor prognosis compared to regular patients. The distribution of NT-proBNP was depicted with natural log transformations, which were depicted with median and interquartile range.The distribution of ln (NT-proBNP) levels in patients with END and poor prognosis is shown in Fig. 2. Based on the incidence of END, we divided the patients into the END group (n = 43) and the Non-END group (n = 282). Compared to patients without END, the NIHSS score, NT-proBNP, and sICH ratios in END patients were considerably higher, and the changes in infarct distribution and fasting blood glucose (FBG) were statistically significant (P < 0.05). As indicated in Table 1, other indicators did not alter significantly (P > 0.05) during the same period.
Furthermore, multivariable binary Logistic regression analysis included age and variables with a P < 0.05 in univariate analysis. As is shown in Table 2, the results after adjusting for the distribution of age, FBG, and infarction showed that the baseline NIHSS score (OR = 1.059, 95%CI:1.005 ~ 1.117, P = 0.033) and ln(NT-proBNP) (OR = 1.450, 95%CI: 1.072 ~ 1.963, P = 0.016) were the independent influencing factors of END after intravenous thrombolysis in AIS patient.
Secondary results
The three months of patient follow-up data were used to determine the poor and good post-AIS prognosis. Based on the results, the data was divided into poor (n = 98) and good prognosis (n = 227). Age, baseline NIHSS score, NT-proBNP, FBG, and baseline systolic blood pressure were all greater in the group with a poor prognosis than in the group with a good prognosis, as did the proportions of sICH and history of hypertension. The TOAST categorization varied between the two groups, and all variations were statistically significant (P < 0.05). There were no additional indicators with a significant difference (P > 0.05), as demonstrated in Table 3.
Furthermore, multivariate binary logistic regression analysis (MBLR) was conducted for the significant variables (P < 0.05) in the univariate regression analysis. According to the results of the MBLR analysis, the baseline NIHSS score (OR = 1.113, 95%CI: 1.059 ~ 1.169, P < 0.001), sICH (OR = 4.062, 95%CI: 1.182 ~ 13.957, P = 0.026), ln(NT-proBNP) (OR = 1.767, 95%CI: 1.347 ~ 2.317, P < 0.001) and FBG (OR = 1.141, 95%CI: 1.021 ~ 1.275, P = 0.020) were all independently associated with poor prognosis at three months, as demonstrated in Table 4.
In addition, the data was used to perform the receiving operating characteristic (ROC) to identify the predictive values of NT-proBNP along with NHISS and FBG. According to ROC curve analysis, the area under the curve (AUC) of ln(NT-proBNP) for END was 0.689 (95% CI: 0.604 ~ 0.775, P < 0.001), the cut-off value (6.64), the sensitivity (48.84%), and the specificity (84.04%). The AUC of NIHSS for predicting END was 0.671 (95%CI: 0.577 ~ 0.765, P < 0.001), the cut-off value (7.00), the sensitivity (67.44%), and the specificity (63.83%). When the two were combined (AUC 0.718, 95%CI: 0.631 ~ 0.805, P < 0.001) for predicting END, the cut-off value was 0.12, the sensitivity was 72.09%, and the specificity was 63.83%. On the other hand, the cut-off value (7.00), the sensitivity (74.49%), the specificity (74.45%), and the AUC NIHSS was 0.758 (95%CI: 0.698 ~ 0.818, P < 0.001) for the poor prognosis. FBG had an AUC of 0.657 (95%CI: 0.593 ~ 0.721, P < 0.001), the optimum cut-off value was 6.51, sensitivity was 54.08%, and specificity was 68.72%. Furthermore, with a cut-off value of 5.12, a sensitivity of 79.59%, and a specificity of 60.35%, the AUC for ln(NT-proBNP) to predict poor prognosis was 0.735 (95%CI: 0.674 ~ 0.796, P < 0.001) and when combined with NIHSS (AUC 0.780, 95%CI: 0.724 ~ 0.836, P < 0.001), the cut-off value was 0.27, with a sensitivity of 75.51% and specificity of 72.25%. Thus, NT-proBNP, like the NIHSS score, is a reliable predictor of adverse outcomes after intravenous thrombolysis in patients with AIS. When combined with the NIHSS, the model's predictive value can be further improved (Fig. 3).
