Abstract
Background
Intraplaque hemorrhage (IPH) is a hallmark of carotid plaque vulnerability. We aim to investigate the association between IPH and recurrent ipsilateral ischemic stroke.
Methods
Patients with a recent stroke or transient ischemic attack (TIA) were prospectively recruited and underwent an ultrasonographic examination and carotid HR VWMRI on the side consistent with symptoms. Carotid plaque was defined as carotid intima-media-thickness (IMT) by ultrasound≥1.5 mm. IPH was determined that the ratio of the plaque signal intensity relative to that of adjacent muscle was > 1.5. All enrolled patients were clinically followed until an ipsilateral ischemic stroke, TIA, carotid endarterectomy (CEA)/carotid artery stenting (CAS), or death within 12 months. Univariate analysis was used to analyze the correlation between clinical characteristics and IPH. Kaplan-Meier survival analysis and a log-rank test were used to compare recurrence-free survival time between the IPH and non-IPH groups. Cox regression models evaluated IPH as the predictor of ipsilateral stroke recurrence.
Results
A total of 171 patients (mean age, 60.13 ± 10.04 years; 118 males) were included in the final analysis. Thirty-two patients (18.7%) showed carotid IPH. During the follow-up, patients with carotid IPH suffered 60.9% (14 of 23) of recurrent ipsilateral strokes and 60.0% (3 of 5) TIA. Multivariate Cox regression analysis proved IPH as a strong predictor of ipsilateral stroke; the adjusted hazard ratio (HR) was 6.64 (95% confidence interval [CI], 2.84–15.54, P < 0.001). Meanwhile, Cox regression analysis also proved that IPH could predict recurrent ischemic events; the adjusted HR was 8.08 (95% CI, 3.65–17.91, P < 0.001).
Conclusions
Carotid intraplaque hemorrhage is strongly associated with recurrent ischemic events and could predict recurrent ipsilateral stroke.
Similar content being viewed by others
Introduction
Atherosclerotic lesions are the leading cause of ischemic stroke, especially in the Chinese population [1, 2]. China National Stroke Registry (CNSR) study has shown that atherosclerotic stroke accounts for 60% of the etiology of ischemic stroke [3]. Several clinical studies have confirmed that carotid atherosclerosis stenosis is significantly correlated with the onset of acute ischemic stroke and clinical prognosis [4]. Consequently, the measurement of carotid artery stenosis has been used in many clinical trials for the risk stratification of stroke patients in the past 20 years [5,6,7].
More studies have confirmed that the vulnerable plaque features of carotid atherosclerosis are the potential risk factors for most ischemic events and are significantly correlated with the recurrence of ischemic events [8,9,10,11,12,13]. Therefore, identifying the vulnerable characteristics of carotid artery plaques based on advanced medical imaging technology and exploring the imaging markers that can predict the risk of stroke recurrence have become research focuses in recent years.
However, there are still few studies on the incidence of extracranial carotid intraplaque hemorrhage (IPH) in acute atherosclerotic ischemic stroke patients and whether there is a significant relationship between IPH and the recurrence of ipsilateral stroke. Our study aims to find the correlation between IPH and ipsilateral stroke recurrence, intending to identify the high-risk factors of stroke recurrence and make individualized prevention strategies.
Methods
Study population
Our study was an observational study of single-center. Patients with a recent stroke or transient ischemic attack (TIA) were recruited in Beijing Tiantan Hospital from June 2011 to December 2014. All patients underwent an ultrasonographic examination and carotid high-resolution vessel wall MRI (HR VWMRI) scan on the side consistent with symptoms within 7 days after admission. According to the recent guidelines from AHA/ASA, patients received standardized medical therapy for secondary stroke prevention and were followed up for 12 ±1 months. All patients fulfilled the following criteria: (1) age ≥ 18 years old; (2) onset of symptoms within the last 14 days; (3) National Institutes of Health Stroke Scale score (NIHSS) ≤ 22 when enrolled; (4) carotid intima-media-thickness (IMT) by ultrasound≥1.5 mm. Exclusion criteria: (1) non-cerebral infarction disease including intracranial hemorrhage (intracranial parenchymal hemorrhage, subarachnoid hemorrhage, subdural hematoma or epidural hematoma), tumor, infection, etc.; (2) suspected cardiogenic embolism or previous history of congenital heart disease, rheumatic heart disease, atrial fibrillation, etc.; (3) suspected non-atherosclerotic vascular lesions ((i.e., vasculitis, arterial dissection, or Moyamoya disease); (4) undergoing emergent thrombolysis or interventional treatments; (5) previous history of carotid endarterectomy or carotid stenting; (6) suffering from severe hepatic or renal insufficiency; (7) life expectancy < 1 year; (8) contraindication to MRI examination (including claustrophobia, metal placement in the body, etc.); (9) Severe stenosis or occlusion of the ipsilateral intracranial carotid artery or middle cerebral artery confirmed by magnetic resonance artery (MRA). The Ethics Committee of Beijing Tiantan Hospital approved this study. All study subjects had signed informed consent.
