Abstract
Background
C1q/TNF-related protein 9 (CTRP9) and adiponectin (APN) have beneficial metabolic regulatory and vasoprotective effects. This study explored alteration of CTRP9 and APN multimers during onset of ischemic stroke and development, to provide novel clinical and experimental basis for recognition and prevention of ischemic stroke.
Methods
There were 269 patients with ischemic stroke and 182 control subjects included in this study. Serum levels of CTRP9 and APN multimers in different disease stages were measured.
Results
Serum CTRP9, total APN (tAPN), and high-molecular weight (HMW) APN decreased gradually in stage I (acute stage, within 72 h of onset) of ischemic stroke and increased during stage III (11th day to one month) and stage IV (1 month after), compared to control. In the non-hyperlipidemia group, serum CTRP9, tAPN, and HMW were decreased in ischemic stroke patients compared to control (P < 0.05). Serum CTRP9 is closely related to serum tAPN and HMW (r = 0.992, 0.991). Serum CTRP9 are protective against ischemic stroke (OR = 0.400, 95% CI 0.197–0.810, P < 0.05).
Conclusions
Lower serum CTRP9, tAPN, LMW, and HMW are significantly associated with increased ischemic stroke risk in non-hyperlipidemia subjects. CTRP9, tAPN, and HMW isoforms may be valuable clinical indicators for patients with ischemic stroke.
Similar content being viewed by others
Background
Cerebral ischemic stroke refers to localized ischemic softening or necrosis of brain parenchyma caused by cerebral circulation disorders, secondary ischemia, and hypoxic pathological changes [1]. Stroke, the second leading cause of death in the world only after ischemic heart disease, is the leading cause of disability in adults [2]. Cerebral ischemic stroke accounts for 87% of all strokes [3].
It has been well-established that adipose tissue has active secretory functions [4]. It secretes various adipokines involved in many pathophysiological processes, such as inflammation, energy metabolism, apoptosis, and aging [4,5,6]. Among the adipokines, leptin, adiponectin (APN), and C1q/TNF-related proteins (CTRPs) all play active roles in energy metabolism regulation, vasomotion modulation, platelet activation, and inflammation reaction [7,8,9]. Serum APN exists in three major forms: trimer (low-molecular weight, LMW), hexamer (middle-molecular weight, MMW), and multimer (high-molecular weight, HMW) which contains a bouquet of 12–18 (possibly more) monomers [10]. Different APN isoforms, known to exert tissue-specific biological function and to activate distinct signaling pathways, do not interconvert in circulation [11,12,13,14]. Of the three APN isoforms, the HMW multimer represents the majority of total adiponectin (tAPN) and is the major bioactive isoform [15]. Substantial evidence supports CTRP9 and APN as beneficial molecules against obesity-related cardiovascular diseases and glycolipid disorders [7, 16]. However, the potential implications of them in clinical settings have not been well documented. In this study, we determined the levels of serum CTRP9 and different APN multimers in patients of different stroke stages and non-stroke subjects, to define the association of CTRP9/APN multimer levels, as well as dynamic changes after onset, with ischemic stroke, therefore to explore their potentials as risk factors and/or biomarkers. Accomplishment of this study would help to identify high-risk individuals of stroke onset and developing worse outcomes. The association between APN and CTRP9 with stroke may also provide new insights into the pathology of stroke onset and following staging.
Methods
Study sample
There were 269 patients with ischemic stroke recruited from departments of neurology, geriatrics, and emergency medicine of Xijing Hospital (Xi’an, China) from October 2016 to November 2017. All patients met the diagnostic criteria of the “Guidelines for the Prevention of Stroke in Patients with Stroke and Transient Ischemic Attack” (American Heart Association and American Stroke Association, 2014) [1]. Infarction was confirmed by CT or MRI. All patients were classified into four subgroups based upon established ischemic stroke staging criteria [17]. There were 57 cases of hyperacute or acute stage (Stage I, < 72 h of infarction), 54 cases of subacute stage (Stage II, 4–10 days after onset), 38 cases of chronic stage (Stage III, 11–30 days after onset), and 115 cases of late chronic stage (Stage IV, > 1 month after onset). Five cases of undetermined onset date were excluded from the stage subgroup analyses.
