Abstract
Background
Spinal Muscular Atrophy (SMA) is characterized by progressive and predominantly proximal and axial muscle atrophy and weakness. Respiratory muscle weakness results in impaired cough with recurrent respiratory tract infections, nocturnal hypoventilation, and may ultimately lead to fatal respiratory failure in the most severely affected patients. Treatment strategies to either slow down the decline or improve respiratory muscle function are wanting.
Objective
The aim of this study is to assess the feasibility and efficacy of respiratory muscle training (RMT) in patients with SMA and respiratory muscle weakness.
Methods
The effect of RMT in patients with SMA, aged ≥ 8 years with respiratory muscle weakness (maximum inspiratory mouth pressure [PImax] ≤ 80 Centimeters of Water Column [cmH2O]), will be investigated with a single blinded randomized sham-controlled trial consisting of a 4-month training period followed by an 8-month open label extension phase.
Intervention
The RMT program will consist of a home-based, individualized training program involving 30-breathing cycles through an inspiratory and expiratory muscle training device. Patients will be instructed to perform 10 training sessions over 5–7 days per week. In the active training group, the inspiratory and expiratory threshold will be adjusted to perceived exertion (measured on a Borg scale). The sham-control group will initially receive RMT at the same frequency but against a constant, non-therapeutic resistance. After four months the sham-control group will undergo the same intervention as the active training group (i.e., delayed intervention). Individual adherence to the RMT protocol will be reviewed every two weeks by telephone/video call with a physiotherapist.
Main study parameters/endpoints
We hypothesize that the RMT program will be feasible (good adherence and good acceptability) and improve inspiratory muscle strength (primary outcome measure) and expiratory muscle strength (key secondary outcome measure) as well as lung function, patient reported breathing difficulties, respiratory infections, and health related quality of life (additional secondary outcome measures, respectively) in patients with SMA.
Discussion
RMT is expected to have positive effects on respiratory muscle strength in patients with SMA. Integrating RMT with recently introduced genetic therapies for SMA may improve respiratory muscle strength in this patient population.
Trial registration
Retrospectively registered at clinicaltrial.gov: NCT05632666.
Similar content being viewed by others
Background
Spinal muscular atrophy (SMA) is a severe neuromuscular disease caused by a homozygous deletion of the survival motor neuron-1 (SMN) gene [1,2,3,4], which leads to cellular SMN protein deficiency. SMA has an incidence of about 1 in 6000–12,000 live births [5]. The main characteristic of SMA is the degeneration of alpha motor neurons in the anterior horns of the spinal cord, resulting in progressive muscle weakness of axial muscles and muscles of the arms and legs with a mild to severely reduced life expectancy in the majority of patients [6, 7]. SMA is classified into four types based on age at onset and highest acquired motor milestone [2, 4, 8,9,10,11]. In the last few years, SMN-augmenting genetic therapies have been introduced, including SMN-gene therapy (Zolgensma®) and therapies that modify SMN2-splicing (Spinraza® and Risdiplam) [12]. Efficacy studies have demonstrated, on average, improved motor function, survival, and overall muscle strength, but the respiratory outcomes vary, with most studies showing no significant improvement in lung function parameters in patients with SMA types 2 and 3 [13,14,15,16].
Respiratory problems are among the principal challenges in clinical care for patients with SMA [6]. Weakness of respiratory muscles requires daily interventions and thereby profoundly affects quality of life [8, 17,18,19]. Progressive decline of vital capacity and cough strength causes respiratory failure in virtually all children with SMA type 1 [20,21,22]. In more chronic types of SMA (type 2 and type 3), weakness or dysfunction of the respiratory musculature leads to severe respiratory complications [20,21,22]. These include reduced cough strength and poor secretion clearance resulting in recurrent respiratory tract infections, reduced chest wall and pulmonary compliance with restrictive lung function decline, alveolar hypoventilation, and, finally, chronic respiratory failure leading to premature death [20,21,22].
In healthy subjects and patients with pulmonary diseases, kyphoscoliosis, or Duchenne’s Muscular Dystrophy, respiratory muscle training (RMT) has been shown to improve respiratory muscle strength as well as endurance [23,24,25,26]. Little is known, however, about therapeutic benefits of RMT in patients with SMA. A pre-experimental study in three children with SMA showed that inspiratory muscle training was safe, feasible and acceptable and improved inspiratory muscle strength and peak inspiratory flow [27]. Importantly, none of these children had received any form of SMN-augmenting therapy that has also been shown to exert positive effects on overall muscle strength in some patients with SMA [24].
To further investigate treatment efficacy of RMT in SMA, we have designed a randomized controlled trial to study the efficacy of a 4-month home-based RMT program in patients with SMA including patients that were recently started on SMN-augmenting therapy.
Methods
Aim
The aim of this study is to assess the feasibility and efficacy of respiratory muscle training (RMT) in patients with SMA and respiratory muscle weakness. We hypothesize that an individualized incremental home-based RMT program will be feasible and may improve inspiratory muscle strength, expiratory muscle strength, lung function and patient reported breathing difficulties in patients with SMA.