Clinical information assessment
We obtained the patients’ pre-existing conditions from themselves, their relatives, or caregivers. We recorded the clinical assessments for ischemic cerebrovascular events (stroke/TIA), cardiovascular risk factors, and medications during recruitment. We defined the present conditions following the definitions recommended by the related international guidelines: hypertension was determined by the diagnosis at discharge or a history of hypertension [14]; diabetes mellitus was defined by the diagnosis at discharge or a history of diabetes mellitus [15]; hyperlipidemia was defined as low-density lipoprotein (LDL) ≥1.7 mmol/L at admission or a history of hyperlipidemia or receiving lipid-lowering treatment or diagnosis at discharge [16]; coronary artery disease was defined as the previous history of angina pectoris or myocardial infarct [17]; smoking history was defined as continuous or cumulative smoking for more than 6 months in one’s life [18]; family history of stroke was defined as at least one of a patient’s first-grade relatives having a history of stroke [19]. The stroke etiology was determined after the diagnostic work-up of patients, including a US carotid examination, echocardiogram, or a 24-h electrocardiogram.
Carotid MR imaging protocol
To define the symptomatic carotid artery, all patients underwent a routine brain MRI scan and carotid ultrasonographic screen. Then, they were performed on a 3 T MR scanner with 8-channel phase array carotid coils [12]. A standardized multisequence protocol was performed for the carotid artery bifurcation on the symptomatic side by acquiring three-dimensional time of flight (3D-TOF), T1-weighted (T1W), T2-weighted (T2W), and magnetization-prepared rapid acquisition gradient echo (MP-RAGE) sequences [12]. The contrast material was gadolinium diethylenetriamine pentametric acid (GD-DTPA) at an injection dose of 0.1 mmol/Kg. In addition, delayed T1WI scanning was performed for about 5 minutes after intravenous injection. Finally, spectral preservation attenuated inversion recovery (SPAIR) was used in the black blood technique to enhance the contrast between the vessel wall and the surrounding tissue. Therefore, the total scanning time was about 35 minutes. The imaging parameters are as follows [12], (1) 3D-TOF MRA: repetition Time (TR)/echo Time (TE) = 20/4.9 ms, Flip Angle (FA) = 20°; Field of view (FOV) = 140 × 140 mm, matrix size = 256 × 256, slice thickness = 1.0 mm; (2) T1W: TR/TE = 800/10 ms, FA = 90°, FOV = 140 mm × 140 mm, Matrix size = 256 × 256, slice thickness = 2.0 mm; (3) T2W: TR/TE = 4800/50 ms, FA = 90°; FOV = 140 mm × 140 mm, matrix size = 256 × 256, slice thickness = 2.0 mm; (4)MP-RAGE: TR/TE = 8.8/5.3 ms, FA = 15°, FOV = 140 mm × 140 mm, Matrix size = 256 × 256, slice thickness = 1.0 mm.
MRI image analysis
The custom-designed software CASCADE (Vascular Imaging Lab, University of Washington) [20] was used to review the MR vessel wall images of carotid arteries [12]. The lumen and outer vessel boundaries were outlined manually on T2 -weighted images. Then, the lumen area, wall area, total vessel area, and maximum wall thickness were automatically calculated. The reference values were defined as the normal vessels located in the contralateral portion or proximal to the stenotic position. The parameters of plaque were defined as follows: 1) wall area (WA) = vessel area (VA) − lumen area (LA); 2) remodeling index (RI) = vessel area at stenotic lesion/ vessel area at reference vessel. The presence of calcification, lipid-rich necrotic core (LRNC), and IPH were detected and measured quantitatively using the published criteria [21, 22]. In particular, IPH was demonstrated by a hyperintense region in the plaque on TOF, T1W, and MP-RAGE sequence images. Meanwhile, the volumes, percent of wall volume, and component volumes were calculated from the measured areas according to the published criteria [12]. If there were multiple plaques in the symptomatic artery, the plaque leading to the highest degree of stenosis was selected for analysis [23]. The luminal stenosis of carotid arteries was measured using the algorithm of North American Symptomatic Carotid Endarterectomy Trial Collaborators (NASCET) [4] and was divided into 0, 1 to 29%, 30 to 49%, and ≥ 50% categories [12]. The image quality was divided into four grades [24]: grade 1 = the image of the carotid lumen and the outside of the blood vessel was not clear, or the signal-noise ratio (SNR) was low, and there were noticeable motion artifacts; Grade 2 = the carotid artery wall was visible in the image, but the wall structure and the external contour of the vessel could not be judged, and SNR was low, or there were motion artifacts; Grade 3 = the carotid artery wall structure, lumen, and outside vascular contour were clear, the blood flow signal in the lumen was not wholly suppressed, and SNR was high, accompanied by a small amount of movement or swallowing artifacts; Grade 4 = the carotid artery wall structure, lumen, and outside vascular contour were clear, the blood flow signal in the lumen was wholly suppressed, and SNR was high without apparent artifacts. Therefore, the image quality of≤grade 2 was excluded from the statistical analysis. Two experienced senior neuroimaging physicians reviewed all slices of the sequences of carotid plaques on HR VWMRI, blinded to the clinical information and the diffusion-weighted images (DWI). Consensus interpretation was accepted for the final analysis if the two readers’ interpretations were different. According to the reproducibility test, twenty patients were randomly selected from the study population every 2 months to test the consistency of inter-reader and intrareader in measuring carotid plaque morphology and carotid plaque compositions, and the final database was accepted if intraclass correlation coefficients (ICC) > 0.80 [23].
