Abstract
Background
Cerebral atherosclerotic stenosis (CAS) is a significant factor in the development of acute ischemic stroke (AIS). Previous studies have reported that cytokines are involved in atherosclerotic diseases, although the relationship between serum levels of the chemokine RANTES (regulated on activation, normal T-cell expressed and secreted) and the presence of CAS remains unclear.
Methods
In total, 127 participants (65 non-AIS controls and 62 patients with AIS) were involved in this study. CAS was defined as the presence of ≥ 50% stenosis in major intracranial or extracranial artery by a Digital Substraction Angiography (DSA) examination, and we classified all participants into four groups according to stroke and CAS status. Serum concentrations of 8 cytokines, including RANTES, were measured by the Human ProcartaPlex Multiplex Immunoassay Kit.
Results
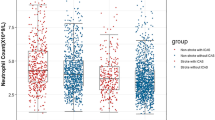
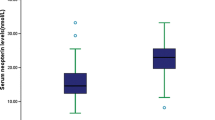
Seventy-eight participants (61.41%) had CAS, of which 39 cases with AIS and 39 case with non-AIS. Patients with CAS had higher RANTES levels compared to non-CAS patients in both the non-AIS group (10.54 ± 0.80 vs. 13.20 ± 0.71, p = 0.016) and stroke group (11.96 ± 0.87 vs. 15.03 ± 0.75, p = 0.011), and multivariate logistic regression analysis showed that the RANTES level is independently associated with CAS in both the non-AIS group (adjusted odds ratio (OR), 1.07; 95% CI, 1.02–1.12, P = 0.004) and stroke group (adjusted OR, 1.32; 95% CI, 1.10–1.58, P = 0.003).
Conclusion
Patients with CAS have higher levels of serum RANTES than non-CAS patients regardless of stroke status suggesting that RANTES may play an important role in the formation of CAS.
Similar content being viewed by others
Introduction
Cerebrovascular disease is a major health problem worldwide and one of the three major causes of death [1, 2]. In cerebrovascular diseases, the incidence of ischemic stroke is as high as 87% and its disability rate exceeds 50% [3, 4]. Cerebral atherosclerotic stenosis (CAS) affects both intra- and extra-cranial arteries and is widely recognized as a critical risk factor for ischemic stroke [5]. Patients with CAS-related acute ischemic stroke often have poor outcomes and increased risk of stroke recurrence [6]. Prior studies have reported that 23% of ischemic strokes are caused by large artery atherosclerosis [7], and the risk of recurrent stroke in these patients is 11–33% and increases with the degree of vascular stenosis.[8,9,10] The Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) trial reported a stroke recurrence rate of up to 17% despite medical intervention, leading to increased disability rates [11]. Identifying patients with a high risk of CAS is essential to the prevention of both occurrence and recurrence of stroke.
The chemokine regulated on activation, normal T-cell expressed and secreted (RANTES) is involved in the migration of inflammatory cells to the inner membrane and plays an important role in the formation of plaque [12]. In particular, RANTES recruits leukocytes to sites of inflammation, promotes lymphocyte and macrophage mobilization and chemotaxis, and further stimulates the release of mediators. Studies have shown significant correlations between RANTES levels and atherosclerotic plaque progression [12], markers of heart failure [13], and even acute coronary syndromes [14]. In a prospective study, elevated RANTES concentrations were used to predict the occurrence of ischemic stroke [15]. However, studies on the association between serum cytokine levels of RANTES and the risk of CAS are limited.
In this study, we aimed to assess the relationship between the concentration of serum RANTES and the presence of CAS, which may serve as a biomarker for identifying patients with high risk of CAS.