Study subjects without ischemic stroke (n = 182) were recruited from the departments of neurology and geriatrics of Xijing hospital concurrently. CT or MRI verified all control individuals were absent of ischemic or infarct lesions.
To avoid potential confounding factors and control heterogeneity of the study sample, we excluded subjects with any of the following conditions: (1) presence or history of cerebral hemorrhage without pre-existing ischemic stroke; (2) history of ischemic encephalopathy of other causes; (3) previous or current diagnosis of coronary heart disease or coronary syndrome.
The study was approved by the Institutional Review Board of Xijing Hospital (Approval No.: XJ20160443). All study participants gave written consent for study participation.
Clinical and laboratory examination
About 4ml of peripheral blood was collected immediately after confirmation of ischemic stroke in the emergency department, or at 6:00 a.m. the next morning of admission. The blood was placed into EDTA tube for 2 h at room temperature, and centrifuged for 15 min at 3000 r/min. The serum supernatant was stored in a labelled cryopreservation tube at –80 °C freezer until laboratory assays. Hospital records documenting clinical course and laboratory assays were reviewed after patient discharge. We did not have information of height and weight due to difficulty in obtaining accurate values for commonly bedridden patients. Hyperlipidemia was diagnosed according to 2016 Chinese guideline for the management of dyslipidemia in adults [18]. Specifically, patients with total cholesterol ≥ 6.2, low-density lipoprotein (LDL) cholesterol ≥ 4.1, high-density lipoprotein (HDL) cholesterol < 1.0, or blood triglyeride ≥ 2.3 were diagnosed as hyperlipidemia. The transcranial Doppler ultrasound and carotid ultrasound were used to determine the presence of intracranial (stenosis) and extracranial (plaque) atherosclerosis.
Serum CTRP9 and APN concentrations were measured by use of double antibody sandwich avidin–biotin peroxdase complex-enzyme-linked immunosorbent assay (ABC-ELISA) kit (Shanghai Xitang, China) per manufacturer’s protocol. Each blood sample was measured twice and the measurements were averaged for subsequent analyses.
Statistical analysis
Data management and statistical analyses were performed using Excel 2010 and SPSS 19.0. Continuous observations were expressed as mean ± SD. Difference between groups were tested by Student’s t test or t' test. The skew-distributed data were subjected to Mann–Whitney U test or Kruskal–Wallis H (K) test. Counting data are conveyed as count (percentage) and subjected to chi-square test. Spearman correlation tested the correlation of quantitative data between groups. Risk factors were determined by use of logistic regression. P values less than 0.05 were considered significant.
When comparing the differences of serum APN/CTRP9 between stroke and non-stroke groups, we applied propensity score to control potential between-group imbalance of confounding parameters. Specifically, multivariable logistic regression was used to derive a propensity score for stroke onset of all subjects. When comparing the between-group differences of APN/CTRP9, the inverse probability weighting approach [19] based on the propensity scores was applied for the purpose of adjustment.
Results
Table 1 reveals the baseline characteristics of the study subjects. There was no significant difference in serum CTRP9 and APN levels between infarct versus control groups (p > 0.05). Age, being of male gender, tobacco use, alcohol consumption rate, proportion of family history of cardiovascular and cerebrovascular diseases, systolic blood pressure, diastolic blood pressure, fasting serum glucose, glycosylated hemoglobin, levels of homocysteine, prevalence of diabetes mellitus, hypertension, atrial fibrillation, vitamin B12 deficiency, arrhythmia, stenosis, and occlusion of intracranial and carotid artery, and atherosclerosis were all greater in the ischemic stroke group compared to control (p < 0.05) before adjustment was applied. After adjustment for propensity score derived using all foregoing characteristics except APN isoforms and CTRP9, we did not find any characteristics showing significant association with stroke onset. The concentration of total cholesterol, HDL cholesterol, LDL cholesterol, and the ratio of hepatic adipose infiltration were decreased in the ischemic stroke group compared to control (p < 0.01; Table 1).