Study setting
We will conduct this study at the outpatient department of the Netherlands SMA center, and the Child Development and Exercise Center at the University Medical Center Utrecht (UMCU), The Netherlands. All members of the study team, consisting of physicians, physiotherapists, lung function technicians, clinical exercise physiologists and nurses, have broad experience with SMA due to the national cohort study that is carried out in this center since 2010 [28].
Study design
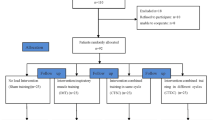
The study protocol was designed using the recommendations of the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidelines [29]. The RESISTANT study is an investigator-initiated, monocenter study consisting of two parts (see Fig. 1).
Part 1 (0–4 months): a single blinded randomized sham-controlled trial (RCT)
In the first part of the study, we will determine the feasibility and efficacy of respiratory muscle training (RMT) in patients with SMA. The active treatment group will receive inspiratory muscle training starting at a therapeutic intensity of 30% of maximum inspiratory mouth pressure (PImax) and expiratory muscle training starting at a therapeutic intensity of 30% of maximum expiratory mouth pressure (PEmax) for 4 months [30]. The sham-control group will receive the same training protocol but with a low (10% of PImax and PEmax) non-therapeutic intensity. Both groups will receive supervision through two-weekly telephone/video calls with a physiotherapist.
Patients will be stratified prior to randomization based on PImax (group 1: PImax < 60 Centimeters of Water Column [cmH2O], group 2: PImax ≥ 60 cmH2O, 60 cmH2O was the median PImax in the group of patients used for the natural history study [31]) and then randomly allocated to the intervention or sham-control group. We will use a variable block randomization method with allocation concealment in a centralized system for randomization. The lung function analyst is blinded for treatment allocation. A data analyst (RvE) will design and sign the data analysis plan in advance. The data will be analyzed according to the analysis plan by a physiotherapist (KK) who is not blinded for treatment allocation. The physiotherapists who will perform the two-weekly telephone calls (KK and EH) are not blinded for treatment allocation. Patients will know that there are two treatment groups, and they are informed that it is not yet known which treatment is most effective.
Part 2 (5–12 months): open label extension phase
In the second part of the study, the sham-control group will be provided with a supervised RMT at a therapeutic intensity of 30% of PImax and PEmax and we will explore the long-term effects of RMT on the occurrence of respiratory infections, health related quality of life and feasibility in the active treatment and sham-controlled group.
Participants will visit our outpatient department every 4 months for 12 months after inclusion for assessment of primary and secondary outcome measures. This study is currently ongoing; the first participant was included on 2–2-2021. We expect study completion in the first quarter of 2023.
Participants
Recruitment
We will recruit patients with SMA from the Dutch national SMA registry, that contains detailed clinical data of more than 400 patients [11].
Eligibility criteria
Patients with SMA (any type) will be invited to participate. Inclusion criteria are:
-
Age ≥ 8 years;
-
Respiratory muscle weakness (PImax ≤ 80 cmH2O [32]);
-
Maintenance dose (≥ 2 months) Spinraza® or (≥ 2 months) Risdiplam or no treatment;
-
Given oral and written informed consent when ≥ 16 years old and additional informed consent by the parents or legal representative if the participant is < 16 years old.
Exclusion criteria are:
-
Inability to perform respiratory and/or lung-function testing;
-
Inability to understand Dutch or English;
-
A history of pneumothorax or symptomatic low cardiac output syndrome;
-
Treatment period < 2 months of Spinraza® or Risdiplam.
Sample size
Based on a previous report on inspiratory muscle training in patients with neuromuscular diseases (n = 27, 18 patients with Duchenne Muscular Dystrophy [DMD] and 9 patients with SMA) [17] indicating a mean improvement in PImax of 28 cmH2O difference (Standard Deviation [SD] ± 26.27), we assume a mean difference between active and sham-treated patients after 4 months of 20 cmH2O (SD 25.0). To detect this effect size with 80% power and two-sided alpha of 5%, 50 patients are needed (25 per group).
Intervention
Inspiratory muscle training
For the inspiratory muscle training (IMT) we use the POWERbreathe KHP2 [33]. Clinical research has shown high participant motivation and adherence to training with the POWERbreathe KHP2 thanks to the on-screen feedback [34]. Furthermore, healthcare professionals can review participant progress by tracking up to 30 of the participants training sessions which the KHP2 is able to store. This data can be scrolled through to monitor progress. The electronic, variable, tapered flow valve ensures maximum training benefit. It is easy to use, easy to clean and training improvements can be easily monitored [33].
Expiratory muscle training
For the expiratory muscle training (EMT) we use the Threshold Inspiratory Muscle Trainer (IMT) (Philips Respironics) in reverse. Use of the Threshold Positive Expiratory Pressure (PEP) (Philips Respironics) is one method to perform EMT. However, the maximal expiratory resistance of the Threshold PEP is limited to 20 cmH20 [35]. To overcome this limitation in expiratory resistance, we chose the reverse use of the Threshold IMT [35,36,37]. This device contains, at its end, a valve closed by the positive pressure of a spring, which can be graded from 9 to 42 cmH2O and allows resistance changes by 1 cmH2O increments. The reverse Threshold IMT has a one-way spring-loaded valve, that closes during expiration and requires that participants exhale hard enough, to open the valve and let the air go out. This device provides constant pressure for expiratory muscle training, regardless of how quickly or slowly the participant breathes, and the optimal loading pressure can be adjusted, based upon the individual characteristics of the participant [35, 37, 38].
