Abstract
Background
The Dural Arteriovenous Fistulas (DAVFs) secondary to cerebral venous sinus thrombosis (CVST) are rather rare. The aim of present study is to investigate the clinical and radiological features, and treatment outcome of DAVFS in patients following CVST.
Methods
Data about demographic information, clinical presentations, radiological findings, as well as treatment and outcome of DAVFs sequence to CVST were collected to analysis from January 2013 to September 2020 in this retrospective study.
Results
Fifteen patients with DAVFs after CVST were included in the study. The median age was 41 years (range17-76 years). Ten patients (66.67%) were male and 6 patients (33.33%) were female. The median duration of presenting CVST was 182 days (Range 20–365). Mean time from diagnosis of CVST to confirmation of DAVFs was 97 days (range 36–370 days). The most common manifestations of DAVFs following CVST were headache and visual disturbance seen in 7 patients respectively. Five patients had pulsatile tinnitus (%) and 2 had nausea/vomiting. The DAVFs are most frequently located at the transverse/sigmoid sinus (7/15, 46.67%), followed by the superior sagittal the sinus and confluence sinus (6/15, 40.00%) respectively. Angiography of DAVFs revealed Board type I in seven (46.7%) patients, Board type II and III in 4(26.7%) patients, respectively. The Cognard I was noted in seven (46.7%), Cognard IIa and IV in 3 patients, IIb and III in one patient, respectively. The main feeding arteries of DAVFs most commonly originate from the branches of the external carotid artery in 6 (40.0%) patients. The other DAVFs are conjointly supplied by multiple feeders from internal and external carotid artery and vertebral arteries. Fourteen (93.33%) patients were treated with endovascular embolization and none of the patients had permanent deficits during follow-up.
Conclusion
Intracranial DAVFs following CVST are rare presentations. Most patients have a good outcome after timely interventional therapy. Continued observation and follow-up of (DSA) are important to find DAVFs secondary to CVST.
Similar content being viewed by others
Background
Intracranial dural arteriovenous fistulas (DAVFs) are pathologic shunts between the pachymeningeal arteries and dural venous sinuses and/or cerebral veins [1, 2]. DAVFs represent 10 − 15% of all intracranial cerebrovascular malformations. The exact etiology of the DAVFs remains completely uncertain. They are thought to be acquired and may be related to intracranial venous stenosis or sinus thrombosis, venous hypertension, traumatic head injury, brain surgery, inflammation, tumor, and other factors [3, 4].
Despite there is a clear link between CVST and DAVFs. However, whether CVST is the cause or the result of DAVFs is still controversial [5]. Most investigators believe that CVST can appear during DAVFs, CVST is a result of DAVFs [6]. Nevertheless, there are also a few reports considered that DAVFs can be secondary to CVST [7, 8]. Under rare circumstances, patients with DAVFs have been reported to occur after CVST due to venous hypertension caused by impaired venous outflow [9, 10].
The clinical presentations are not specific and vary with the location and the venous drainage pattern of DAVFs. DAVFs can present with ocular symptoms, headache, tinnitus, and neurologic deficits [11, 12]. The definite diagnosis of DAVFs depends on DSA.
Treatment options of DAVFs are based on their expected clinical course and patient status. DAVFs treatments include conservative management, selective endovascular treatment, surgical ligation as well as stereotactic radiosurgery [11, 13].
The development of DAVFs following CVST is uncommon. To date, few studies involving DAVFs cases after CVST have been published [4,5,6]. Low occurrence of DAVFs secondary to CVST results in limited data available to understand the clinical characteristics of these lesions. In the light of this, we presented a new cohort of CVST patients with concomitant DAVFs retrospectively. We described all the retrieved clinical features, angiographic findings, as well as therapeutic outcomes from 15 CVST patients with complicated with DAVFs for updating purposes.
Methods
Study population and baseline data collection
This study was approved by the Ethics Committee and Institutional Review Board of Xuanwu Hospital of Capital Medical University. All patients or legal guardians provided informed consent for participation. Data supporting this study are available from the first author upon reasonable request.