Outcome and follow-up
Patients or their authorized proxies were visited by telephone interview at 3, 6, 9, and 12 months after discharge. The primary outcome was the recurrence of ipsilateral ischemic stroke, defined as a sudden onset of focal neurological deficit lasting > 24 hours in the ipsilateral carotid territory [25]. The terminating points were TIA, carotid endarterectomy (CEA)/carotid artery stenting (CAS), or death [26]. All recurrent ischemic events were confirmed according to the clinical medical records, which were defined as ischemic stroke, TIA, acute coronary syndrome, and peripheral artery disease [26]. Two experienced stroke neurologists reviewed patients’ medical documents to ensure a reliable diagnosis of recurrent ischemic stroke. If the patient’s information was missing or the patient died without hospitalization, two neurologists made the decision based on the medical documents. Any death of patients was confirmed by checking the hospital medical records or local citizen registration. In addition, the following events were also recorded over the entire follow-up period: 1) new atrial fibrillation; 2) other stroke (contralateral stroke/posterior circulation stroke); 3) other vascular events (cardiovascular events/peripheral vascular events); 4) death from non-vascular events; 5) hemorrhage. Two trained stroke neurologists in Beijing Tian Tan hospital completed all the telephone follow-ups of patients.
Statistical analysis
Continuous variables were described by means (standard deviations [SDs]) or medians (interquartile ranges [IQRs]), and categorical data were expressed as frequency and percentage. Cohen’s kappa coefficient and ICC were assessed for inter-reader and intrareader reproducibility. Differences between the IPH and non-IPH groups were analyzed using Student’s t-test or Mann–Whitney U- test for continuous variables according to a normal distribution and chi-squared test or Fisher exact test for categorical variables. By calculating hazard ratio (HR) and 95% confidence interval (CI), we used multivariable Cox regression models to study predictors of stroke recurrence with a P < 0.05 and parameters with clinical significance in univariate analysis. Kaplan-Meier survival analysis and a log-rank test were used to compare recurrence-free survival time with the IPH and non-IPH groups. Two-sided tests were used for all statistical data, with a P < 0.05 considered statistically significant. All statistical analysis was conducted by the SPSS version 22.0 (IBM, NY, USA).
Results
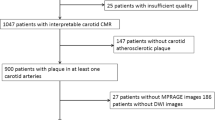
A total of 191 patients were enrolled from June 2011 to December 2014, and 20 patients were excluded due to the following reasons: 1) the clinical messages of 5 patients were lost; 2) 9 patients with poor image qualities; 3) 2 patients withdrew from the study; 4) 4 patients determined cardioembolic stroke etiology during the follow-up. Thus, a total of 171 patients (mean age, 59.66 ± 10.06 years) were included in the final statistical analysis, including 118 males (69.0%) (Fig. 1). The mean follow-up time was (10.39 ± 2.66) months. During the follow-up, 17 patients underwent CEA/CAS, and 11 patients suffered recurrent neurological events before undergoing CAS. Meanwhile, twenty-eight patients (16.4%) occurred ipsilateral cerebral ischemic events, including twenty-three patients (13.5%) with ipsilateral cerebral stroke and five patients (2.9%) with TIA. Intraplaque hemorrhage detected by HR VWMRI was found in 32 of 171 patients (Fig. 2).
