Abstract
Background
Acute encephalopathy with biphasic seizures and late reduced diffusion (AESD) and mild encephalopathy associated with excitotoxicity (MEEX) are the most frequent acute encephalopathies in pediatric patients in Japan. AESD typically presents with biphasic seizures and delayed reduced diffusion in the subcortical area, called bright tree appearance (BTA), on radiological examination. In patients with AESD, arterial spin labeling (ASL) shows decreased cerebral blood flow (CBF) in the hyperacute stage and increased CBF in the acute stage, suggesting the usefulness of ASL for the early diagnosis of AESD. Additionally, proton magnetic resonance spectroscopy (MRS) shows elevated glutamate (Glu) and glutamine (Gln) in AESD. MEEX is a group of mild encephalopathies with transient elevation of Gln on MRS similar to that in AESD; however, MEEX does not include any clinical biphasic course or abnormalities, including BTA on diffusion-weighted imaging. Although the usefulness of ASL for AESD has been reported, there are no reports for patients with MEEX. In this study, we report our experience with a 4-year-old girl diagnosed with MEEX who showed unique findings on ASL.
Case presentation
The patient was a 4-year-old girl admitted to the emergency room with febrile status epilepticus. Considering the possibility of AESD, vitamin therapy was initiated. ASL-MR imaging (MRI) of the brain performed on the second day showed increased blood flow in the frontal, temporal, and occipital regions with spared central sulcus, which indicated AESD with central sparing. The patient was diagnosed with AESD, and the treatment included pulse steroid therapy and immunoglobulin therapy from day 3. The patient remained mildly unconscious but gradually became conscious by day 7 with no seizures. Brain MRI performed on day 8 did not show any characteristic AESD findings, such as BTA. Furthermore, MRS showed elevated Gln, which, along with the clinical course, led to the diagnosis of MEEX. The patient was discharged on day 16 without obvious sequelae.
Conclusions
ASL may be useful in the early diagnosis of MEEX as well as AESD, facilitating early intervention.
Similar content being viewed by others
Background
Acute encephalopathy with biphasic seizures and late reduced diffusion (AESD) is the most frequent subtype of pediatric encephalopathy in Japan, with an estimated 100–200 cases per year [1]. AESD is characterized by a biphasic clinical course and delayed imaging findings [2, 3]. The first symptom of AESD is a prolonged febrile seizure (early seizure), after which the disturbance of consciousness tends to improve (almost resolved in 20–30% of cases) [4]. However, the seizures recur within 4–6 days (late seizures), and the disturbance of consciousness worsens [4]. After a second seizure cluster, aphasia, loss of spontaneity, and stereotypic movements become apparent [2]. Magnetic resonance imaging (MRI) shows no abnormality between days 1 and 2, but it shows restricted diffusion—high signal intensity on diffusion-weighted imaging (DWI) and low signal intensity on the apparent diffusion coefficient (ADC) map—of subcortical white matter usually with bifrontal predominance, which is called bright tree appearance (BTA) during days 3 to 9 [3, 5]. Interestingly, BTA, in most cases, is not observed in the pre- and post-central gyri, which is called “central sparing” [2, 6]. Since the perirolandic regions of infants aged between 1 and 2 are considered resistant to ischemic injury [7], the presumed pathophysiological implication of central sparing is thought to be ischemic damage [5]. In addition, MR spectroscopy (MRS) in patients with AESD show elevated glutamate (Glu) from days 1 to 4, followed by elevated glutamine (Gln) from days 4 to 12 [8].
Mild encephalopathy associated with excitotoxicity (MEEX) is characterized by impaired consciousness lasting > 24 h with a prolonged febrile seizure but without late seizures and BTA on MRI. Similar to AESD, MEEX also shows Glu elevation followed by subacute Gln elevation on MRS. Therefore, AESD and MEEX are considered part of the same disease spectrum characterized by excitotoxicity caused by the Glu/Gln complex [9].