All subjects were assigned to four subgroups: subjects without ischemic stroke and hyperlipidemia (Group I, n = 115), ischemic stroke patients without hyperlipidemia (Group II, n = 182), control subjects with hyperlipidemia (Group III, n = 67) and patients with hyperlipidemia (Group IV, n = 87). Serum CTRP9, tAPN, HMW APN and LMW APN were significantly decreased in the hyperlipidemic group compared to the non-hyperlipidemic group (p < 0.01, Table 2). The non-hyperlipidemic group exhibited significantly decreased serum levels of CTRP9, tAPN, and HMW APN in Group II compared to Group I (p < 0.05, Table 3). Quantitative data and ordinal data between two non-hyperlipidemia subgroups demonstrated positive correlation between serum CTRP9 concentration and serum levels of all APN isoforms (including tAPN, HMW, MMW, and LMW APN), age, HDL, and vitamin B12 (p < 0.01), and negative correlation with diastolic blood pressure and serum triglyceride levels (p < 0.01, Table 4).
In the non-hyperlipidemic group, thirteen independent variables (age, sex, smoking status, drinking history, family history, hypertension, diabetes mellitus, homocysteine, vitamin B12 and CTRP9, HMW, MMW, LMW) were included in the binary logistic regression for analyzing ischemic stroke risk factors. Age exceeding 60 [OR (95% CI), 3.305 (1.573–6.947), p < 0.01], smoking [2.434 (1.074–5.520), p < 0.05], hypertension [3.557 (1.742–7.263), p < 0.001] and vitamin B12 deficiency [2.653 (1.206–5.835), p < 0.05] were independent risk factors to ischemic stroke. High CTRP9 concentration was a protective factor against ischemic stroke [0.400 (0.197—0.810); p < 0.05] (Table 5).
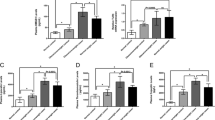
The levels of serum CTRP9 and various APN isoforms in patients at different stages of ischemic stroke were compared against control. Serum CTRP9, tAPN, and HMW APN decreased gradually after ischemic stroke onset, which decreases in stage II and stage III were statistically significant (p < 0.05), and restored in Stage IV. MMW and LMW APN exhibited significant transient increase in Stage I. (p < 0.05, Table S1).
Discussion
To the best of our knowledge, this is the first study comprehensively investigating the association of serum CTRP9 and APN isoforms with cerebral ischemic stroke onset and stages in a clinical setting. We reported several significant findings. First, the serum concentration of CTRP9, tAPN, and HMW APN significantly decrease in ischemic stroke patients without hyperlipidemia. Elevated CTRP9 level is likely to exert independently protective effect against stroke onset. Second, serum CTRP9, tAPN, and HMW APN gradually decrease initially and eventually restored by the late stage infarct period.
Systematic inflammatory response is actively involved in cerebral infarction development [20]. Cerebral infarction shares common pathological mechanisms with cardiovascular diseases such as coronary heart disease [21]. Clinical trials demonstrated that increased CTRP9 is an independent protective factor for coronary heart disease [22]. To exclusively determine the role of serum CTRP9 and APN in cerebral infarct pathophysiology, we patients of excluded coronary heart disease from this study.
A long list of predisposing factors are associated with ischemic stroke risk, including but not limited to age, hypertension, type 2 diabetes, obesity, hyperlipidemia, and heart diseases, etc. [1, 23]. More than 40% of stroke patients have no such risk condition [24]. Obesity, a well-documented modifiable risk factor, is characterized by derangements of adipose-derived hormones [23, 25]. APN (also known as Acrp30, AdipoQ, GBP-28, and apM1) is the most abundant peptide secreted by adipocytes, and plays a central role in obesity-related diseases [26]. Studies have demonstrated that APN has insulin-sensitizing, anti-atherogenic, and anti-inflammatory effects [27, 28].