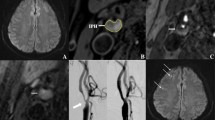
A 64-year-old male patient presented with a right ischemic stroke. IPH is demonstrated by the hyperintensity on (a) 3D-TOF, (b) T1W, (d) MPRAGE, and hyperintensity on (c) T2W (white arrow). There is a hyperintensity lesion in the blood-supply area of the right carotid artery on (d) DWI (green arrow) and no evident ipsilateral intracranial vascular stenosis or occlusion on (e) MRA. DWI, diffusion-weighted images; MRA, magnetic resonance artery; 3D-TOF, three-dimensional time of flight; T1W, T1-weighted; T2W, T2-weighted; MPRAGE, magnetization-prepared rapid acquisition gradient-echo
There were no significant differences in gender, hypertension, hyperlipidemia, coronary heart disease, prior stroke/TIA, family history of stroke, alcohol consuming, serum cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C) between the IPH group and the non-IPH group. However, there were significant differences in age (mean, 64.50 ± 9.60 vs 59.13 ± 9.90, P = 0.006), diabetes mellitus (43.8% vs 25.9%, P = 0.045), smoking history (68.8% vs 46.8%, P = 0.025), NIHSS on admission (3 [0–8] vs 5 [0–18], P = 0.004), pre-admission mRS (1 [0–3] vs 1 [0–5], P = 0.030), hs-CRP (7.71 [0.10–17.90] mg/L vs 3.72 (0.00–31.90) mg/L, P = 0.002), and hcy (18.99 [9.20–75.00] umol/L vs 14.10 [1.11–41.20] umol/L, P = 0.028) between the two groups (Table 1). And Multivariate Logistic regression analysis showed that the factors associated with IPH were age (OR,1.06, 95% CI, 1.01–1.11; P = 0.11), Hcy (OR,1.06, 95% CI, 1.01–1.11; P = 0.012), hs-CRP (OR,1.09, 95% CI, 1.01–1.12; P = 0.022) (Table 1).
Between the IPH group and the non-IPH group, there was a significant difference in the percentage of stenosis degree (31.10 [4–43]% vs 36.35 [6–58]%,P < 0.001). There was no significant difference in vessel area (VA) between the two groups (P = 0.244 > 0.05). However, there were significant differences in the lumen area (LA) (14.30 [2.64–31.96] mm2 vs 16.67 [2.06–42.38] mm2, P = 0.040), wall area (WA) (73.58 [33.10–160.57] mm2 vs 53.22 [28.03–116.38] mm2, P < 0.001), normalized wall index (NMI) (0.69 [0.53–0.97] vs 0.53(0.40–0.94), P < 0.001), wall thickness (WI) (4.40 [3.07–11.35] mm vs 2.65 (1.00–7.56) mm, P < 0.001), plaque area (PA) (29.90 [14.24–71.35] mm2 vs 22.93 [5.61–74.29] mm2, P = 0.001), and remodeling index (RI) (0.72 ± 0.08 vs 0.75 ± 0.08, P = 0.012). At the same time, there were significant differences in the presence of plaque components between the two groups, including calcification (81.3% vs 48.9%, P = 0.001), loose matrix (65.6% vs 58.0%, P = 0.014), and LRNC (100% vs 77.0%, P = 0.003) (Table 1).
Univariate Cox regression analysis showed that several factors were associated with recurrent stroke, including age (HR, 1.05, 95% CI, 1.01–2.00; P = 0.029), taking aspirin until primary outcome (HR, 0.38, 95% CI, 0.16–0.89; P = 0.027), taking statins until primary outcome (HR, 0.27, 95% CI, 0.09–0.78; P = 0.016), and the plaque characteristics such as loose matrix (HR, 4.50, 95% CI, 1.60–12.11; P = 0.003), LRNC (HR, 2.73, 95% CI, 1.21–6.19; P = 0.016),and IPH (HR, 6.40, 95% CI, 2.77–14.82; P < 0.001) (Table 2).
And Multivariate Cox regression analysis showed that the factors associated with recurrent stroke were loose matrix (HR, 4.08, 95% CI, 1.48–11.24; P = 0.007) and IPH (HR, 8.68, 95% CI, 3.62–20.85; P < 0.001). However, the medication of aspirin used until the primary outcome was the protective factor of recurrent stroke (HR, 0.24, 95% CI, 0.10–0.60; P = 0.002) (Table 2).