Arterial spin labeling (ASL) is a noninvasive MR cerebral blood flow (CBF) imaging method that does not use a contrast agent [10]. ASL in patients with AESD shows decreased CBF in the hyperacute phase (8–22 h after early seizures) but increased CBF in the acute phase (within 24 h after late seizures or on days 3–5 after early seizures) [6, 11]. The usefulness of ASL for AESD has been reported; however, it has not been confirmed in patients with MEEX [6, 11, 12]. In this study, we report our experience of a case involving a 4-year-old girl diagnosed with MEEX, which demonstrated unique findings on ASL.
Case presentation
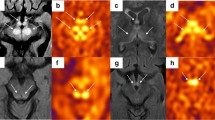
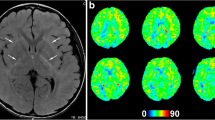
A 4-year-old girl with no significant medical history was brought to the emergency department with febrile status epilepticus. Approximately 10 h after the onset of fever, generalized tonic–clonic convulsions appeared, lasting approximately 50 min. After the generalized seizures subsided, clonic convulsions of the left upper extremity continued in clusters. Midazolam, fosphenytoin sodium hydrate, and levetiracetam were administered. After the convulsions sudsided, in about 90 min, the patient remained mildly unconscious; therefore, head computed tomography and cerebrospinal fluid examination were performed, but no abnormalities were found. Human parainfluenza virus type 3 and human rhinovirus type C were co-detected using FilmArray® Respiratory 2.1 panel and in-house reverse transcription loop-mediated isothermal amplification assays from nasopharyngeal specimen, and they were thought to be the source of the fever. Because of the possibility of AESD, vitamin therapy (vitamin B1 100 mg/day, vitamin B6 20 mg/kg/day, and L-carnitine 30 mg/kg/day) was administered on the first day [13]. Although brain MRI including T1, T2, DWI, and ADC map on the second day (33 h after early seizure) showed no abnormalities (Fig. 1A–C), ASL showed increased blood flow in the frontal, temporal, and occipital regions with spared central sulcus, similar to the central sparing of AESD (Fig. 2A–C). In addition, electroencephalogram (EEG) showed high-voltage slow waves, especially in the right occipital region (Fig. 3A). The patient was diagnosed with AESD because of the prolonged disturbance of consciousness and ASL results at 33 h that pointed to a diagnosis of AESD in the acute stage. Therefore, she was treated with pulse steroid therapy and immunoglobulin therapy in addition to vitamin therapy from the third day. The patient remained mildly unconscious, but she gradually became conscious by the seventh day, and no late seizures occurred. A brain MRI performed on the eighth day did not show BTA; however, the cerebrum was generally and mildly atrophic (Fig. 1D–F), and this could possibly have been caused by pulse steroid therapy. Additionally, ASL performed on the eighth day showed no apparent abnormal CBF distribution (Fig. 2D–F). Furthermore, Gln elevation was observed on MRS on the eighth day (Fig. 3B). This finding together with the clinical course led to a diagnosis of MEEX. The vitamin cocktail treatment was completed after a total of 10 days. Although EEG still showed slow waves in the occipital lobe, there were no problems in motor function, conversation, or the state of consciousness. The patient was discharged on the sixteenth day without obvious sequelae.
Discussion and conclusions
In this study, the MRI on the second day (33 h after early seizure) showed abnormal blood flow distribution on ASL, which led us to suspect AESD and to commence early treatment, resulting in discharge without sequelae. Although several scoring models for AESD and MEEX have been advocated, there are currently no validated biomarkers in the early phase, making it difficult to distinguish between AESD, MEEX, and early complex febrile seizures [4, 6]. Previous reports have shown that blood flow measurement using ASL can be effective in identifying seizures [10]. It is also known that in the first 4 h after a seizure, there is evidence of focal hypoperfusion [14, 15]. To date, there have been a few reports on the utility of ASL in patients with AESD [6, 11]. Most patients with AESD show decreased CBF in the hyperacute phase (8–22 h after first seizure) and increased CBF in the acute phase, suggesting that ASL may be useful for the early diagnosis of AESD but should be performed > 8 h after the onset of early seizures to exclude the possibility of hypoperfusion due to seizures [6, 11]. Conversely, ASL in patients with complex febrile seizures shows variable blood flow patterns without central sparing [16]. In our case, the MRI performed 33 h after the first seizure showed increased blood flow in the frontal, temporal, and occipital regions with spared central sulcus, which was similar to that observed in patients with AESD in the acute phase (Table 1).