Leaving aside consistently reported protection, the relationship between serum APN level and risk of ischemic stroke is inconsistent in clinical studies [23]. Decreased APN level has been associated with increased ischemic stroke risk independently or indirectly [29,30,31]. In contrast, the Northern Manhattan Study (NOMAS) reported association between lower circulating APN level and decreased ischemic stroke risk [32]. A recent meta-analysis also reported a positive association between serum APN level and ischemic stroke risk [33]. In this study we found significant association of APNs/CTRP9 with stroke in subjects with normal blood lipids, which association was not the case in the whole sample. This implies that the major confounding factor behind the inconsistency may be the heterogeneity across the study samples.
The etiology of the inconsistency across studies may also arise from the coexistence of multiple APN isoforms and their temporal patterns in infarct subtypes and stages, given that different metabolic disorders may underlie the same diagnosis of cerebral ischemic stroke. Total concentrations aside, isoform distribution must be considered in the interpretation of serum APN level [34]. A case–control study demonstrated that serum APN concentration in large artery atherosclerotic (LAA) subtype of ischemic stroke patients significantly decreased in non-stroke subjects compared to non-LAA stroke patients, with no significant difference between non-LAA patients and non-stroke subjects [35]. The observed trend in this study that CTRP9, tAPN, and HMW APN decreased gradually and increased to baseline during ischemic stroke is consistent with evidence suggesting that CTRP9 and HMW unequivocally harbor protective effect within the nervous system [36,37,38]. CTRP9, a novel adipokine first reported in 2009, expresses predominantly in adipose tissue [39]. As a paralog of APN, CTRP9 shares 51% amino acid sequence with APN [39]. CTRP9 regulates energy metabolism, modulates vasomotion, protects endothelial cells, inhibits platelet activation and pathological vascular remodeling, stabilizes atherosclerotic plaques, and protects the heart [40].
Emerging evidence suggests that CTRP9 exerts neuroprotective effects upon central nervous system diseases [41]. CTRP9 has a high affinity to APN receptor 1 (AdipoR1), which is widely expressed in the central nervous system, especially on neurons [42, 43]. AdipoR1 agonists protect the brain against ischemic stroke and intracerebral hemorrhage via inhibiting neuronal apoptosis [36, 44]. Nevertheless, some (albeit few) researches reported the association of serum CTRP9 level with ischemic stroke risk in humans [45]. The levels of serum CTRP9 and tAPN in patients at early stage (within 3 days) of ischemic stroke were decreased compared to control [45]. Heretofore, no investigation focused on the pattern of APN/CTRP9 response to stages of cerebral ischemic stroke to the best of our knowledge.
Closely related to abnormal adipose metabolism, hyperlipidemia is an important and independent risk factor for ischemic stroke [1, 3]. As expected, tAPN, HMW APN, and CTRP9 levels in the hyperlipidemic group were decreased compared to the non-hyperlipidemic group. To avoid the confounding variable of lipid concentration, a subgroup analysis per blood lipid level was completed in this study. In the non-hyperlipidemic group, the levels of CTRP9, tAPN, and HMW APN were decreased in stroke patients compared to control. This implies that decreased levels of CTRP9 and APN may be risk factors of ischemic stroke independent of hyperlipidemia. Previously we reported that the HMW APN isoform is most closely associated with cardiovascular diseases-related biochemical indicators, total cholesterol, high-density lipoprotein cholesterol, and uric acid, and therefore an independent predictor of cardiovascular diseases [15]. Meanwhile, high serum CTRP9 is an independent protective factor for metabolic syndrome, and is correlate with decreased hyperlipidemia indicators such as cholesterol, triglyceride, and low density lipoprotein [46]. In this study, binary logistic regression in the non-hyperlipidemic group revealed age > 60, smoking, hypertension, and low vitamin B12 were risk factors of ischemic stroke, while increased CTRP9 was a protective factor of cerebral infarction. When CTRP9 was removed from the initial model, high concentration of HMW exerted protective factor for ischemic stroke (details not presented). In the non-hyperlipidemic subgroup, serum CTRP9 were positively correlated with APN (including tAPN, HMW, MMW, and LMW APN), age, HDL, and vitamin B12, and negatively correlated with diastolic blood pressure and triglyceride.