After adjusting for confounding factors (age, gender, NIHSS on admission, pre-admission mRS, hypertension, diabetes mellitus, coronary heart disease, hyperlipidemia, previous history of stroke, smoking history, and family history of stroke, stenotic degree, aspirin used for 1 year, statins used for 1 year, plaque calcification, loose matrix, lipid-rich necrotic core, and plaque fibrous cap rupture), the prevalence of IPH still could predict recurrent ischemic events (adjusted HR, 6.64, 95% CI, 2.84–15.54; P < 0.001) and ipsilateral recurrent ischemic stroke (adjusted HR,8.08, 95% CI, 3.65–17.91, P < 0.001) (Table 3).
In the IPH group, 14 patients suffered recurrent ipsilateral strokes, and three patients suffered TIA (Table 1). Kaplan–Meier analysis showed that IPH was associated with ipsilateral recurrent stroke (P < 0.001; Fig. 3a) and recurrent cerebral ischemic events at 12 months compared to the non-IPH group (P < 0.001; Fig. 3b).
Survival analysis (Kaplan–Meier plot) figures confirm the predictive value of IPH for (a) stroke and (b) all cerebral ischemic events. The x-axis represents the time of follow-up in months. The y-axis represents the proportion of patients who had ipsilateral stroke recurrence. IPH, intraplaque hemorrhage; HR, hazard ratio
Discussion
Our study investigated the association between IPH and ipsilateral recurrent ischemic stroke. Schindler et al. [13] found that carotid IPH could be more than 50% in patients with symptomatic carotid stenosis. However, the incidence of carotid IPH in our study was 18.71% (32 of 171). We considered that the proportion of patients with mild stenosis in our study was much higher than in other studies, and only nine patients had a degree of stenosis > 50% in our study. Saam et al. [27] found that most atherosclerotic carotid lesions defined by American Heart Association type VI (AHA-LT6) were 37.0%, while the incidence of IPH was 84.4%. Subsequently, Saam et al. [28] found that the incidence of IPH was as high as 49.0% in symptomatic carotid atherosclerosis patients in a meta-analysis.
Some studies have confirmed the strong correlation between IPH and acute stroke [26, 29]. However, there were seldom studies on the relationship between IPH and neurological deficit. Our study found a significant difference in NIHSS on admission (P = 0.004) between the IPH and non-IPH groups. Still, patients with IPH might have less neurological impairment than patients without IPH. We don’t know the reason for the result, and it would be a meaningful research focus.
Concerning the risk factors for IPH, some studies have found that the prevalence of carotid IPH would increase with age increasing and was associated with multiple cardiovascular risk factors (e.g., advanced age, male sex, presence of carotid stenosis, HDL) [30, 31]. Nevertheless, Catalano et al. [32] analyzed atherosclerotic burden and plaque composition quantitatively and found that a history of hypercholesterolemia, diabetes, hypertension or smoking did not correlate with IPH.
Our study found that the factors such as age, Hcy, and hs-CRP were associated with IPH, and the combination of such risk factors might predict the existence of IPH. Due to the limitations of our study, we did not conduct further research. We hope that future extensive sample studies can explore the predictors of IPH to implement intervention measures as soon as possible.
Some studies have confirmed that the prevalence of IPH is associated with plaque morphology and degree of stenosis [33,34,35], which is also found in our research. We found that the patients with IPH might have more severe stenosis of the carotid artery, larger plaque area, more oversized WI, and less RI. At the same time, IPH might be related to the plaque components such as calcification, loose matrix, and LRNC. Because of the limited research ability, we had no further study on this result, and it would be our research direction in the future.
Although some studies have confirmed that the degree of carotid stenosis is associated with stroke recurrence [4], it is not sufficient to accurately predict the risk of stroke recurrence [36]. Our study did not find that carotid stenosis was associated with stroke recurrence owing to the lower percentage of patients with stenosis (> 50%), which was only 5.3% (9 of 171).
About 20% of patients with first ischemic stroke would undergo recurrent ischemic vascular events within 1 year [37]. Studies have confirmed that IPH was significantly associated with stroke recurrence [38, 39]. Deng, F et al. [40] also confirmed that the presence of IPH in carotid plaque, LRNC, and FCR shown on MRI were strong predictors of recurrent stroke events in a recent meta-analysis; the hazard ratios were 7.14 (95%CI, 4.32–11.82), 5.68 (95%CI, 2.40–13.47), and 2.73 (95%CI, 1.04–7.16), respectively. In our study, 13.5% of patients with carotid atherosclerosis occurred ipsilateral cerebral stroke, and 16.4% occurred ischaemic cerebrovascular events (stroke and TIA). The recurrence rate of an ipsilateral stroke in patients with IPH could be 6.30 times higher than in patients without IPH even after adjusting for multiple cardiovascular risk factors, medication (taking aspirin or statins), and other vulnerable plaque features (such as calcification, loose matrix, LRNC, and FCR).