Method
Study population
We prospectively recruited acute ischemic stroke (AIS) and non-AIS control patients who were hospitalized in the Hospital from July 2019 to December 2019. AIS was diagnosed according to clinical symptoms and imaging (magnetic resonance/computer tomography) within 14 days of symptom onset. Non-AIS patients were hospitalized due to dizziness and headache, and did not suffer an acute stroke in the past 6 months. Exclusion criteria was (1) age ≤ 30 years; (2) incomplete neuroimaging data and laboratory tests; (3) prior thrombolysis or thrombectomy; (4) atrial fibrillation, cardioembolism and serious peripheral arterial disease; (5) recent local and systemic infectious disease(s); (6) intracranial and extracranial arterial stenosis caused by arterial dissection, arteritis, moyamoya disease, and muscle fiber dysplasia; and (7) a history of malignant tumors, severe liver or kidney dysfunction, or systemic disease. This study has been approved by the Tongji Hospital Ethics Committee (No. TJ-IRB20210107) and conducted according to the Declaration of Helsinki. All participants provided informed consent.
Definition of CAS
Cerebrovascular images were obtained from all participants, and CAS was defined and determined by 50–99% stenosis or occlusion by digital subtraction angiography (DSA) examinations in at least one major arterial segment, including the intracranial or extracranial segment of the carotid artery (ICA) or vertebral artery (VA), M1 middle cerebral artery (MCA) and basilar artery (BA). A significantly stenotic vessel segment is considered a responsible CAS when an infarction lesion occurs in its blood supply area. In the current study, we assessed the location and severity of responsible CAS.
Clinical assessments
Clinical data were collected from the clinical record and included age, gender, history of smoking and drinking, and medical history (e.g., prior ischemic stroke/TIA, coronary heart disease, hypertension, diabetes mellitus, hyperlipidemia). Hypertension was defined as systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg and/or prior treatment with antihypertensive medication [16]. Diabetes mellitus was defined as glycosylated hemoglobin A1c (HbA1c) ≥ 6.5%, two-hour plasma glucose ≥ 11.1 mmol/L after an oral glucose tolerance test, or self-reported history of diabetes mellitus [11, 17, 18]. Laboratory data included serum levels of fasting triglycerides, total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), creatinine, glomerular filtration rate (eGFR), fasting plasma glucose (FPG), and HbA1c platelet indexes. All data were collected in a standard manner in the laboratory of the hospital within 24 h of enrollment.
Measurement of serum cytokine level
Peripheral blood samples were collected within 24 h of admission with vacutainer tubes containing coagulant accelerator (Becton–Dickinson, San Jose, USA) and immediately centrifuged at 1,278 g for 15 min at 4 °C. Serum samples were stored at − 80 °C until analysis. The concentrations of serum cytokines (including TNF-α, IL-6, MMP-9, MMP-1, IP-10, RANTES VEGF, and IL-23,) were analyzed using the Human ProcartaPlex Multiplex Immunoassay Kit (Affymetrix, eBioscience, USA) according to the manufacturer’s instructions.
Statistical analysis
Statistical analyses were performed using IBM SPSS 22.0 software (IBM Corp., Armonk, NY, USA), and P < 0.05 was considered statistically significant. Categorical variables are represented as frequencies (percentages), whereas continuous variables are represented as mean ± standard error of the mean (SEM) or median [interquartile range]. Non-AIS vs. AIS groups or non-CAS vs. CAS groups were compared using a chi-square test, two-sided t-test or Mann–Whitney U-test according to their distributions. The Kruskal–Wallis H test was utilized to explore the association between serum RANTES levels and CAS features. Multivariate logistic regression analyses were used to determine the relationship between serum RANTES levels and CAS. Odds ratio (OR) was adjusted by variables that had a significant clinical correlation (including age, gender, smoking history, drinking history, and medical history) and variables with P < 0.05 by univariate logistic regression analysis. Finally, receiver operating characteristic (ROC) curve analysis was used to determine whether serum RANTES predicts CAS, and the area under the ROC curve (AUC) is reported.
Results
Baseline characteristics and serum cytokines levels of participants
One hundred twenty-seven participants were included in the study analysis (65 [51.18%] AIS patients and 62 [48.82%] non-AIS controls). Thirty-nine (30.71%) AIS patients and 39 (30.71%) non-AIS controls with at least one segment of CAS, while 26 (20.47%) AIS patients and 23 (18.11%) non-AIS controls were absent of CAS. Baseline characteristics are illustrated in Table 1. Compared to controls, patients with acute ischemic stroke were younger overall, but demonstrated lower levels of both total cholesterol (TC) and high-density lipoprotein (HDL). No significant differences between baseline characteristics were found between the CAS and non-CAS groups in patients with or without AIS.