We included patients with confirmed cranial DAVFs who were initially admitted to the Xuanwu Hospital of Capital Medical University because of CVST from January 2013 to September 2020. The patients’ records were reviewed retrospectively for detailed data about demographic characteristics, clinical presentations, imaging results, as well as treatment and outcome. CVST was diagnosed according to international guidelines [14]. The diagnosis of CVST and DAVFs was confirmed by DSA. Individual DAVFs were categorized according to the Borden classification [15] and the Cognard classification [16]. The follow-up period was calculated from the time of initiation of treatment to the last visit or death. Good outcome was defined as modified Rankin scale (mRS) of 0–2.
Results
Patient characteristics
The total of 308 patients diagnosed CVST were searched from the database of our hospital. Twenty-two patients with suspected DAVFs and CVST were reviewed. Fifteen patients confirmed presence of CVST and DAVFs and were included in the analysis. The remaining 7 patients were excluded due to coexistence DAVF with CVST on admission (n = 4) and incomplete data (n = 3). The features of DAVFs following CVST summarized in Table 1. Overall, 15 patients with DAVFs secondary to CVST were included, which definite diagnosis by DSA. The median age at the time of diagnosis was 41 years (range17-76 years). Nine patients (60.0%) were male and 6 patients (40.0%) were female. The median duration of presenting CVST was 182 days (Range 20–365). The detail clinical data of all patients are shown in Table 2.
CVST characteristics
The most common presentation of CVST was headache reported in 8 (53.33%) patients. The followed clinical presentations included diplopia or visual difficulty in 6 (40.00%), nausea/vomiting in 5 (33.33%), limb weakness, and tinnitus in 2 (13.33%) respectively. Less common symptoms such as ophthalmodynia, dysarthria, unsteady gait, seizure were also noted. Papilledema was present in 9 (60.00%) patients.
The most common locations of CVST were the superior sagittal and the transverse sinus in 10 patients (66.67%) respectively, followed by the sigmoid sinus in 8 (53.33%) and the confluence of sinuses in 5 (33.33%) on radiologic evaluation. Also, the straight sinus and the jugular vein thrombosis were seen in 2 (13.33%) patients. Only one patient displayed cortical venous thrombosis.
DAVFs characteristics
The mean time from diagnosis of CVST to confirmation of DAVFS was 97 days (range 36–370 days). Five (33.33%) patients demonstrated the formation of DAVFS between 3 and 5 months after CVST. There were 4 (26.66%) cases that confirmed DAVFs between 6 and 11 months. Six (40.00%) patients developed DAVFs more than one year following CVST.
When these CVST patients were diagnosed with DAVFS, the most common manifestations of DAVFs were headache and visual disturbance seen in 7 patients respectively. Five patients had pulsatile tinnitus (%) and 2 had nausea/vomiting. Other presentations included varicose veins in the scalp, ophthalmodynia, exophthalmos, dysarthria, unsteady gait, sensory disturbance.
A total of 10 patients (66.67%) completed lumbar puncture and increased intracranial pressure (> 180 mmH2O) was observed in these patients at the time of occurrence of DAVFs. The cytological and biochemical results of cerebrospinal fluid (CSF) were normal.
Anatomic location and type of DAVFs
The DAVFs are most frequently located at the transverse/sigmoid sinus (7/15, 46.67%), followed by the superior sagittal sinus and confluence of the sinus (6/15, 40.00%) respectively. Other locations included occipital sinus and jugular foramen in one patient each.
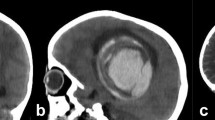
Angiographic classification of DAVFs included Board type I in seven (46.7%) patients, Board type II and III in 4 (26.7%) patients, respectively. According to Cognard’s method, 7 DAVFs (46.7%) were categorized as Cognard I, Cognard IIa, and IV in 3 patients, IIb and III in one patient, respectively. The main feeding arteries of DAVFs most commonly originate from the branches of the external carotid artery (ECA) in 6 (40.0%) patients. The other DAVFs are conjointly supplied by multiple feeders from the internal carotid artery (ICA) and external carotid artery, external carotid and vertebral arteries (VBA), or ECA, ICA, and VBA in 3 (20.0%) patients, respectively. Brain MRI and DSA images of case 3 are presented in Fig. 1.