Diabetes was surprisingly not among the risk factors of stroke in this study. One of the possible reasons may rest in the fact that patients with coronary heart disease were excluded from this study. Coronary atherosclerosis is the central pathology of coronary heart diseases. As a component of metabolic syndrome, diabetes has extensive crosstalk with hyperlipidemia and atherosclerosis. Therefore, exclusion of patients with coronary heart diseases would simultaneously exclude diabetes patients with more complications. The remaining patients with “benign diabetes”, a term coined presently to represent potential diabetic subtypes imposing less damage to other organs, may contribute to lack of association between diabetes and stroke risk.
Several limitations exist in the current work. First, this is a cross-sectional study, making it difficult to establish causal inference. Second, the sample size is not very large. Further studies with larger sample size are warranted. Third, the effect of concurrent diseases and current active medications were not considered, which may affect serum CTRP9 and/or APN levels and thereby act as confounding factors. This may also underlie the observed paradox of lower total cholesterol and LDL cholesterol, as well as lower prevalence of hyperlipidemia in patients than in controls, as shown in Table 1.
Conclusions
In summary, serum CTRP9 was closely correlated to tAPN and HMW APN. Increased serum CTRP9 is an independent protective factor for ischemic stroke. Reduced serum CTRP9, tAPN, and HMW APN may impose increased ischemic stroke risk, and may serve as biomarkers of early ischemic stroke stage. Assays of these molecules’ concentrations may increase precision of diagnosis, subtyping, and staging of cerebral ischemic stroke. Large-scale multiple-center collaborative efforts investigating the effects of CTRP9 and APN upon cardiovascular and cerebrovascular diseases are warranted.
Availability of data and materials
All data is ready to be uploaded on request.
Abbreviations
- ABC-ELISA:
-
Avidin–Biotin Peroxdase Complex-enzyme-linked Immunosorbent Assay
- APN:
-
Adiponectin
- CI:
-
Confidence interval
- CTRP:
-
C1q/TNF-Related Protein
- HDL:
-
High-density lipoprotein
- HMW:
-
High-molecular weight
- LAA:
-
Large artery atherosclerotic
- LDL:
-
Low-density lipoprotein
- LMW:
-
Low-molecular weight
- MMW:
-
Middle-molecular weight
- tAPN:
-
Total adiponectin
References
Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160–236. https://doi.org/10.1161/str.0000000000000024.
Evans MA, Broughton BRS, Drummond GR, Ma H, Phan TG, Wallace EM, et al. Amnion epithelial cells - a novel therapy for ischemic stroke? Neural Regen Res. 2018;13(8):1346–9. https://doi.org/10.4103/1673-5374.235223.
Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, et al. Heart disease and stroke statistics-2018 update: a report from the american heart association. Circulation. 2018;137(12):e67–492. https://doi.org/10.1161/cir.0000000000000558.
Choe SS, Huh JY, Hwang IJ, Kim JI, Kim JB. Adipose tissue remodeling: its role in energy metabolism and metabolic disorders. Front Endocrinol (Lausanne). 2016;7:30. https://doi.org/10.3389/fendo.2016.00030.
El-Lebedy DH, Ibrahim AA, Ashmawy IO. Novel adipokines vaspin and irisin as risk biomarkers for cardiovascular diseases in type 2 diabetes mellitus. Diabetes Metab Syndr. 2018;12(5):643–8. https://doi.org/10.1016/j.dsx.2018.04.025.
Acquarone E, Monacelli F, Borghi R, Nencioni A, Odetti P. Resistin: a reappraisal. Mech Ageing Dev. 2019;178:46–63. https://doi.org/10.1016/j.mad.2019.01.004.
Ohashi K, Shibata R, Murohara T, Ouchi N. Role of anti-inflammatory adipokines in obesity-related diseases. Trends Endocrinol Metab. 2014;25(7):348–55. https://doi.org/10.1016/j.tem.2014.03.009.