Participant timeline
The study schedule is presented in Table 1. Before the first visit, participants will be recruited for enrollment by a research nurse. Patients who express interest in participating receive a patient information letter and an appointment with the physiotherapist. At the first visit (M0), the physiotherapist further determines whether patients are eligible for participation. After signing the informed consent form, participants are weighed and their length is determined, followed by lung function tests. If PImax > 80 cmH2O, participants are excluded for the study. If PImax ≤ 80 cmH2O, participants will be stratified (group 1 < 60 cmH2O, group 2 ≥ 60 cmH2O) and then randomly allocated to either the active treatment group or the sham-controlled group.
All participants (and parents) will be instructed by a trained physiotherapist on the use of both devices at the first visit (M0). Participants are instructed to aim for 10 training sessions per week, divided over 5 to 7 days. A minimum of 6 hours in between training sessions is recommended. Per training session, the participant breathes 30 times through the POWERbreathe and 30 times through the reverse Threshold IMT. If necessary, the participant may take a break, with a maximum of 60 seconds after 10 or 15 breaths. After each session they fill in a diary, which contains information about the intensity of the training and the perceived exertion (Borg scale 0–10).
In the active treatment group, the intensity of the training is set at M0 at 30% of PImax and PEmax and will be increased or decreased based on level of perceived exertion. Participants are instructed to increase the intensity with 1–5 cmH2O if they score a perceived exertion of 0–4 and decrease the intensity if they score a perceived exertion of 7–10. If they score a perceived exertion of 5 or 6, the intensity will not be adjusted. The intensity of the training in the sham-controlled group will be set at M0 at 10% of PImax and PEmax and will remain the same during the first 4 months of training. After 4 months we will provide the sham-controlled group with the same training regime as the active treatment group.
Data collection
Baseline measures
We will record the following baseline data: gender, age, SMA type, number of SMN2 copies, type of SMN augmenting therapy, use of other medication, co-morbidities, ambulatory level according to the modified Hoffer classification [39], use of ventilatory support and use of Airway Clearance Techniques (ACT) (Airstacking [AS] or Mechanical insufflation-exsufflation [MI-E]).
Outcome measures
This study investigates the feasibility and efficacy of respiratory muscle training in patients with SMA. The lung function analysts, who are blinded for treatment allocation, administer the questionnaires (health related quality of life, Medical Research Council [MRC] dyspnea and dyspnea immediately after lung function measure), perform lung function tests, and measure respiratory muscle strength, in a fixed order.
Feasibility
Feasibility will be determined based on adherence and acceptability. Adherence is defined as the completion rate of the estimated number of training sessions over 4 months (≥ 80% of the participants have fulfilled the prescribed treatment = good adherence). Adherence will be monitored by a patient diary, two weekly telephone- or video calls with a physiotherapist and the number of training sessions in the POWERbreathe KHP2. Acceptability is defined as the willingness to continue the training (≥ 5 = good acceptability) and will be assessed with a Borg Scale (0–10) at M4, M8 and M12 by the physiotherapist.
Efficacy: primary and key secondary outcome measure
PImax and PEmax
To measure the efficacy, we will examine changes in respiratory performance over time in both groups. Measurements of PImax and PEmax is a simple assessment of global respiratory muscle strength in a clinical setting and the test is responsive to evaluate changes within subjects. PImax and PEmax in kiloPascal (kPa) is assessed conform the European Respiratory Society/American Thoracic Society (ERS/ATS) recommendations [32]. PImax and PEmax will be converted to cmH2O by multiplying the value in kPa by 10.197. Reference values of Wilson et al. [40] will be used to calculate % of predicted.
Efficacy: secondary outcome measures
To additionally investigate the effect of the respiratory muscle training on daily life functioning, lung function and respiratory infections we use the following measures:
Health related quality of life
Health related quality of life will be measured with the 36-item Short Form Health Survey (SF36) for adults and the Pediatric Quality of Life Inventory (PedsQL) for children. The SF36 Health Survey is composed of 36 questions and standardized response choices, organized into eight multi-item scales: physical functioning (PF), role limitations due to physical health problems (RP), bodily pain (BP), general health perceptions (GH), vitality (VT), social functioning (SF), role limitations due to emotional problems (RE), and general mental health (MH). All raw scale scores are linearly converted to a 0 to 100 scale, with higher scores indicating higher levels of functioning or well-being [41]. The scores of the different scales will be summarized into a physical component summary (PCS) and a mental component summary (MCS) [42]. The PedsQL generic score scale consists of 23 items and has a child self-report format for ages 5–7, 8–12, and 13–18 years. The items are scored on a five-point Likert-scale, ranging from ‘never a problem’ to ‘almost always a problem’ (corresponding scores 100, 75, 50, 25 or 0) and are organized into four multidimensional scales: physical functioning, emotional functioning, social functioning, and school functioning and three summary scores: total scale score, physical health summary score, and psychosocial health summary score. A higher PedsQL score indicates a better quality of life [43].