AIS patients had higher levels of MMP-1 and RANTES compared to non-AIS patients (p < 0.05), and in patients with AIS, those with CAS had higher levels of RANTES and IP-10. Non-AIS controls with CAS, however, had higher levels of RANTES and IL-23 (Table 2).
Higher serum RANTES levels were associated with the presence of CAS
The multivariable logistic regression analysis showed that serum RANTES was independently associated with CAS in all subjects (Table S1). In non-AIS patients, RANTES was significantly associated with CAS (unadjusted OR: 1.169; 95% CI: 1.02–1.34; p = 0.022 and adjusted OR: 1.041; 95% CI: 1.02–1.12; p = 0.004). In AIS patients, RANTES was significantly associated with CAS (unadjusted OR: 1.17; 95% CI: 1.03–1.33; p = 0.017), and the adjusted odds ratio of RANTES was statistically significant (OR: 1.024; 95% CI: 1.008–1.041; p = 0.003; Table 3).
Seventy-eight (61.4%) participants had CAS, including 50 (64.1%) patients with single CAS, 23 (29.5%) patients with 2 CAS, and 5 (6.4%) patients with ≥ 2 CAS (Table S2). RANTES concentration was not significantly different among participants with differing stenosed cerebral vessels. The effect of serum RANTES levels on location and severity of responsible CAS was also analyzed, although was not significantly associated.
Discussion
In the current study, we demonstrate that patients with AIS have elevated serum RANTES levels compared to patients with non-AIS. Although the AIS and no-AIS subjects were not matched for age, importantly, the multivariate regression analysis excluded the impact of this difference on our results. Interestingly, RANTES appears independently associated with CAS and aids in CAS identification regardless of stroke status. Indeed, the concentration of RANTES as a marker in the atherosclerosis process may distinguish individuals with an increased risk of CAS and may be a potential target for future drug intervention.
Regarding the correlation between RANTES and ischemic stroke, our results are similar to previous studies that the serum RANTES levels increase in AIS [19, 20]. However, the role of RANTES in ischemic stroke remains controversial. As reported, the neurons could produce RANTES which has the potential to directly or indirectly protect neurons by producing neurotrophic factors in the area around the infarct after ischemic stroke, [21] although other studies demonstrate opposite results [22,23,24]. As a pro-inflammatory chemokine, RANTES could recruit white blood cells to the infarct area and exacerbate cerebral infarction volume. These altering functions may be due to the functional differences of RANTES secreted by different cell types or the varying receptors involved in signal modulation [25]. Further research to clarify its mechanism is warranted.
Atherosclerosis is increasingly recognized as a chronic inflammatory disease. Indeed, inflammation plays an important role in all stages of atherosclerosis and provides a significant pathophysiological mechanism for the formation and rupture of atherosclerotic plaque. Previous studies have reported RANTES in atherosclerotic plaques [26,27,28] and different cell types, including macrophages, CD8 + T cells, smooth muscle cells and platelets [29, 30]. The expression of CCL5 could promote the proliferation of SMCs and convert them into a synthetic phenotype that causes atherosclerosis, thereby promoting atherosclerosis. Rodent studies have demonstrated RANTES overexpression in atherosclerotic lesions, and genetic deletion of the gene responsible for RANTES receptor CCR5 reduces the atherosclerosis progression [31]. Furthermore, elevated levels of serum RANTES are associated with coronary artery stenosis [31], carotid artery disease and peripheral artery disease. An ARIC carotid MRI study also demonstrated that higher RANTES levels are associated with higher lipid core volume, which may lead to the formation of high-risk plaques [32]. Indeed, higher levels of RANTES were found in patients with symptomatic intracranial arteriosclerosis compared with asymptomatic intracranial arteriosclerosis [33, 34]. Here, we report peripheral serum RANTES levels are closely associated to the presence of CAS, and serum RANTES level effectively identify the presence of CAS. While there was not a significant relationship between CAS characteristics, this may be due to the small sample size, and additional therefore research is warranted.