Mancuso P, Curtis JL, Freeman CM, Peters-Golden M, Weinberg JB, Myers MG, et al. Ablation of the leptin receptor in myeloid cells impairs pulmonary clearance of streptococcus pneumoniae and alveolar macrophage bactericidal function. Am J Physiol Lung Cell Mol Physiol. 2018;315(1):L78-l86. https://doi.org/10.1152/ajplung.00447.2017.
Wei WY, Ma ZG, Zhang N, Xu SC, Yuan YP, Zeng XF, et al. Overexpression of CTRP3 protects against sepsis-induced myocardial dysfunction in mice. Mol Cell Endocrinol. 2018;476:27–36. https://doi.org/10.1016/j.mce.2018.04.006.
Magkos F, Sidossis LS. Recent advances in the measurement of adiponectin isoform distribution. Curr Opin Clin Nutr Metab Care. 2007;10(5):571–5. https://doi.org/10.1097/MCO.0b013e3282bf6ea8.
Liu M, Liu F. Regulation of adiponectin multimerization, signaling and function. Best Pract Res Clin Endocrinol Metab. 2014;28(1):25–31. https://doi.org/10.1016/j.beem.2013.06.003.
Awazawa M, Ueki K, Inabe K, Yamauchi T, Kubota N, Kaneko K, et al. Adiponectin enhances insulin sensitivity by increasing hepatic IRS-2 expression via a macrophage-derived IL-6-dependent pathway. Cell Metab. 2011;13(4):401–12. https://doi.org/10.1016/j.cmet.2011.02.010.
Schraw T, Wang ZV, Halberg N, Hawkins M, Scherer PE. Plasma adiponectin complexes have distinct biochemical characteristics. Endocrinology. 2008;149(5):2270–82. https://doi.org/10.1210/en.2007-1561.
Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, et al. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem. 2004;279(13):12152–62. https://doi.org/10.1074/jbc.M311113200.
Wang W, Xing W, Zhang H, Ding M, Shang L, Lau WB, et al. Reduced high-molecular-weight adiponectin is an independent risk factor for cardiovascular lesions in hypercholesterolaemic patients. Clin Endocrinol (Oxf). 2013;78(4):539–44. https://doi.org/10.1111/j.1365-2265.2012.04444.x.
Zhao L, Chen S, Sherchan P, Ding Y, Zhao W, Guo Z, et al. Recombinant CTRP9 administration attenuates neuroinflammation via activating adiponectin receptor 1 after intracerebral hemorrhage in mice. J Neuroinflammation. 2018;15(1):215. https://doi.org/10.1186/s12974-018-1256-8.
Chen X-R, Shen T-Z. Imaging of cerebral infarct. Chin J Med Comput Imaging. 2000;6(1):35.
Joint Committee for Developing Chinese guidelines on Prevention and Treatment of Dyslipidemia in Adults. 2016 Chinese guideline for the management of dyslipidemia in adults. Zhonghua Xin Xue Guan Bing Za Zhi. 2016;44(10):833–53. https://doi.org/10.3760/cma.j.issn.0253-3758.2016.10.005.
Curtis LH, Hammill BG, Eisenstein EL, Kramer JM, Anstrom KJ. Using inverse probability-weighted estimators in comparative effectiveness analyses with observational databases. Med Care. 2007;45(10 Supl 2):S103-7. https://doi.org/10.1097/MLR.0b013e31806518ac.
Ramiro L, Simats A, García-Berrocoso T, Montaner J. Inflammatory molecules might become both biomarkers and therapeutic targets for stroke management. Ther Adv Neurol Disord. 2018;11:1756286418789340. https://doi.org/10.1177/1756286418789340.
Egerer E, Siemonsen S, Erbguth F. Acute diseases of the brain and heart : a reciprocal culprit-victim relationship. Med Klin Intensivmed Notfmed. 2018;113(6):456–63. https://doi.org/10.1007/s00063-018-0465-3.
Shi J-H, Cui J-J. The correlation between CTRP9, APN, SAA, hs-CRP levels and coronary plaque stability in patients with coronary heart disease. Chin J Gerontol. 2018;38(14):4.
Meadows KL. Ischemic stroke and select adipose-derived and sex hormones: a review. Hormones (Athens). 2018;17(2):167–82. https://doi.org/10.1007/s42000-018-0034-4.