By Kaplan–Meier survival analysis, we found the estimated probability of recurrent cerebrovascular stroke in patients with IPH was 25.0% at 3 months, 28.4% at 6 months, 38.6% at 9 months, and 48.9% at 12 months. However, the risk of stroke in patients without IPH was only 3.66% at 3 months and 6.68% at 12 months. Some previous studies have confirmed that recurrent cerebrovascular events often occur within 14–90 days after the index stroke [41, 42]. Such results give us a hint that we might take more active and effective interventions on patients with acute ischemic stroke as soon as possible.
Our study found that taking aspirin or statins was the protective factor for stroke recurrence. We also tried to determine whether it was also a protective factor for IPH, which has been confirmed in some studies [43, 44]. Unfortunately, our results were negative. We thought it might be due to the low proportion of taking aspirin or statins in patients with vulnerable carotid plaque. We believe it would be our future research direction.
There are some shortcomings in this study. Firstly, it is well known that atherosclerotic disease is a persistent chronic lesion and will accelerate deterioration with the increasing of time and age. However, we did not detect the change of carotid atherosclerotic plaques in patients accepting standardized secondary prevention strategies for stroke. Secondly, due to the limitations of the funds, we only studied plaques in the extracranial carotid artery carotid bifurcation. We did not exclude the interfering factors of plaques in other parts of extracranial carotid arteries and intracranial arteries, which may lead to errors in the results. Thirdly, we included a sufficient number of variables in the multivariate analysis, and the statistical results might be inevitably biased due to the small sample size.
Nevertheless, our study still put some insight into the future. Firstly, we may need to implement more active and effective intervention strategies for patients with IPH. Secondly, the future research hot point may explore the pathophysiological mechanism of plaque vulnerability and the molecular biomarkers combined with neuroimaging markers for risk stratification of recurrent ischemic events in symptomatic patients.
Conclusions
Compared with non-IPH, the patients with IPH have a higher risk of 1-year ischemic stroke recurrence. The potential risk of IPH should not be overlooked. It will be the future research hot point to understand the underlying pathophysiological mechanism of plaque vulnerability and the molecular biomarkers combined with neuroimaging markers.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due to the reason that there is a potential for disclosure of patient information but are available from the corresponding author on reasonable request.
Abbreviations
- IPH:
-
Intraplaque hemorrhage
- TIA:
-
Transient ischemic attack
- NIHSS:
-
National institutes of health stroke Scale
- mRS:
-
Modified rankin scale
- IQR:
-
Interquartile range
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- hs-CRP:
-
Hypersensitive c-reactive protein
- TC:
-
Cholesterol
- TG:
-
Triglycerides
- LDL-C:
-
Low-density lipoprotein cholesterol
- HDL-C:
-
High-density lipoprotein cholesterol
- Hcy:
-
Homocysteine
- CA:
-
Carotid artery
- LA:
-
Lumen area
- WA:
-
Wall area
- VA:
-
Vessel area
- NWI:
-
Normalized wall index
- WT:
-
Wall thickness
- PA:
-
Plaque area
- RI:
-
Remodeling index
- LRNC:
-
Lipid-rich necrotic core
- FCR:
-
Fibrous cap rupture
- CEA:
-
Carotid endarterectomy
- CAS:
-
Carotid artery stenting
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- LRNC:
-
Lipid-rich necrotic core
- IPH:
-
Intraplaque hemorrhage
References
Liu L, Wang D, Wong KS, Wang Y. Stroke and stroke care in China: huge burden, significant workload, and a national priority. Stroke. 2011;42(12):3651–4.
Herrington W, Lacey B, Sherliker P, Armitage J, Lewington S. Epidemiology of atherosclerosis and the potential to reduce the global burden of Atherothrombotic disease. Circ Res. 2016;118(4):535–46.
Wu S, Wu B, Liu M, Chen Z, Wang W, Anderson CS, et al. Stroke in China: advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 2019;18(4):394–405.
Barnett HJM, Taylor DW, Haynes RB, Sackett DL, Peerless SJ, Ferguson GG, et al. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325(7):445–53.
Orrapin S, Rerkasem K. Carotid endarterectomy for symptomatic carotid stenosis. Cochrane Database Syst Rev. 2017;6(6):Cd001081.
Dharmakidari S, Bhattacharya P, Chaturvedi S. Carotid artery stenosis: medical therapy, surgery, and stenting. Curr Neurol Neurosci Rep. 2017;17(10):77.
Calvet D, Mas JL. Symptomatic carotid stenosis: is stenting as safe and effective as carotid endarterectomy? Curr Opin Neurol. 2017;30(1):22–7.