Brain MRI showed abnormal signal in bilateral cerebral hemisphere. a, b, c: T2-, FLAIR, DWI-weighted images, showing hyperintensity, respectively. The venous phase of DSA (d) showed poor visualization of superior sagittal sinus, left transverse sinus and left sigmoid sinus were not indistinct (red arrows); The follow-up of DSA 3 months later (e) revealed a DAVF in the region of left lateral sinus supplied by the left occipital artery (red arrows); DAVF disappeared completely after embolization (f)
Treatments and outcome
Fourteen (93.33%) patients were treated with endovascular embolization with Onyx, of which 4 (28.57%) patients were incompletely embolized. Only one patient received transverse sinus stenting, and observational follow-up was selected for DAVFs. Angiographic and clinical follow-up was available for all patients. The mean follow-up period was 17 range 8–36 months) months. All patients achieved a good outcome according to the mRS: were asymptomatic (mRS = 0) and slight symptom (mRS = 1) in 6 (46.2%) patients, respectively, mild disability (mRS = 2) in 2 cases. One patient identified new DAVFs in the middle of the superior sagittal sinus during the observation period, and embolization was not performed because it was asymptomatic. None of the patients had permanent deficits and recurrence during follow-up (Table 2).
Discussion
Development of DAVFs subsequent to CVST is a rare chronic complication that should not be ignored. This cohort of 15 patients with DAVFs after CVST adds to the knowledge of this rare chronic condition. Our present study demonstrated: DAVFs can occur as the result of previously CVST and was a delayed consequence. In accordance with literature reports, the location of DAVFs most frequently involved the transverse/sigmoid sinus (46.67%). The most common clinical presentations of DAVFs were headache and visual disturbance (46.67%). DAVFS was predominantly benign without cortical venous return (66.67%) and had a good prognosis after embolization.
Intracranial dural arteriovenous fistulas (DAVFs) are abnormal anastomoses between meningeal arteries and dural venous sinuses and/or cerebral veins. The specific etiology of the DAVFs remains uncertain. DAVFs are thought to be acquired lesions and are mainly related to intracranial venous stenosis or sinus thrombosis, venous hypertension, trauma, brain surgery, inflammation, tumor, and other factors [11, 12].
Clinical findings show that CVST is closely related to DAVFs. CSVT and DAVFs share many similar epidemiologic and etiologic features, and nonspecific clinical manifestations. However, whether sinus thrombosis is a consequence or a cause of DAVFs is still not fully understood [17, 18]. Some researchers believed that CVST is likely a secondary event after DAVFs [6]. Nevertheless, others suggested that DAVFs are likely a delayed complication of CVST [7, 8], which was consistent with our findings. According to our patient’s DSA, no evidence of any detectable vascular malformation was found when patients were initially diagnosed with CVST. After anticoagulant therapy, the symptoms of most CVST patients were relieved and the imaging of CVST was improved. The new presence of DAVFs was confirmed only after the occurrence of new symptoms such as tinnitus, headache, and ocular symptoms, or DSA was performed after a follow-up period of 3–36 months. DAVFs were caused by CVST. Therefore, CVST precedes and contributes the causative factor in DAVFs formation.
The exact pathogenesis involved in the development of DAVFs after CVST is complex. The venous pressure and drainage pattern have been proposed to be the crucial pathogenic factor of DAVFs formation following CVST [10]. CVST leads to venous outflow obstruction and elevated venous pressure. The continued venous hypertension promoted the enlargement of physiological shunts between the dural artery and venous sinus, and induced angiogenesis [9, 19], which then initiated abnormal arteriovenous communications.
DAVFs often developed in the chronic stage of CVST, and there were few acute cases reported [8]. DAVFs generally developed as early as 3 months after CVST and are located in the same or adjacent site of the thrombosed sinus. Even after systematic anticoagulant therapy, it couldn’t completely prevent the occurrence of DAVFs following CVST. Once CVST patients experience headaches, visual disturbances, especially pulsatile tinnitus during follow-up, clinicians should be alert to the possibility of DAVFs secondary to CVST. However, it should also be noted that DAVFs can be asymptomatic in some cases. In general, the clinical signs and symptoms are mainly related to the location of DAVFs and the pattern of venous drainage [20].