Donnan GA, Fisher M, Macleod M, Davis SM. Stroke Lancet. 2008;371(9624):1612–23.
Badimon L, Cubedo J. Adipose tissue depots and inflammation: effects on plasticity and resident mesenchymal stem cell function. Cardiovasc Res. 2017;113(9):1064–73. https://doi.org/10.1093/cvr/cvx096.
Achari AE, Jain SK. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int J Mol Sci. 2017;18(6):1321. https://doi.org/10.3390/ijms18061321.
Gao LR, Zhang NK, Zhang Y, Chen Y, Wang L, Zhu Y, et al. Overexpression of apelin in Wharton’ jelly mesenchymal stem cell reverses insulin resistance and promotes pancreatic beta cell proliferation in type 2 diabetic rats. Stem Cell Res Ther. 2018;9(1):339. https://doi.org/10.1186/s13287-018-1084-x.
Natsukawa T, Maeda N, Fukuda S, Yamaoka M, Fujishima Y, Nagao H, et al. Significant association of serum adiponectin and creatine kinase-MB levels in ST-segment elevation myocardial infarction. J Atheroscler Thromb. 2017;24(8):793–803. https://doi.org/10.5551/jat.38232.
Matsumoto M, Ishikawa S, Kajii E. Association of adiponectin with cerebrovascular disease: a nested case-control study. Stroke. 2008;39(2):323–8. https://doi.org/10.1161/strokeaha.107.497552.
Chen MP, Tsai JC, Chung FM, Yang SS, Hsing LL, Shin SJ, et al. Hypoadiponectinemia is associated with ischemic cerebrovascular disease. Arterioscler Thromb Vasc Biol. 2005;25(4):821–6. https://doi.org/10.1161/01.ATV.0000157784.25920.a7.
Stott DJ, Welsh P, Rumley A, Robertson M, Ford I, Sattar N, et al. Adipocytokines and risk of stroke in older people: a nested case-control study. Int J Epidemiol. 2009;38(1):253–61. https://doi.org/10.1093/ije/dyn215.
Gardener H, Goldberg R, Mendez AJ, Wright CB, Rundek T, Elkind MS, et al. Adiponectin and risk of vascular events in the Northern Manhattan study. Atherosclerosis. 2013;226(2):483–9. https://doi.org/10.1016/j.atherosclerosis.2012.11.020.
Gorgui J, Gasbarrino K, Georgakis MK, Karalexi MA, Nauche B, Petridou ET, et al. Circulating adiponectin levels in relation to carotid atherosclerotic plaque presence, ischemic stroke risk, and mortality: a systematic review and meta-analyses. Metabolism. 2017;69:51–66. https://doi.org/10.1016/j.metabol.2017.01.002.
Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, et al. Impaired multimerization of human adiponectin mutants associated with diabetes. molecular structure and multimer formation of adiponectin. J Biol Chem. 2003;278(41):40352–63. https://doi.org/10.1074/jbc.M300365200.
Kim BJ, Lee SH, Ryu WS, Kim CK, Yoon BW. Adipocytokines and ischemic stroke: differential associations between stroke subtypes. J Neurol Sci. 2012;312(1–2):117–22. https://doi.org/10.1016/j.jns.2011.08.007.
Zhao L, Zhang JH, Sherchan P, Krafft PR, Zhao W, Wang S, et al. Administration of rCTRP9 attenuates neuronal apoptosis through Adipor1/PI3K/Akt signaling pathway after ICH in mice. Cell Transplant. 2019;28(6):756–66. https://doi.org/10.1177/0963689718822809.
Yang J, Du G, Wang J, Chen J, Yang C, Li J, et al. Reduced serum adiponectin level and risk of poststroke depression in patients with ischemic stroke. J Stroke Cerebrovasc Dis. 2019;28(2):305–10. https://doi.org/10.1016/j.jstrokecerebrovasdis.2018.09.057.