Considering that MEEX and AESD share the same pathophysiology, we assume that an MRI for patients with MEEX should be performed ≥ 8 h after the early seizure as well as AESD. Further research is necessary to prove the above hypothesis. Although we diagnosed our patient with MEEX based on the clinical course and findings of MRI and MRS, ASL in our patient also showed a central sparing pattern similar to that in AESD. This is the first case confirming the usefulness of ASL in the diagnosis of MEEX
In conclusion, considering that AESD and MEEX are within the same spectrum of encephalopathy associated with excitotoxicity, ASL may be useful in the early diagnosis of MEEX as well as AESD, allowing for the initiation of early intervention.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- AESD:
-
Acute encephalopathy with biphasic seizures and late reduced diffusion
- MEEX:
-
Mild encephalopathy associated with excitotoxicity
- BTA:
-
Bright tree appearance
- MRI:
-
Magnetic resonance imaging
- MRS:
-
Magnetic resonance spectroscopy
- ASL:
-
Arterial spin labeling
- CBF:
-
Cerebral blood flow
- DWI:
-
Diffusion-weighted imaging
- ADC:
-
Apparent diffusion coefficient
- EEG:
-
Electroencephalogram
References
Hoshino A, Saitoh M, Oka A, Okumura A, Kubota M, Saito Y, et al. Epidemiology of acute encephalopathy in Japan, with emphasis on the association of viruses and syndromes. Brain Dev. 2012;34:337–43. https://doi.org/10.1016/j.braindev.2011.07.012.
Mizuguchi M, Yamanouchi H, Ichiyama T, Shiomi M. Acute encephalopathy associated with influenza and other viral infections. Acta Neurol Scand. 2007;115:45–56. https://doi.org/10.1111/j.1600-0404.2007.00809.x.
Takanashi J, Oba H, Barkovich AJ, Tada H, Yamanouchi H, et al. Diffusion MRI abnormalities after prolonged febrile seizures with encephalopathy. Neurology. 2006;66:1304–9. https://doi.org/10.1212/01.wnl.0000210487.36667.a5 Discussion 1291.
Takanashi JI, Murofushi Y, Hirai N, Sano K, Matsuo E, Saito K, et al. Prognostic value of MR spectroscopy in patients with acute excitotoxic encephalopathy. J Neurol Sci. 2020;408:116636. https://doi.org/10.1016/j.jns.2019.116636.
Sanefuji M, Ichimiya Y, Kaku N, Sasazuki M, Yonemoto K, Torio M, et al. Vascular pathomechanism in acute encephalopathy with biphasic seizures and late reduced diffusion. J Neurol Sci. 2018;395:141–6. https://doi.org/10.1016/j.jns.2018.10.007.
Uetani H, Kitajima M, Sugahara T, Muto Y, Hirai K, Kuroki Y, et al. Perfusion abnormality on three-dimensional arterial spin labeling in patients with acute encephalopathy with biphasic seizures and late reduced diffusion. J Neurol Sci. 2020;408:116558. https://doi.org/10.1016/j.jns.2019.116558.
Barkovich AJ. MR and CT evaluation of profound neonatal and infantile asphyxia. AJNR Am J Neuroradiol. 1992;13:959–72 (Discussion 973).
Takanashi J, Mizuguchi M, Terai M, Barkovich AJ. Disrupted glutamate-glutamine cycle in acute encephalopathy with biphasic seizures and late reduced diffusion. Neuroradiol. 2015;57:1163–8. https://doi.org/10.1007/s00234-015-1573-x.