Lung function
Lung function testing includes spirometry with measurements of upright (forced) vital capacity ([F]VC) in liters, peak expiratory flow (PEF) in liters per second, forced expiratory volume in 1 s (FEV1) in liters, peak cough flow (PCF) in liters per second, sniff nasal inspiratory pressure (SNIP) in cmH2O and mouth occlusion pressure at 100 ms during quiet breathing (P0.1) in kPa. P0.1 is intended to measure the actual central respiratory drive [44]. P0.1/PImax is the ratio between the respiratory drive and the capacity of the inspiratory muscles and have been suggested as important predictor of impending respiratory muscle fatigue (work of breathing) [44]. Lung function is assessed conform the ERS/ATS recommendations [32]. Global lung function reference equations for VC [45], FVC and FEV1 [46], PEF [47, 48], and P0.1 [44, 49] will be used to calculate % of predicted.
For the use of the reference equations, height in centimeters and weight in kilogram will be needed. Height is assessed using the ulna method [50]. This method is useful for determining the height of wheelchair bound patients and those with curvature of the spine. Weight will be measured with a passive floor lift (Maxi Move, type Arjo).
Patient reported breathing difficulties
Patient reported impact of breathing difficulties will be measured with the Medical Research Council (MRC) dyspnea scale. The dyspnea scale has been in use for many years for grading the effect of breathlessness on daily activities. This scale measures perceived respiratory disability. The MRC dyspnea scale is simple to administer as it allows the patients to indicate the extent to which their breathlessness affects their mobility [51, 52]. Dyspnea immediately after lung function measure and after each training session is measured with a Borg scale ranging from 0–10.
Respiratory infections
Respiratory infection frequency (based on the need for antibiotics and/or hospitalization) will be assessed during the two weekly telephone consultations and during each visit by the physiotherapist. In case of uncertainties the general practitioner, the neurologist or the pharmacy of the patient will be consulted.
Adverse Events (AEs)
All AEs that are reported spontaneously by the participant or observed by the investigator or study staff members are recorded and if necessary, appropriate measures are taken.
Statistical analysis
Continuous variables will be expressed as means with standard deviations or medians with interquartile ranges (whichever is more appropriate), and discrete variables will be expressed as numbers with percentages. The main efficacy population will consist of all patients being randomized and analyzed according to their original treatment allocation, irrespective of actual received treatment or follow-up (intention-to-treat). The primary comparison will be the mean difference in PImax % of predicted at month 4. For the secondary outcome measures we will compare the mean difference in health-related quality of life, PEmax % of predicted, VC % of predicted, FVC % of predicted, FEV1% of predicted, PEF % of predicted, P0.1% of predicted, PCF, SNIP, P0.1/PImax and patient reported breathing difficulties. An ANCOVA model will be used to analyze the differences between groups adjusting for baseline values. Missing data in the outcomes at month 4 will be imputed by the baseline-observation-carried-forward (BOCF) approach. This will be a conservative method because we expect patients’ PImax will improve after training. For the longitudinal data, we will use a mixed model for repeated measurements including a term for visit, treatment, their interaction, and baseline PImax to account for the correlation within subjects. Similar models will be used for the secondary endpoints. We will summarize incidence of respiratory infections and other AEs by treatment group and in all treatment groups combined in frequency tables, coded according to the introductory guide Medical Dictionary for Regulatory Activities (MedDRA) version 21.0 [53].
Data management
The following measures will be taken to assure the confidentiality and anonymity of the participants’ data or documents collected in Castor: a) each participant will be identified in an electronic database by a unique six digit code; b) the list of participant names corresponding to the codes will be stored in a separate encrypted electronic database, safeguarded by the principal investigator; c) only study investigators will have access to the databases and examine individual data or documents; d) all logins will be recorded; e) adopt strict precautions to prevent access to the data or documents by non-authorized persons; f) the handling of data and documents will comply with the General data protection regulation (GDPR) and is further described in the Data Monitoring Plan.
Ethics, dissemination, and safety monitoring
This study is registered in the American registry for clinical studies and trials (NCT05632666; https://clinicaltrials.gov). The investigator obtains written informed consent before study participation from participants and from parents if the participant is < 16 years old.
The trial is monitored by an external independent party (Julius Clinical). Because of the negligible risk classification minimal monitoring will be necessary. All participants are insured by the sponsor in case of harm due to study participation.
The study will be conducted according to the principles of the Declaration of Helsinki, adapted 19–10-2013, and in accordance with the Medical Research Involving Human Subjects Act (WMO). The code of Conduct as agreed upon 2001 by the Dutch organization of Pediatrics will be used. The study is partly done by minors, which means that in any case of resistance the test and research protocol will be terminated. Resistance means that the participant’s behavior obviously differs from or is more excessive compared to participant’s normal behavior. The national rules of the Dutch Association of Pediatrics for protection of minor study participants, are followed during the entire study. The results of this study will be publicly disclosed in several publications in peer reviewed scientific journals related to the topic of this study and orally in conferences concerning this theme.