In this study, we found that in addition to RANTES, other cytokines, such as MMP-1, IP-10 and IL-23, were also different between groups. As previously reported, elevated MMP-1 is associated with AIS [23], while IP-10 and IL-23 are associated with atherosclerosis [35]. This result suggests an inflammatory reaction and accompanied by changes in the expression level of some cytokines regardless of AIS or CAS formation. However, further studies are needed to confirm the stability of this result.
The present study has some limitations. First, this study has a small sample size and is conducted exclusively Chinese patients, which may lead to some selection bias and lack of generalizability outside the Asian population. Therefore, the results need to be interpreted carefully and verified further. Second, the use of antiplatelet drugs and statins after ischemic stroke may affect the concentration of RANTES. However, we minimized the potential effects of stroke and related drugs on RANTES levels by dividing the participants into AIS and non-AIS groups. Finally, we could not present the dynamic change of RANTES levels among patients with acute ischemic stroke in this study since we only measured the serum RANTES levels at a single time point.
Conclusion
In conclusion, patients with CAS had higher levels of serum RANTES compared to patients with non-CAS, which suggests that the RANTES level might play a role in the physiopathological mechanism of CAS formation, although additional studies are needed to verify this result.
Authors’contributions
YPG, QQK and YZ. collected the clinical data. YPG, JZ and HH. processed statistical data. YPG, ZYY and DH. drafted and revised the manuscript. XL. designed and guided the study. All authors read and approved the final manuscript.
Availability of data and materials
The data used to support the findings of this study are available from the corresponding author upon request.
References
Crawford KM, Gallego-Fabrega C, Kourkoulis C, Miyares L, Marini S, Flannick J, Burtt NP, von Grotthuss M, Alexander B, Costanzo MC, et al. Cerebrovascular disease knowledge portal: an open-access data resource to accelerate genomic discoveries in stroke. Stroke. 2018;49(2):470–5.
Katan M, Luft A. Global burden of stroke. Semin Neurol. 2018;38(2):208–11.
Ding Q, Liu S, Yao Y, Liu H, Cai T, Han L: Global, regional, and national burden of ischemic stroke, 1990–2019. Neurology 2021.
Saini V, Guada L, Yavagal DR: Global epidemiology of stroke and access to acute ischemic stroke interventions. Neurology 2021, 97(20 Supplement 2):S6-S16.
Banerjee C, Turan TN. Large artery atherosclerosis: extracranial and intracranial. Semin Neurol. 2017;37(3):307–15.
Al Kasab S, Derdeyn CP, Guerrero WR, Limaye K, Shaban A, Adams HP Jr. Intracranial large and medium artery atherosclerotic disease and stroke. J Stroke Cerebrovasc Dis. 2018;27(7):1723–32.
Ornello R, Degan D, Tiseo C, Di Carmine C, Perciballi L, Pistoia F, Carolei A, Sacco S. Distribution and temporal trends from 1993 to 2015 of ischemic stroke subtypes: a systematic review and meta-analysis. Stroke. 2018;49(4):814–9.
North American Symptomatic Carotid Endarterectomy Trial C, Barnett HJM, Taylor DW, Haynes RB, Sackett DL, Peerless SJ, Ferguson GG, Fox AJ, Rankin RN, Hachinski VC et al: Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. The New England journal of medicine 1991, 325(7):445-453.
Gulli G, Marquardt L, Rothwell PM, Markus HS. Stroke risk after posterior circulation stroke/transient ischemic attack and its relationship to site of vertebrobasilar stenosis: pooled data analysis from prospective studies. Stroke. 2013;44(3):598–604.
Eckstein HH, Kuhnl A, Berkefeld J, Lawall H, Storck M, Sander D. Diagnosis, treatment and follow-up in extracranial carotid stenosis. Dtsch Arztebl Int. 2020;117(47):801–7.