Discussion
END is a common and prominent clinical problem in AIS patients. Patients with clinical symptoms do not respond to treatment causing their families social and economic burden [5, 14, 15]. Some scholars [16] defined the END as that minor ischemic stroke patients with NIHSS scores ≤ 3 points had a slight change in NIHSS score, such as an increase of ≥ 2 points within 24 h after thrombolysis. However, there is no unified definition of END. Most studies define END as an NIHSS score rise of ≥ 4 points or death within 24 h of receiving rt-PA. It is more reasonable to adopt the minimum NIHSS score rise of ≥ 4 points as the criterion of END because the median NIHSS score of patients with AIS included in this study was 6 points. A meta-analysis [17] that included 11 independent trials used the definition to determine the END is an increase of ≥ 4 points in the NIHSS score from admission to 24 h. Additionally, they reported that the overall incidence of END following thrombolytic treatment was around 11.0% and varied from 3.4% to 28.8%. However, this study showed a strong relationship between the NIHSS score at admission and END (13.2%) occurrence and poor prognosis.
Previous studies [7, 8, 18] have demonstrated a strong correlation between END and poor neurological outcomes in AIS patients. In addition, studies [15, 18, 19] have shown that several variables and mechanisms affect END incidence, resulting in poor prognosis and disability or higher mortality in AIS patients. Common etiologies include sICH, malignant cerebral edema, early recurrent stroke, a collateral circulation disorder, and thrombus enlargement at the primary site. Furthermore, Seners et al. [16] confirmed that malignant cerebral edema and sICH are the leading direct causes of END and poor prognosis. In this study, sICH was an independent factor for END and poor prognosis at 3 months. In addition, our study found that sICH accounted for 30.2% of the END group, similar to the 20% ~ 30% of END reported by other studies [20].
The present study demonstrated that elevated blood NT-proBNP were independently associated with END and poor three-month prognosis in AIS patients. The following aspects may suggest that using NT-proBNP level as a biomarker for predicting END and poor prognosis in AIS patients receiving intravenous thrombolysis is feasible (Fig. 4). Accumulating clinical and experimental evidence [21, 22] shows that brain injury affects the heart and vice versa. The catecholamine surge hypothesis is probably the most widely accepted mechanism of brain–heart interaction. Studies [21, 23] have further demonstrated that catecholamine levels increase in response to ischemic stroke or ischemia and rt-PA-induced cerebral ischemia–reperfusion injury. High plasma catecholamine levels can lead to cardiac dysfunction by acting on cardiac adrenergic receptors to induce hypoxia and cardiomyocyte apoptosis. Ventricular dysfunction causes impaired contractility, which causes volume overload in the ventricles, stretches the ventricular myocytes, and raises NT-proBNP levels. AIS patients with ventricular systolic dysfunction may experience worsening cerebral ischemia and a higher risk of death. In addition, earlier studies [23] have demonstrated a link between neurological abnormalities and impaired cardiac function in AIS patients. Therefore, it is reasonable to assume that NT-proBNP is correlated with END and functional outcomes. Furthermore, Zhang et al. [22] demonstrated an association between high NT-proBNP levels and malignant brain edema and mortality in AIS patients after reperfusion therapy. It has been reported that BNP may play a key role in cerebral edema and cardiac function. Elevated BNP increases the permeability of microvascular endothelial cells, shifting albumin from the intravascular to the interstitial space and reducing reabsorption in extravascular areas, leading to brain edema [24]. In addition, BNP promotes the aggregation of inflammatory cells and increases matrix metalloproteinase 9(MMP-9) activity. This increase is usually associated with opening the blood–brain barrier and may lead to accelerated edema [25]. Moreover, studies [23, 26] have shown that NT-proBNP is released in the brain after brain injury and may pass through the blood–brain barrier, leading to an increase in the level of NT-proBNP in the blood, reflecting brain injury. However, its mechanism is still unclear. Moreover, Kim et al. [27] demonstrated that BNP levels in AIS patients were positively correlated with infarct volume. In addition, cardioembolic stroke and atrial fibrillation can both be predicted by high NT-proBNP levels, according to earlier researches [28, 29]. However, the findings of this study demonstrated that atrial fibrillation and cardioembolism were not independent risk factors for END and poor 3-month outcomes, which is consistent with the observations of Visnja et al. [30].
In addition, previous literature reports that [5, 14, 20] advanced age, NIHSS score, large infarction, hypertension, hyperglycemia, time from onset to thrombolysis, hs-CRP, TOAST classification, etc., closely related to poor prognosis. Further, our study showed that infarction distribution in anterior and posterior circulation significantly correlated with END, suggesting that large infarct size influenced END. The related reason may be that the formation of leptomeningeal collateral circulation in patients with higher NIHSS scores is worse, which is connected to the larger infarct volume, reflecting the severity of stroke [31]. According to a recent study, hyperglycemia on admission was strongly related to a poor prognosis in AIS patients and the results were consistent with our study [32]. Previous studies [6] have reported that various risk factors, such as advanced age and large-artery atherosclerotic stroke, lead to poor outcomes in AIS patients after rt-PA. However, it was statistically non-significant in this study. This might be related to single-center studies, small sample sizes, different designs, and sample heterogeneity. Second, we did not measure NT-proBNP levels continuously. Therefore, we could not observe a relationship between dynamic change in NT-proBNP level and functional outcomes. Future, large-sample, and multicenter clinical studies are needed to verify and generalize.