Due to the relatively rare and chronic course, the diagnostic workup is often delayed. These observations also suggest that the follow-up time of CVST should be long enough, at least 3 or more, to have a chance to detect whether new DAVFs occur, and follow-up of DSA is also necessary [21]. DSA can clearly show the location and the numbers of fistulas, the source and number of supply arteries, and the direction of venous drainage. It is still the gold standard for diagnosing DAVFs. It is still the gold standard imaging modality for the diagnosis and classification of DAVFs, especially for CVST combined with DAVFs.
Most of the patients in this study received endovascular therapy, and all patients achieved a good outcome, even including those who did not receive endovascular treatment. None of the patients had permanent deficits and recurrence during follow-up. Treatment strategies for DAVFs includes endovascular treatment, which can be selective transarterial or transvenous embolization, intrasinus stenting, surgical ligation, stereotactic radiosurgery, as well as conservative treatment [11, 13].
Endovascular approaches are nowadays the first-line therapy for most of DAVFs, but the appropriate treatment should determine based on the patient’s clinical presentation, type of lesion, medical comorbidities, and impact on quality of life [3, 13, 22]. Those that are high-grade with cortical venous drainage or symptomatic are candidates for an intervention. In fact, some patients with low grade but symptomatic DAVF also receive interventional treatment. Follow-up observation is reasonable for asymptomatic DAVFs or those without cortical venous drainage.
Limitations
The current study also had its limitations. This is retrospective research, and there may exist selective bias. The small case series, limited follow-up time, and lack of a control group prevent further statistical analysis. However, the study represents a “real-world” cohort, the clinical data and treatment outcomes contribute to raise awareness and to improving the management of DAVFs.
Conclusion
DAVFs could be a rare chronic consequence of CVST. The clinical presentations are not specific, and DSA is the gold standard for diagnosis of DAVFs. Most patients with DAVFs have a good outcome after timely interventional therapy. Therefore, continued clinical observation and follow-up of DSA is important to find whether DAVFs occur.
Data Availability
The raw data s and materials related to this article are available from the corresponding author upon reasonable request.
Abbreviations
- CVST:
-
Cerebral venous sinus thrombosis
- DAVFs:
-
Dural arteriovenous fistulas
- DSA:
-
Digital subtraction angiography
- MRI:
-
Magnetic resonance imaging
- ECA:
-
External carotid artery
- ICA:
-
Internal carotid artery
- VBA:
-
Vertebral arteries
- SSS:
-
Superior sagittal sinus
- SiS:
-
Sigmoid sinus
- TS:
-
Transverse sinus
- SS:
-
Straight sinus
- CS:
-
Confluence of sinuses
- ISS:
-
Inferior sagittal sinus
- JV:
-
Jugular vein
- CV:
-
Cortical vein
- SCV:
-
Superficial cerebral vein
- AphA:
-
Ascending pharyngeal artery
- MHT:
-
Meningohypophyseal trunk
- OccA:
-
Occipital artery
- PMA:
-
Posterior meningeal artery
- PAA:
-
Posterior auricular artery
- MMA:
-
Middle meningeal artery
- STA:
-
Superficial temporal artery
- PCA:
-
Posterior cerebral artery
- SCA:
-
Superior cerebellar artery
- ACA:
-
Anterior cerebral artery
- PMA:
-
Pia meningeal artery
References
Hou K, Ji T, Guo Y, et al. Current status of Endovascular Treatment for Dural Arteriovenous Fistulas in the Superior Sagittal Sinus Region: a systematic review of the literature. World Neurosurg. 2019;122:133–43.
Reynolds MR, Lanzino G, Zipfel GJ. Intracranial Dural Arteriovenous Fistulae Stroke. 2017;48:1424–31.
Gandhi D, Chen J, Pearl M, et al. Intracranial dural arteriovenous fistulas: classification, imaging findings, and treatment. AJNR Am J Neuroradiol. 2012;33:1007–13.
Hamada Y, Goto K, Inoue T, et al. Histopathological aspects of dural arteriovenous fistulas in the transverse-sigmoid sinus region in nine patients. Neurosurgery. 1997;40:452–6.
Pierot L, Chiras J, Duyckaerts C, et al. Intracranial dural arteriovenous fistulas and sinus thrombosis: report of five cases. J Neuroradiol. 1993;20:9–18.