Chen W, Pan Y, Jing J, Zhao X, Liu L, Meng X, Wang Y, Wang Y, Investigators C: Recurrent Stroke in Minor Ischemic Stroke or Transient Ischemic Attack With Metabolic Syndrome and/or Diabetes Mellitus. J Am Heart Assoc 2017, 6(6).
Jongstra-Bilen J, Tai K, Althagafi MG, Siu A, Scipione CA, Karim S, Polenz CK, Ikeda J, Hyduk SJ, Cybulsky MI. Role of myeloid-derived chemokine CCL5/RANTES at an early stage of atherosclerosis. J Mol Cell Cardiol. 2021;156:69–78.
Koh SJ, Kim JY, Hyun YJ, Park SH, Chae JS, Park S, Kim JS, Youn JC, Jang Y, Lee JH. Association of serum RANTES concentrations with established cardiovascular risk markers in middle-aged subjects. Int J Cardiol. 2009;132(1):102–8.
Podolec J, Trabka-Zawicki A, Badacz R, Siedlinski M, Tomala M, Bartus K, Legutko J, Przewlocki T, Zmudka K, Kablak-Ziembicka A. Chemokine RANTES and IL-1beta in mild therapeutic hypothermia-treated patients after out-of-hospital sudden cardiac arrest. Postepy Kardiol Interwencyjnej. 2019;15(1):98–106.
Canoui-Poitrine F, Luc G, Mallat Z, Machez E, Bingham A, Ferrieres J, Ruidavets JB, Montaye M, Yarnell J, Haas B, et al. Systemic chemokine levels, coronary heart disease, and ischemic stroke events: the PRIME study. Neurology. 2011;77(12):1165–73.
Turin TC, Okamura T, Afzal AR, Rumana N, Watanabe M, Higashiyama A, Nakao Y, Nakai M, Takegami M, Nishimura K, et al. Hypertension and lifetime risk of stroke. J Hypertens. 2016;34(1):116–22.
Lo JW, Crawford JD, Samaras K, Desmond DW, Kohler S, Staals J, Verhey FRJ, Bae HJ, Lee KJ, Kim BJ, et al. Association of prediabetes and type 2 diabetes with cognitive function after stroke: A STROKOG Collaboration study. Stroke. 2020;51(6):1640–6.
Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, Fang MC, Fisher M, Furie KL, Heck DV, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2014;45(7):2160–236.
Terao S, Yilmaz G, Stokes KY, Russell J, Ishikawa M, Kawase T, Granger DN. Blood cell-derived rantes mediates cerebral microvascular dysfunction, inflammation, and tissue injury after focal ischemia-reperfusion. Stroke. 2008;39(9):2560–70.
Hutanu A, Iancu M, Maier S, Balasa R, Dobreanu M. Plasma biomarkers as potential predictors of functional dependence in daily life activities after ischemic stroke: a single center study. Ann Indian Acad Neurol. 2020;23(4):496–503.
Tokami H, Ago T, Sugimori H, Kuroda J, Awano H, Suzuki K, Kiyohara Y, Kamouchi M, Kitazono T, Investigators R. RANTES has a potential to play a neuroprotective role in an autocrine/paracrine manner after ischemic stroke. Brain Res. 2013;1517:122–32.
Yilmaz G, Granger DN. Leukocyte recruitment and ischemic brain injury. Neuromolecular Med. 2010;12(2):193–204.
Victoria ECG, Toscano ECB, Oliveira FMS, de Carvalho BA, Caliari MV, Teixeira AL, de Miranda AS, Rachid MA. Up-regulation of brain cytokines and metalloproteinases 1 and 2 contributes to neurological deficit and brain damage in transient ischemic stroke. Microvasc Res. 2020;129: 103973.
Sorce S, Bonnefont J, Julien S, Marq-Lin N, Rodriguez I, Dubois-Dauphin M, Krause KH. Increased brain damage after ischaemic stroke in mice lacking the chemokine receptor CCR5. Br J Pharmacol. 2010;160(2):311–21.
Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol. 2010;87(5):779–89.