Discussion
Most studies on the effect of SMN-augmenting genetic therapies on respiratory outcomes, do not show significant improvement in lung function parameters in patients with SMA types 2 and 3 [13,14,15,16]. Respiratory muscle training (RMT) has been shown effective in patients with pulmonary diseases, kyphoscoliosis, or DMD [23,24,25,26]. There are two studies who included patients with SMA, however the groups of patients with SMA were small (n = 9, 33% of total number of patients [17] and n = 3, 37% of total number of patients [27]) and distinction was not made between the results for the DMD and SMA patients. None of these patients with SMA received any form of SMN-augmenting therapy. To further study the efficacy of a 4-month home-based RMT program in patients with SMA, we designed a randomized controlled trial.
The diaphragm acts as the primary inspiratory muscle and accounts for 70% of the inspired air volume during regular breathing [54]. In patients with SMA, intercostal respiratory muscles are weak while the diaphragm is relatively spared [55, 56] resulting in lower expiratory muscle strength (PEmax) compared to inspiratory muscle strength (PImax) [31]. Therefore, RMT may perhaps be expected to particularly benefit rescue of expiratory muscle function. Here, however, we chose PImax as the primary outcome measure for the following specific reason: it turned out that reference values to identify patients with respiratory muscle weakness and calculate the required sample size are only available for this particular outcome measure (i.e., (PImax ≤ 80 cmH2O; [32]). As a result, it may be difficult to reach the primary endpoint of this study.
Training intensity of at least 30% of PImax and PEmax are necessary to increase the strength of respectively the inspiratory and expiratory muscles [30]. RMT has been studied before in patients with neuromuscular diseases [30, 57], however almost all these studies were conducted in patients with Amyotrophic Lateral Sclerosis (ALS) or myopathies, such as DMD [30, 57]. Frequency of training, duration of the interventions and the intensity of the training programs varied considerably [30, 57]. Only few studies included patients with SMA and none of the studies provided separate data for patients with SMA [30]. In a pre-experimental study with eight participants, including three patients with SMA, participants performed inspiratory muscle training twice a day, 5 days a week, 30 breaths per session, for six weeks [27]. Here, we chose to copy the training intensity and frequency used in this study [27].
Studies suggest that an IMT protocol of training twice a day with PImax as guidance of resistance over a period of three to six months can have a positive effect on inspiratory muscle strength in patients with neuromuscular diseases [57]. Patients who are treated with the SMN2-splicing modifying drugs Spinraza® or Risdiplam have their follow up visits in our center every four months. To minimize patient burden, we have opted to combine visits for the RESISTANT trial with these therapy follow-up visits, and we have chosen a training period of 4 months.
Lastly, a recent study on fatigability of respiratory muscles in patients with SMA observed that perceived exertion, measured with an OMNI scale, did not correlate with objective exertion [58]. The OMNI scale has only been validated in children and adults during motor activities [59, 60] and may not detect exertion of the respiratory muscles. Here, we have therefore chosen to monitor the response on respiratory muscle loading with experienced intensity of the training and perceived dyspnea measured with a Borg scale [61].
In conclusion, we will conduct a single blinded randomized sham-controlled trial to investigate the feasibility and efficacy of respiratory muscle training in patients with SMA and respiratory muscle weakness. We hypothesize that RMT is feasible and that it will improve inspiratory and expiratory muscle strength.
Availability of data and materials
Not applicable.
Abbreviations
- ACT:
-
Airway clearance techniques
- AE:
-
Adverse events
- ALS:
-
Amyotrophic lateral sclerosis
- AS:
-
Airstacking
- BOCF:
-
Baseline-observation-carried-forward
- BP:
-
Bodily pain
- cmH2O:
-
Centimeters of water column
- DMD:
-
Duchenne muscular dystrophy
- EMT:
-
Expiratory muscle training
- ERS/ATS:
-
European respiratory society/ American thoracic society
- FEV1:
-
Forced expiratory volume in 1 s
- (F)VC:
-
(Forced) vital capacity
- GDPR:
-
General data protection regulation
- GH:
-
General health perceptions
- IMT:
-
Inspiratory muscle training
- kPa:
-
Kilopascal
- M:
-
Month
- MCS:
-
Mental component scale
- MEDRA:
-
Medical dictionary for regulatory activities
- METC:
-
Medical ethics research committee
- MH:
-
General mental health
- MI-E:
-
Mechanical insufflation-exsufflation
- MRC:
-
Medical research council
- P0.1:
-
Mouth occlusion pressure at 100 ms during quiet breathing
- PCF:
-
Peak cough flow
- PCS:
-
Physical component scale
- PedsQL:
-
Pediatric quality of life inventory
- PEF:
-
Peak expiratory flow
- PEmax:
-
Maximum expiratory mouth pressure
- PEP:
-
Positive expiratory pressure
- PF:
-
Physical functioning
- PImax:
-
Maximum inspiratory mouth pressure
- RCT:
-
Randomized sham-controlled trial
- RE:
-
Role limitations due to emotional problems
- RESISTANT:
-
Respiratory Muscle Training in Patients with Spinal Muscular Atrophy
- RMT:
-
Respiratory muscle training
- RP:
-
Role limitations due to physical health problems
- SD:
-
Standard deviation
- SF:
-
Social functioning
- SF36:
-
36-Item short form health survey
- SMA:
-
Spinal muscular atrophy
- SMN:
-
Survival motor neuron
- SNIP:
-
Sniff nasal inspiratory pressure
- SPIRIT:
-
Standard protocol items: recommendations for interventional trials
- UMCU:
-
University medical center Utrecht
- VT:
-
Vitality
- WMO:
-
Medical Research Involving Human Subjects Act
References
Wadman R, Vrancken A, van den Berg L, van der Pol W. Dysfunction of the neuromuscular junction in spinal muscular atrophy types 2 and 3. Am Acad Neurol. 2012;79:2050–5.