Conclusions
In summary, this study found that NT-proBNP is an independent risk factor for END and poor prognosis in AIS patients following rt-PA and has a particular predictive value for poor prognosis. The measurement of NT-proBNP may help clinicians improve the early identification of high-risk AIS groups, adjust the treatment plan in time, and improve the clinical prognosis.
Availability of data and materials
Further clinical data are available from the corresponding author upon reasonable request.
Abbreviations
- END:
-
Early neurological deterioration
- FBG:
-
Fasting blood glucose
- sICH:
-
Symptomatic intracranial hemorrhage
- NIHSS:
-
National Institutes of Health Stroke Scale
- TIA:
-
Transient ischemic attack
- TOAST:
-
Trial of Org 10,172 in Acute Stroke Treatment
- LAA:
-
Large-artery atherosclerosis
- SAO:
-
Small-artery occlusion
- CE:
-
cardioembolism
References
Tu WJ, Zhao Z, Yin P, et al. Estimated burden of stroke in China in 2020. JAMA Netw Open. 2023;6(3): e231455.
GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. 2021;20(10):795–820.
Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2019;50(12):344-344e418.
Liu YL, Yin HP, Qiu DH, et al. Multiple hypointense vessels on susceptibility-weighted imaging predict early neurological deterioration in acute ischaemic stroke patients with severe intracranial large artery stenosis or occlusion receiving intravenous thrombolysis[J]. Stroke Vasc Neurol. 2020;5(4):361–7.
Sabir Rashid A, Huang-Link Y, Johnsson M, et al. Predictors of early neurological deterioration and functional outcome in acute ischemic stroke: the importance of large artery disease, hyperglycemia and inflammatory blood biomarkers[J]. Neuropsychiatr Dis Treat. 2022;18:1993–2002.
Marto JP, Lambrou D, Eskandari A, et al. Associated factors and long-term prognosis of 24-hour worsening of arterial patency after ischemic stroke. Stroke. 2019;50(10):2752–60.
Kwan J, Hand P. Early neurological deterioration in acute stroke: clinical characteristics and impact on outcome[J]. QJM. 2006;99(9):625–33.
Geng HH, Wang Q, Li B, et al. Early neurological deterioration during the acute phase as a predictor of long-term outcome after first-ever ischemic stroke[J]. Medicine (Baltimore). 2017;96(51):e9068.
Daniels LB, Maisel AS. Natriuretic peptides[J]. J Am Coll Cardiol. 2007;50(25):2357–68.
Berntsson J, Zia E, Borné Y, et al. Plasma natriuretic peptides and incidence of subtypes of ischemic stroke[J]. Cerebrovasc Dis. 2014;37(6):444–50.
Pan H, Feng K, Fu M, Ge W, Zhou C. Correlation between the B-type Natriuretic Peptide before thrombolysis and prognosis in patients with ischemic stroke. Clin Neurol Neurosurg. 2021;211: 107021.
Adams HP Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment[J]. Stroke. 1993;24(1):35–41.
de LosRíoslaRosa F, Khoury J, Kissela BM, et al. Eligibility for intravenous recombinant tissue-type plasminogen activator within a population: the Effect of the European Cooperative Acute Stroke Study (ECASS) III Trial[J]. Stroke. 2012;43(6):1591–5.
Yu WM, Abdul-Rahim AH, Cameron AC, et al. The incidence and associated factors of early neurological deterioration after thrombolysis: results from SITS registry. Stroke. 2020;51(9):2705–14.
Wang L, Cheng Q, Hu T, et al. Impact of Stress hyperglycemia on early neurological deterioration in acute ischemic stroke patients treated with intravenous thrombolysis[J]. Front Neurol. 2022;13:870872.
Seners P, Baron JC. Revisiting “progressive stroke”: incidence, predictors, pathophysiology, and management of unexplained early neurological deterioration following acute ischemic stroke[J]. J Neurol. 2018;265(1):216–25.
Hou X, Chen W, Xu H, et al. The rate of early neurological deterioration occurring after thrombolytic therapy: A meta-analysis[J]. Brain Behav. 2019;9(2):e01210.