Hirai N, Yoshimaru D, Moriyama Y, Yasukawa K, Takanashi JI. A new infectious encephalopathy syndrome, clinically mild encephalopathy associated with excitotoxicity (MEEX). J Neurol Sci. 2017;380:27–30. https://doi.org/10.1016/j.jns.2017.06.045.
Telischak NA, Detre JA, Zaharchuk G. Arterial spin labeling MRI: Clinical applications in the brain. J Magn Reson Imaging. 2015;41:1165–80. https://doi.org/10.1002/jmri.24751.
Kuya K, Fujii S, Miyoshi F, Ohno K, Shinohara Y, Maegaki Y, et al. A case of acute encephalopathy with biphasic seizures and late reduced diffusion: Utility of arterial spin labeling sequence. Brain Dev. 2017;39:84–8. https://doi.org/10.1016/j.braindev.2016.07.003.
Ando K. MR spectroscopy even has power to predict outcome of acute encephalopathy. J Neurol Sci. 2020;408: 116637. https://doi.org/10.1016/j.jns.2019.116637.
Fukui KO, Kubota M, Terashima H, Ishiguro A, Kashii H. Early administration of vitamins B1 and B6 and l-carnitine prevents a second attack of acute encephalopathy with biphasic seizures and late reduced diffusion: A case control study. Brain Dev. 2019;41:618–24. https://doi.org/10.1016/j.braindev.2019.02.015.
Farrell JS, Gaxiola-Valdez I, Wolff MD, David LS, Dika HI, Geeraert BL, et al. Postictal behavioural impairments are due to a severe prolonged hypoperfusion/hypoxia event that is COX-2 dependent. eLife. 2016;5:e19352. https://doi.org/10.7554/eLife.19352.
Farrell JS, Colangeli R, Wolff MD, Wall AK, Phillips TJ, George A, et al. Postictal hypoperfusion/hypoxia provides the foundation for a unified theory of seizure-induced brain abnormalities and behavioral dysfunction. Epilepsia. 2017;58:1493–501. https://doi.org/10.1111/epi.13827.
Hirano K, Fukuda T. Abnormal cerebral blood flow distributions during the post-ictal phase of febrile status epilepticus in three pediatric patients measured by arterial spin labeling perfusion MRI. No To Hattatsu. 2016;48:213–7.
Hautala M, Arvila J, Pokka T, Mikkonen K, Koskela U, Helander H, et al. Respiratory viruses and febrile response in children with febrile seizures: A cohort study and embedded case-control study. Seizure. 2021;84:69–77. https://doi.org/10.1016/j.seizure.2020.11.007.
Kasai M, Shibata A, Hoshino A, Maegaki Y, Yamanouchi H, Takanashi JI, et al. Epidemiological changes of acute encephalopathy in Japan based on national surveillance for 2014–2017. Brain Dev. 2020;42:508–14. https://doi.org/10.1016/j.braindev.2020.04.006.
Acknowledgements
We thank the patient and the patient’s family whose help and participation made this work possible.
Funding
This work was supported in part by the Japan Agency for Medical Research and Development, AMED (Grant No: JP21fk0108117). The funding agency played no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
Author information
Authors and Affiliations
Contributions
YN, SK, MN, KO, TK, TK, and MO contributed to the study concept and design. YN, SK, HT, SI, AK, NK, MA, DS, NO, KH, TK, KO, TK, and MO contributed to clinical data acquisition. YN, SK, MN, KO, and MO contributed to drafting the manuscript and figures. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethical Committee of the Showa General Hospital (Approval No. REC-094) in Japan and was conducted in accordance with the tenets of the Declaration of Helsinki.
Consent for publication
Written informed consent for publication of the clinical information and materials of the patient in this study were obtained from the patient’s parents.
Competing interests
All authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Nakajima, Y., Kobayashi, S., Tanoue, H. et al. Cerebral blood flow abnormalities with central sparing on arterial spin labeling in mild encephalopathy associated with excitotoxicity: a case report. BMC Neurol 22, 403 (2022). https://doi.org/10.1186/s12883-022-02942-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-022-02942-5