Finkel RE, Sejersen T, Mercuri E, Study W, Bertini E. 218th ENMC International Workshop: Revisiting the consensus on standards of care in SMA. Neuromuscul Disord. 2017;27(6):596–605.
KNGF, Spierziekten Nederland. Kinderfysiotherapie en fysiotherapie bij SMA (Brochure). 2017. Available from: https://www.spierziekten.nl/fileadmin/user_upload/VSN/documenten/Hulpverlenersinformatie/Fysiotherapiebrochures/F014-Fysiotherapie-bij-SMA-metbrieven.pdf.
Lunn MR, Wang CH. Spinal muscular atrophy. Lancet. 2008;371:2120–33.
Verhaart IEC, Robertson A, Wilson IJ, Aartsma-Rus A, Cameron S, Jones CC, et al. Prevalence, incidence and carrier frequency of 5q-linked spinal muscular atrophy - a literature review. Orphanet J Rare Dis. 2017;12(1):1–15.
Wang CH, Finkel RS, Bertini ES, Schroth M, Simonds A, Wong B, et al. Consensus statement for standard of care in spinal muscular atrophy. J Child Neurol. 2007;22(8):1027–49.
Stam M, Wadman RI, Wijngaarde CA, Bartels B, Asselman FL, Otto LAM, et al. Protocol for a phase II, monocentre, double-blind, placebo-controlled, cross-over trial to assess efficacy of pyridostigmine in patients with spinal muscular atrophy types 2–4 (SPACE trial). BMJ Open. 2018;8: e019932.
Mongiovi P, Dilek N, Garland C, Hunter M, Kissel JT, Luebbe E, et al. Patient reported impact of symptoms in spinal muscular atrophy (PRISM-SMA). Neurology. 2018;91(13):E1206–14.
Bartels B, Habets L, Stam M, Wadman R, Wijngaarde C, Schoenmakers M, et al. Assessment of fatigability in patients with spinal muscular atrophy: development and content validity of a set of endurance tests. BMC Neurol. 2019;19:1–10.
Bartels B, De Groot JF, Habets LE, Wijngaarde CA, Vink W, Stam M, et al. Fatigability in spinal muscular atrophy: Validity and reliability of endurance shuttle tests. Orphanet J Rare Dis. 2020;15(1):1–9.
Wadman RI, Wijngaarde CA, Stam M, Bartels B, Otto LAM, Lemmink HH, et al. Muscle strength and motor function throughout life in a cross-sectional cohort of 180 patients with spinal muscular atrophy types 1c–4. Eur J Neurol. 2018;25(3):512–8.
Finkel RS, Mercuri E, Darras BT, Connolly AM, Kuntz NL, Kirschner J, et al. Nusinersen versus Sham Control in Infantile-Onset Spinal Muscular Atrophy. N Engl J Med. 2017;377(18):1723–32.
Paul GR, Gushue C, Kotha K, Shell R. The respiratory impact of novel therapies for spinal muscular atrophy. Pediatr Pulmonol. 2021:56(4):687-796.
Heitschmidt L, Pichlmaier L, Eckerland M, Steindor M, Olivier M, Fuge I, et al. Nusinersen does not improve lung function in a cohort of children with spinal muscular atrophy – a single-center retrospective study. Eur J Paediatr Neurol. 2021;31:88–91.
Audic F, De La Banda MGG, Bernoux D, Ramirez-Garcia P, Durigneux J, Barnerias C, et al. Effects of nusinersen after one year of treatment in 123 children with SMA type 1 or 2: a French real-life observational study. Orphanet J Rare Dis. 2020;15(1):1–10.
Erdos J, Wild C. Mid- and long-term (at least 12 months) follow-up of patients with spinal muscular atrophy (SMA) treated with nusinersen, onasemnogene abeparvovec, risdiplam or combination therapies: A systematic review of real-world study data. Eur J Paediatr Neurol. 2022;39(March):1–10.
Koessler W, Wanke T, Winkler G, Nader A, Toifl K, Kurz H, et al. 2 Years’ experience with inspiratory muscle training in patients with neuromuscular disorders. Chest. 2001;120(3):765–9.
Winkler G, Zifko U, Nader A, Frank W, Zwick H, Toifl K, et al. Dose-dependent effects of inspiratory muscle training in neuromuscular disorders. Muscle Nerve. 2000;23(8):1257–60.
DiMarco AF, Kelling JS, DiMarco MS, Jacobs I, Shields R, Altose MD. The effects of inspiratory resistive training on respiratory muscle function in patients with muscular dystrophy. Muscle Nerve. 1985;8(4):284–90.
Gozal D, Thiriet P. Respiratory muscle training in neuromuscular disease: long-term effects on strength and load perception. Med Sci Sports Exerc. 1999;31(11):1522–7.