Braunersreuther V, Zernecke A, Arnaud C, Liehn EA, Steffens S, Shagdarsuren E, Bidzhekov K, Burger F, Pelli G, Luckow B, et al. Ccr5 but not Ccr1 deficiency reduces development of diet-induced atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2007;27(2):373–9.
Veillard NR, Kwak B, Pelli G, Mulhaupt F, James RW, Proudfoot AE, Mach F. Antagonism of RANTES receptors reduces atherosclerotic plaque formation in mice. Circ Res. 2004;94(2):253–61.
Schober A, Manka D, von Hundelshausen P, Huo Y, Hanrath P, Sarembock IJ, Ley K, Weber C. Deposition of platelet RANTES triggering monocyte recruitment requires P-selectin and is involved in neointima formation after arterial injury. Circulation. 2002;106(12):1523–9.
Zernecke A, Weber C. Chemokines in atherosclerosis: proceedings resumed. Arterioscler Thromb Vasc Biol. 2014;34(4):742–50.
Appay V, Rowland-Jones SL. RANTES: a versatile and controversial chemokine. Trends Immunol. 2001;22(2):83–7.
Braunersreuther V, Steffens S, Arnaud C, Pelli G, Burger F, Proudfoot A, Mach F. A novel RANTES antagonist prevents progression of established atherosclerotic lesions in mice. Arterioscler Thromb Vasc Biol. 2008;28(6):1090–6.
Virani SS, Nambi V, Hoogeveen R, Wasserman BA, Coresh J, Gonzalez F 2nd, Chambless LE, Mosley TH, Boerwinkle E, Ballantyne CM. Relationship between circulating levels of RANTES (regulated on activation, normal T-cell expressed, and secreted) and carotid plaque characteristics: the atherosclerosis risk in communities (ARIC) carotid MRI study. Eur Heart J. 2011;32(4):459–68.
Shalhoub J, Viiri LE, Cross AJ, Gregan SM, Allin DM, Astola N, Franklin IJ, Davies AH, Monaco C. Multi-analyte profiling in human carotid atherosclerosis uncovers pro-inflammatory macrophage programming in plaques. Thromb Haemost. 2016;115(5):1064–72.
Montecucco F, Lenglet S, Gayet-Ageron A, Bertolotto M, Pelli G, Palombo D, Pane B, Spinella G, Steffens S, Raffaghello L, et al. Systemic and intraplaque mediators of inflammation are increased in patients symptomatic for ischemic stroke. Stroke. 2010;41(7):1394–404.
Munjal A, Khandia R. Atherosclerosis: orchestrating cells and biomolecules involved in its activation and inhibition. Adv Protein Chem Struct Biol. 2020;120:85–122.
Acknowledgements
We thank Dr. Guo Li and Mr. Renjie Feng for kindly participating in the data analysis.
Funding
This study was supported by the National Nature Science Foundation of China (81,771,341 to X. Luo), the Key Research and Development Program of Hubei Province (2020BCA070 to X. Luo), the Application Foundation Frontier Special Project of Wuhan Science and Technology Bureau (2,020,020,601,012,226 to X. Luo), and the Flagship Program of Tongji Hospital (2019CR106 to X. Luo).
Author information
Authors and Affiliations
Contributions
YPG, QQK and YZ collected the clinical data. YPG, JZ and HH processed statistical data. YPG, ZYY and DH drafted and revised the manuscript. XL designed and guided the study. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study has been approved by the Tongji Hospital Ethics Committee (No. TJ-IRB20210107) and conducted according to the Declaration of Helsinki. All participants have provided informed consent.
Consent for publication
Not Applicable.
Competing interests
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Table S1. Multivariate logistic regression analysis showing the predictors for the CAS. Table S2. CAS characteristics of patients with and without AIS
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Guo, Y., Kong, Q., Zhang, Y. et al. Elevated RANTES levels are associated with increased risk of cerebral atherosclerotic stenosis. BMC Neurol 23, 39 (2023). https://doi.org/10.1186/s12883-023-03079-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-023-03079-9