Che F, Wang A, Ju Y, et al. Early neurological deterioration in acute ischemic stroke patients after intravenous thrombolysis with alteplase predicts poor 3-month functional prognosis - data from the Thrombolysis Implementation and Monitor of acute ischemic stroke in China (TIMS-China)[J]. BMC Neurol. 2022;22(1):212.
Ji X, Tian L, Yao S, et al. A systematic review of body fluids biomarkers associated with early neurological deterioration following acute ischemic stroke[J]. Front Aging Neurosci. 2022;14:918473.
Nair SB, Somarajan D, Pillai RK, et al. Predictors of early neurological deterioration following intravenous thrombolysis: difference between risk factors for ischemic and hemorrhagic worsening[J]. Ann Indian Acad Neurol. 2022;25(4):627–33.
Zhao YH, Gao H, Pan ZY, et al. Prognostic value of NT-proBNP after ischemic stroke: a systematic review and meta-analysis of prospective cohort studies[J]. J Stroke Cerebrovasc Dis. 2020;29(4): 104659.
Zhang X, Yan S, Zhong W, et al. Early NT-ProBNP (N-Terminal Probrain Natriuretic Peptide) elevation predicts malignant edema and death after reperfusion therapy in acute ischemic stroke patients[J]. Stroke. 2021;52(2):537–42.
Zhang KJ, Jin H, Xu R, et al. N-Terminal pro-brain natriuretic peptide is associated with hemorrhagic transformation and poor outcomes in patients with stroke treated with intravenous thrombolysis[J]. Front Mol Neurosci. 2021;14: 758915.
Kuhn M. Endothelial actions of atrial and B-type natriuretic peptides. Br J Pharmacol. 2012;166(2):522–31.
Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, Wang X, Lo EH. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nat Med. 2006;12(4):441–5.
Ru D, Yan Y, Li B, et al. BNP and NT-proBNP concentrations in paired cerebrospinal fluid and plasma samples of patients with traumatic brain injury[J]. J Surg Res. 2021;266:353–60.
Kim SH, Lee JY, Park SH, et al. Plasma B-type natriuretic peptide level in patients with acute cerebral infarction according to infarction subtype and infarction volume[J]. Int J Med Sci. 2013;10(1):103–9.
Zhao J, Zhang Y, Yuan F, et al. Diagnostic value of N-terminal pro B-type natriuretic peptide for nonvalvular atrial fibrillation in acute ischemic stroke patients: a retrospective multicenter case-control study[J]. J Neurol Sci. 2020;414:116822.
Faura J, Bustamante A, Reverté S, et al. Blood biomarker panels for the early prediction of stroke-associated complications[J]. J Am Heart Assoc. 2021;10(5): e018946.
Padjen V, Bodenant M, Jovanovic DR, et al. Outcome of patients with atrial fibrillation after intravenous thrombolysis for cerebral ischaemia[J]. J Neurol. 2013;260(12):3049–54.
Zhang K, Luan J, Li C, et al. Nomogram to predict hemorrhagic transformation for acute ischemic stroke in Western China: a retrospective analysis[J]. BMC Neurol. 2022;22(1):156.
Tsivgoulis G, Katsanos AH, Mavridis D, et al. Association of baseline hyperglycemia with outcomes of patients with and without diabetes with acute ischemic stroke treated with intravenous thrombolysis: a propensity score-matched analysis from the SITS-ISTR registry[J]. Diabetes. 2019;68(9):1861–9.
Acknowledgements
We are grateful to all who participated in this study.
Funding
National Health Commission Brain Prevention Committee "Research and Promotion Project of Appropriate Technology Intervention for High-risk Groups of Stroke in China" (GN-2018R0009) and Xuzhou Promoting Science and Technology Innovation Project (KC22241).
Author information
Authors and Affiliations
Contributions
Zhuang Zhu, Bilal Muhammad, Ning Gu, Tian-Yue Meng, and Shu Kan conceived the research, drafted the manuscript, and statistical analysis. Bo Du, Ying-Feng Mu, Yan-Bo Cheng reviewed and revised the manuscript. Shi-Guang Zhu and De-Qin Geng revised the manuscript and designed the study. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The Medical Ethics Committee of the Affiliated Hospital of Xuzhou Medical University approved this study (approval number: XYFY2018-KL038-01). Written informed consent was obtained individually. We confirm that all methods in our study were performed in accordance with the relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhu, Z., Muhammad, B., Du, B. et al. Elevated NT-proBNP predicts unfavorable outcomes in patients with acute ischemic stroke after thrombolytic therapy. BMC Neurol 23, 203 (2023). https://doi.org/10.1186/s12883-023-03222-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-023-03222-6