Aslan GK, Gurses HN, Issever H, Kiyan E. Effects of respiratory muscle training on pulmonary functions in patients with slowly progressive neuromuscular disease: a randomized controlled trial. Clin Rehabil. 2014;28(6):573–81.
Laghi F, Tobin MJ. Disorders of the respiratory muscles. Am J Respir Crit Care Med. 2003;168(1):10–48.
Wanke T, Toifl K, Merkle M, Formanek D, Lahrmann H, Zwick H. Inspiratory muscle training in patients with Duchenne muscular dystrophy. Chest. 1994;105(2):475–82.
Harver A, Mahler DA, Daubenspeck JA. Targeted inspiratory muscle training improves respiratory muscle function and reduces dyspnea in patients with chronic obstructive pulmonary disease. Ann Intern Med. 1989;111(2):117–24.
Keens TG, Krastins IRB, Wannamaker EM, Levison H, Crozier DN, Bryan AC. Ventilatory muscle endurance training in normal subjects and patients with cystic fibrosis. Am Rev Respir Dis. 1977;116(5):853–60.
Hornstein S, Inman S, Ledsome JR. Ventilatory muscle training in kyphoscoliosis. Spine (Phila Pa 1976). 1987;12(9):859–63.
Human A, Morrow B. Inspiratory muscle training in children and adolescents living with neuromuscular diseases: a pre-experimental study. S Afr J Physiother. 2021;77(1):1–10.
Wadman RI, Stam M, Gijzen M, Lemmink HH, Snoeck IN, Wijngaarde CA, et al. Association of motor milestones, SMN2 copy and outcome in spinal muscular atrophy types 0–4. J Neurol Neurosurg Psychiatry. 2017;88(4):364–7.
Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža-Jerić K, et al. Spirit 2013 statement: Defining standard protocol items for clinical trials. Chin J Evid Based Med. 2013;13(12):1501–7.
Silva I, Pedrosa R, Azevedo A, Forbes A, Fregonezi G, Dourado Junior M, et al. Respiratory muscle training in children and adults with neuromuscular disease (Review). Cochrane Database Syst Rev. 2019;9(9):CD011711.
Veldhoen ES, Wijngaarde CA, Hulzebos EHJ, Wösten-van Asperen RM, Wadman RI, van Eijk RPA, et al. Natural history of respiratory muscle strength in spinal muscular atrophy: a prospective national cohort study. Orphanet J Rare Dis. 2022;17(1):70.
Laveneziana P, Albuquerque A, Aliverti A, Babb T, Barreiro E, Dres M, et al. ERS statement on respiratory muscle testing at rest and during exercise. Eur Respir J. 2019;53:1801214.
POWERbreathe. POWERbreathe K2. 2022. Available from: https://www.powerbreathe.com/product/k2/. [Cited 2022 Nov 30].
McDonald T, Stiller K. Inspiratory muscle training is feasible and safe for patients with acute spinal cord injury. J Spinal Cord Med. 2019;42(2):220–7.
Menzes K, Nascimento L, Avelino P, Polese J, Salmela L. A review on respiratory muscle training devices. J Pulm Respir Med. 2018;08(02):451.
Suzuki S, Sato M, Okubo T. Expiratory muscle training and sensation of respiratory effort during exercise in normal subjects. Thorax. 1995;50(4):366–70.
Matsuo Y, Yanagisawa Y, Cahalin LP. Brief research report: the feasibility of expiratory resistive loading using the threshold inspiratory muscle training device. Cardiopulm Phys Ther J. 2014;25(3):92–5.
Koninklijke Philips Electronics. Pressure that’s good for you. Threshold PEP and IMT. 2013. Available from: https://www.philips.co.uk/c-dam/b2bhc/master/whitepapers/copd/1036501_ThresholdIMT_SalesAid.pdf.
Schoenmakers MAGC, Gulmans VAM, Gooskens RHJM, Helders PJM. Spina bifida at the sacral level: More than minor gait disturbances. Clin Rehabil. 2004;18(2):178–85.
Wilson SH, Cooke NT, Edwards RHT, Spiro SG. Predicted normal values for maximal respiratory pressures in caucasian adults and children. Thorax. 1984;39:535–8.
Aaronson NK, Muller M, Cohen PDA, Essink-Bot ML, Fekkes M, Sanderman R, et al. Translation, validation, and norming of the Dutch language version of the SF-36 health survey in community and chronic disease populations. J Clin Epidemiol. 1998;51(11):1055–68.
Ware JE, Kosinski M, Keller SD. SF-36 Physical and Mental Health Summary Scales: a User’s Manual. Boston; 1994 Dec. Available from: https://www.researchgate.net/publication/292390260.
Bastiaansen D, Koot HM, Bongers IL, Varni JW, Verhulst FC. Measuring quality of life in children referred for psychiatric problems: Psychometric properties of the PedsQLTM 4.0 generic core scales. Qual Life Res. 2004;13(2):489–95.
Mellies U, Stehling F, Dohna-Schwake C. Normal values for inspiratory muscle function in children. Physiol Meas. 2014;35(10):1975–81.
Hall GL, Filipow N, Ruppel G, Okitika T, Thompson B, Kirkby J, et al. Official ERS technical standard: global lung function initiative reference values for static lung volumes in individuals of european ancestry. Eur Respir J. 2021;57(3):2000289.
Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multı-ethnic reference values for spirometry for the 3–95 year age range: the global lung function 2012 equations: report of the Global Lung Function Initiative (GLI), ERS task force to establish improved lung function reference values. Eur Respir J. 2012;40(6):1324–43.
Koopman M, Zanen P, Kruitwagen CLJJ, Van Der Ent CK, Arets HGM. Reference values for paediatric pulmonary function testing: The Utrecht dataset. Respir Med. 2011;105(1):15–23.
Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Report working party standardization of lung function tests, european community for steel and coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl. 1993;16:5–40.
Criée CP. Empfehlungen der Deutschen Atemwegsliga zur Messung der inspiratorischen Muskelfunktion. Pneumologie. 2003;57:98–100.
Madden AM, Tsikoura T, Stott DJ. The estimation of body height from ulna length in healthy adults from different ethnic groups. J Hum Nutr Diet. 2012;25(2):121–8.
Fletcher CM, Clifton M, Fairbairn AS, Fry J, Gilson C, Higgings ITT, et al. Standardized questionnaire on respiratory symptoms. Br Med J. 1960;1665.
Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–6.
International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). Introductory Guide MedDRA Version 21.0. 2018. Available from: https://admin.meddra.org/sites/default/files/guidance/file/intguide_21_0_english.pdf.
Fayssoil A, Behin A, Ogna A, Mompoint D, Amthor H, Clair B, et al. Diaphragm: Pathophysiology and Ultrasound Imaging in Neuromuscular Disorders. J Neuromuscul Dis. 2018;5(1):1–10.
Boentert M, Wenninger S, Sansone VA. Respiratory involvement in neuromuscular disorders. Neuromuscul Dis Muscle. 2017;30(5):529–37.
Schroth MK. Special considerations in the respiratory management of spinal muscular atrophy. Pediatrics. 2009;123(SUPPL. 4):245–50.
Human A, Corten L, Jelsma J, Morrow B. Inspiratory muscle training for children and adolescents with neuromuscular diseases: A systematic review. Neuromuscul Disord. 2017;27(6):503–17.
Kant-Smits K, Hulzebos EHJ, Habets LE, Asselman F, Veldhoen ES, van Eijk RPA, et al. Respiratory muscle fatigability in patients with spinal muscular atrophy. Pediatr Pulmonol. 2022;57:3050.
Bulthuis M, van Empelen R, Heimeriks I, Dronkers J. Het gebruik van een belastingschaal bij kinderen tussen de 8 en 12 jaar voor het meten van subjectief ervaren belasting tijdens training. Nederlands tijdschrijft voor Kinderfysiotherapie. 2010;22(67):24–31.
Robertson RJ, Goss FL, Boer NF, Peoples JA, Foreman AJ, Dabayebeh IM, et al. Children’s OMNI scale of perceived exertion: mixed gender and race validation. Med Sci Sports Exerc. 2000;32(2):452–8.
Just N, Bautin N, Danel-Brunaud V, Debroucker V, Matran R, Perez T. The Borg dyspnoea score: a relevant clinical marker of inspiratory muscle weakness in amyotrophic lateral sclerosis. Eur Respir J. 2010;35(2):353–60.
Acknowledgements
We are grateful to the patients with SMA who will participate in this study, to Jeroen Jeneson who helped us formulate the English language correctly, and to the Prinses Beatrix Spierfonds, without them we cannot conduct this research.
Funding
This study will be funded by the Prinses Beatrix Spierfonds. The Prinses Beatrix Spierfonds is not involved in the design of the study nor in writing this manuscript.
Author information
Authors and Affiliations
Contributions
Study concept and design were conducted by FA, BB, EH, EV, LvdP and RvE. Collection of data is done by KK and EH. Technical, administrative, and material support was provided by FA and BB. Drafting of the manuscript was done by KK, EH and BB. Critical revision of the manuscript was performed by FA, EV, LvdP and RvE. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Medical Research Ethics Committee Utrecht (METC Utrecht) reviewed the study in accordance with the Dutch Medical Research Involving Human Subjects Act (WMO) and other applicable Dutch and European regulations. Based on the requirements of the WMO, the METC Utrecht approved this study. Oral and written informed consent will be obtained when ≥ 16 years old and additional written informed consent will be obtained by the parents or legal representative if the participant is < 16 years old.
Consent for publication
Not applicable.
Competing interests
LvdP serves on the scientific advisory board for SMA Europe, is a member of the Branaplam data monitoring committee (DMC) for Novartis, provides ad hoc consultancy for Biogen and AveXis (Novartis), and receives research support from the Prinses Beatrix Spierfonds, Vriendenloterij and Stichting Spieren voor Spieren.
BB receives research support from Prinses Beatrix Spierfonds, Stichting Spieren voor Spieren and Health Holland, all non-profit foundations. His employer receives fees for SMA-related consultancy activities for Biogen, Novartis, Scholar Rock and Roche.
All other authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kant-Smits, K., Bartels, B., Asselman, FL. et al. The RESISTANT study (Respiratory Muscle Training in Patients with Spinal Muscular Atrophy): study protocol for a randomized controlled trial. BMC Neurol 23, 118 (2023). https://doi.org/10.1186/s12883-023-03136-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-023-03136-3