Bai H, Zhao L, Liu H, Guo H, Guo W, Zheng L, et al. Adiponectin confers neuroprotection against cerebral ischemia-reperfusion injury through activating the cAMP/PKA-CREB-BDNF signaling. Brain Res Bull. 2018;143:145–54. https://doi.org/10.1016/j.brainresbull.2018.10.013.
Wong GW, Krawczyk SA, Kitidis-Mitrokostas C, Ge G, Spooner E, Hug C, et al. Identification and characterization of CTRP9, a novel secreted glycoprotein, from adipose tissue that reduces serum glucose in mice and forms heterotrimers with adiponectin. FASEB J. 2009;23(1):241–58. https://doi.org/10.1096/fj.08-114991.
Yang C, Fan F, Sawmiller D, Tan J, Wang Q, Xiang Y. C1q/TNF-related protein 9: a novel therapeutic target in ischemic stroke? J Neurosci Res. 2019;97(2):128–36. https://doi.org/10.1002/jnr.24353.
Li Q, Zhu Z, Wang C, Cai L, Lu J, Wang Y, et al. CTRP9 ameliorates cellular senescence via PGC1alpha/AMPK signaling in mesenchymal stem cells. Int J Mol Med. 2018;42(2):1054–63. https://doi.org/10.3892/ijmm.2018.3666.
Thundyil J, Pavlovski D, Sobey CG, Arumugam TV. Adiponectin receptor signalling in the brain. Br J Pharmacol. 2012;165(2):313–27. https://doi.org/10.1111/j.1476-5381.2011.01560.x.
Kambara T, Shibata R, Ohashi K, Matsuo K, Hiramatsu-Ito M, Enomoto T, et al. C1q/tumor necrosis factor-related protein 9 protects against acute myocardial injury through an adiponectin receptor I-AMPK-dependent mechanism. Mol Cell Biol. 2015;35(12):2173–85. https://doi.org/10.1128/mcb.01518-14.
Xu N, Zhang Y, Doycheva DM, Ding Y, Zhang Y, Tang J, et al. Adiponectin attenuates neuronal apoptosis induced by hypoxia-ischemia via the activation of AdipoR1/APPL1/LKB1/AMPK pathway in neonatal rats. Neuropharmacology. 2018;133:415–28. https://doi.org/10.1016/j.neuropharm.2018.02.024.
Lin X-D, Shao A-M. Analysis of correlation between serum CTRP9 APN level and carotid atherosclerosis in patients with ACI. Zhejiang Clinical Medical Journal. 2015;17(1):3.
Hwang YC, Woo OhS, Park SW, Park CY. Association of serum C1q/TNF-Related Protein-9 (CTRP9) concentration with visceral adiposity and metabolic syndrome in humans. Int J Obes (Lond). 2014;38(9):1207–12. https://doi.org/10.1038/ijo.2013.242.
Acknowledgements
None
Funding
This research was supported by National Natural Science Foundation of China (No. 81470413, 81270401, 82070263, and 81700327), and Fund Program for the Scientific Activities of Selected Returned Overseas Professionals in Shanxi Province (2018–1059).
Author information
Authors and Affiliations
Contributions
YQZ drafted the manuscript. YWZ, XGL, and YQZ conducted the statistical analyses. JLD, CL, XMW, and RL recruited study participants, as well as collected blood samples and clinical records. CL and XMW processed the blood samples and conducted laboratory measurements. RL and XGL designed the study and finalized the manuscript. WQW, WBL and HFZ helped in improving the manuscript. WBL and HFZ were responsible for the methodology. WQW managed the data and was in charge of data curation. The author(s) read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Institutional Review Board of Xijing Hospital (Approval No.: XJ20160443). All study participants gave written consent for study participation.
Consent for publication
Not applicable.
Competing interests
On behalf of all authors, the corresponding author states that there is no conflict of competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Serum concentration of CTRP9 and APN in patients of different infarct stages.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, YQ., Zhang, YW., Dai, JL. et al. Serum CTRP9 and high-molecular weight adiponectin are associated with ischemic stroke. BMC Neurol 22, 429 (2022). https://doi.org/10.1186/s12883-022-02967-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-022-02967-w