Kannath SK, Rajan JE, Mukherjee A, et al. Factors Predicting spontaneous thrombosis of aggressive cranial dural arteriovenous fistulas. World Neurosurg. 2017;103:821–828e2.
Micieli JA, Derkatch S, Pereira VM, et al. Development of dural arteriovenousfistulas after cerebral venous sinus thrombosis. J euroophthalmol. 2016;36:53–7.
Chen JG, Li ZX, Zhang DF, et al. Cerebral venous sinus thrombosis complicated with acute developmentof dural arteriovenous fistula: a case report. J Clin Neurosci. 2017;44:225–6.
Uranishi R, Nakase H, Sakaki T. Expression of angiogenic growth factors in dural arteriovenous fistula. J Neurosurg. 1999;91:781–6.
Kusaka N, Sugiu K, Katsumata A, et al. The importance of venous hypertension in the formation of dural arteriovenous fistulas: a case report of multiple fistulas remote from sinus thrombosis. Neuroradiology. 2001;43:980–4.
Baharvahdat H, Ooi YC, Kim WJ, et al. Updates in the management of cranial dural arteriovenous fistula. Stroke Vasc Neurol. 2019;5:50–8.
Corbelli I, De Maria F, Eusebi P, et al. Dural arteriovenous fistulas and headache features: an observational study. J Headache Pain. 2020;21:6.
Signorelli F, Della Pepa GM, Sabatino G, et al. Diagnosis and management of dural arteriovenous fistulas: a 10 years single-center experience. Clin Neurol Neurosurg. 2015;128:123–9.
Saposnik G, Barinagarrementeria F, Brown RD, et al. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:1158–92.
Borden JA, Wu JK, Shucart WA. A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg. 1995;82:166–79.
Cognard C, Gobin YP, Pierot L, et al. Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology. 1995;194:671–80.
Nishijima M, Takaku A, Endo S, et al. Etiological evaluation of duralarteriovenous malformations of the lateral and sigmoid sinuses based on histopathological examinations. J Neurosurg. 1992;76:600–6.
Elhammady MS, Ambekar S, Heros RC. Epidemiology, clinical presentation, diagnostic evaluation, and prognosis of cerebral dural arteriovenous fistulas. Handb Clin Neurol. 2017;143:99–105.
Terada T, Higashida RT, Halbach VV, et al. Development of aquired arteriovenous fistula in rats due to venous hypertension. J Neurosurg. 1994;80:884–9.
Melo Neto JF, Pelinca da Costa EE, et al. Cerebral venous drainage in patients with dural arteriovenous fistulas: correlation with clinical presentation. J Neurosurg. 2020;13:1–9.
Ferro JM, Coutinho JM, Jansen O, et al. Dural Arteriovenous Fistulae after cerebral venous thrombosis. Stroke. 2020;51:3344–7.
Chen CC, Cho YD, Yoo DH, et al. Endovascular management of multiple intracranial dural arteriovenous fistulas. J Neuroradiol. 2019;46:390–7.
Acknowledgements
We appreciate all patients and physicians for their cooperation in this project.
Funding
This study was supported by the Capital Medical University in 2017 (17JL-02) and the National Key Research and Development Program of China (2016YFC1300600; No. 2016YFC0901004).
Author information
Authors and Affiliations
Contributions
Xiaoqin Huang: manuscript drafting and revision, and study concept and design. Huixin Shen: collected and analyzed the data, drafted and revised the manuscript. Chunqiu Fan and Jian Chen: collection, assembly, and interpretation of the data. Ran Meng: manuscript revising and supervised the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee and Institutional Review Board of Xuanwu Hospital, Capital Medical University (Beijing, China) based on the guidelines of the 1964 Declaration of Helsinki. Informed consent was obtained from all patients or their legal guardians before participation.
Consent for publication
Not applicable.
Competing interests
All authors declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Huang, X., Shen, H., Fan, C. et al. Clinical characteristics and outcome of dural arteriovenous fistulas secondary to cerebral venous sinus thrombosis: a primary or secondary event?. BMC Neurol 23, 131 (2023). https://doi.org/10.1186/s12883-023-03141-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-023-03141-6