Jiang P, Chen Z, Hippe DS, Watase H, Sun B, Lin R, et al. Association between carotid bifurcation geometry and atherosclerotic plaque vulnerability: a Chinese atherosclerosis risk evaluation study. Arterioscler Thromb Vasc Biol. 2020;40(5):1383–91.
Zhao X, Li R, Hippe DS, Hatsukami TS, Yuan C. Chinese atherosclerosis risk evaluation (CARE II) study: a novel cross-sectional, multicentre study of the prevalence of high-risk atherosclerotic carotid plaque in Chinese patients with ischaemic cerebrovascular events-design and rationale. Stroke Vasc Neurol. 2017;2(1):15–20.
Yang D, Ji Y, Wang D, Watase H, Hippe DS, Zhao X, et al. Comparison of carotid atherosclerotic plaques between subjects in northern and southern China: a Chinese atherosclerosis risk evaluation study. Stroke Vasc Neurol. 2020;5(2):138–45.
Saba L, Saam T, Jäger HR, Yuan C, Hatsukami TS, Saloner D, et al. Imaging biomarkers of vulnerable carotid plaques for stroke risk prediction and their potential clinical implications. Lancet Neurol. 2019;18(6):559–72.
Zhao X, Hippe DS, Li R, Canton GM, Sui B, Song Y, et al. Prevalence and characteristics of carotid artery high-risk atherosclerotic plaques in Chinese patients with cerebrovascular symptoms: a Chinese atherosclerosis risk evaluation II study. J Am Heart Assoc. 2017;6(8):e005831.
Schindler A, Schinner R, Altaf N, Hosseini AA, Simpson RJ, Esposito-Bauer L, et al. Prediction of stroke risk by detection of hemorrhage in carotid plaques: Meta-analysis of individual patient data. JACC Cardiovasc Imaging. 2020;13(2 Pt 1):395–406.
Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and Management of High Blood Pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension. 2018;71(6):e13–e115.
Punthakee Z, Goldenberg R, Katz P. Definition, classification and diagnosis of diabetes, prediabetes and metabolic syndrome. Can J Diabetes. 2018;42(Suppl 1):S10–s15.
Keevil JG, Cullen MW, Gangnon R, McBride PE, Stein JH. Implications of cardiac risk and low-density lipoprotein cholesterol distributions in the United States for the diagnosis and treatment of dyslipidemia: data from National Health and nutrition examination survey 1999 to 2002. Circulation. 2007;115(11):1363–70.
Ricci C, Wood A, Muller D, Gunter MJ, Agudo A, Boeing H, et al. Alcohol intake in relation to non-fatal and fatal coronary heart disease and stroke: EPIC-CVD case-cohort study. Bmj. 2018;361:k934.
Boehme AK, Esenwa C, Elkind MS. Stroke risk factors, genetics, and prevention. Circ Res. 2017;120(3):472–95.
Eriksson H, Wirdefeldt K, Åsberg S, Zelano J. Family history increases the risk of late seizures after stroke. Neurology. 2019;93(21):e1964–70.
Kerwin W, Xu D, Liu F, Saam T, Underhill H, Takaya N, et al. Magnetic resonance imaging of carotid atherosclerosis: plaque analysis. Top Magn Reson Imaging. 2007;18(5):371–8.
Cai JM, Hatsukami TS, Ferguson MS, Small R, Polissar NL, Yuan C. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. CIRCULATION. 2002;106(11):1368–73.
Xu WH, Li ML, Gao S, Ni J, Yao M, Zhou LX, et al. Middle cerebral artery intraplaque hemorrhage: prevalence and clinical relevance. Ann Neurol. 2012;71(2):195–8.
Chung JW, Hwang J, Lee MJ, Cha J, Bang OY. Previous statin use and high-resolution magnetic resonance imaging characteristics of intracranial atherosclerotic plaque: the intensive statin treatment in acute ischemic stroke patients with intracranial atherosclerosis study. STROKE. 2016;47(7):1789–96.
Li D, Zhao H, Chen X, Chen S, Qiao H, He L, et al. Identification of intraplaque haemorrhage in carotid artery by simultaneous non-contrast angiography and intraPlaque haemorrhage (SNAP) imaging: a magnetic resonance vessel wall imaging study. Eur Radiol. 2018;28(4):1681–6.
Marnane M, Prendeville S, McDonnell C, Noone I, Barry M, Crowe M, et al. Plaque inflammation and unstable morphology are associated with early stroke recurrence in symptomatic carotid stenosis. STROKE. 2014;45(3):801–6.
Hosseini A, Kandiyil N, Macsweeney S, Altaf N, Auer D. Carotid plaque hemorrhage on magnetic resonance imaging strongly predicts recurrent ischemia and stroke. Ann Neurol. 2013;73(6):774–84.
Saam T, Underhill HR, Chu B, Takaya N, Cai J, Polissar NL, et al. Prevalence of American Heart Association type VI carotid atherosclerotic lesions identified by magnetic resonance imaging for different levels of stenosis as measured by duplex ultrasound. J Am Coll Cardiol. 2008;51(10):1014–21.
Saam T, Hetterich H, Hoffmann V, Yuan C, Dichgans M, Poppert H, et al. Meta-analysis and systematic review of the predictive value of carotid plaque hemorrhage on cerebrovascular events by magnetic resonance imaging. J Am Coll Cardiol. 2013;62(12):1081–91.
Yang D, Liu Y, Han Y, Li D, Wang W, Li R, et al. Signal of carotid Intraplaque hemorrhage on MR T1-weighted imaging: association with acute cerebral infarct. AJNR Am J Neuroradiol. 2020;41(5):836–43.
Singh N, Moody AR, Zhang B, Kaminski I, Kapur K, Chiu S, et al. Age-specific sex differences in magnetic resonance imaging-depicted carotid Intraplaque hemorrhage. STROKE. 2017;48(8):2129–35.
Larson AS, Benson JC, Brinjikji W, Savastano L, Lanzino G, Huston J 3rd, et al. Variations in the presence of carotid Intraplaque hemorrhage across age categories: what age groups are Most likely to benefit from plaque imaging? Front Neurol. 2020;11:603055.
Catalano O, Bendotti G, Mori A, De Salvo M, Falconi M, Aloi TL, et al. Evolving determinants of carotid atherosclerosis vulnerability in asymptomatic patients from the MAGNETIC observational study. Sci Rep. 2021;11(1):2327.
Truijman MT, de Rotte AA, Aaslid R, van Dijk AC, Steinbuch J, Liem MI, et al. Intraplaque hemorrhage, fibrous cap status, and microembolic signals in symptomatic patients with mild to moderate carotid artery stenosis: the plaque at RISK study. STROKE. 2014;45(11):3423–6.
Zhu C, Tian X, Degnan AJ, Shi Z, Zhang X, Chen L, et al. Clinical significance of Intraplaque hemorrhage in low- and high-grade basilar artery stenosis on high-resolution MRI. AJNR Am J Neuroradiol. 2018;39(7):1286–92.
Yamada K, Yoshimura S, Shirakawa M, Uchida K, Nakahara S, Nishida S, et al. Asymptomatic moderate carotid artery stenosis with intraplaque hemorrhage: progression of degree of stenosis and new ischemic stroke. J Clin Neurosci. 2019;63:95–9.
Rothwell PM, Eliasziw M, Gutnikov SA, Fox AJ, Taylor DW, Mayberg MR, et al. Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. LANCET. 2003;361(9352):107–16.
Serena J, Segura T, Roquer J, García-Gil M, Castillo J. The ARTICO study: identification of patients at high risk of vascular recurrence after a first non-cardioembolic stroke. BMC Neurol. 2015;15:28.
Kwee RM, van Oostenbrugge RJ, Mess WH, Prins MH, van der Geest RJ, ter Berg JW, et al. MRI of carotid atherosclerosis to identify TIA and stroke patients who are at risk of a recurrence. J Magn Reson Imaging. 2013;37(5):1189–94.
Lu M, Peng P, Cui Y, Qiao H, Li D, Cai J, et al. Association of Progression of carotid Artery Wall volume and recurrent transient ischemic attack or stroke: a magnetic resonance imaging study. Stroke. 2018;49(3):614–20.
Deng F, Mu C, Yang L, Li H, Xiang X, Li K, et al. Carotid plaque magnetic resonance imaging and recurrent stroke risk: a systematic review and meta-analysis. Medicine (Baltimore). 2020;99(13):e19377.
Johansson E, Cuadrado-Godia E, Hayden D, Bjellerup J, Ois A, Roquer J, et al. Recurrent stroke in symptomatic carotid stenosis awaiting revascularization: a pooled analysis. NEUROLOGY. 2016;86(6):498–504.
Marnane M, Ni Chroinin D, Callaly E, Sheehan OC, Merwick A, Hannon N, et al. Stroke recurrence within the time window recommended for carotid endarterectomy. NEUROLOGY. 2011;77(8):738–43.
Mujaj B, Bos D, Selwaness M, Leening MJG, Kavousi M, Wentzel JJ, et al. Statin use is associated with carotid plaque composition: the Rotterdam study. Int J Cardiol. 2018;260:213–8.
Konishi T, Funayama N, Yamamoto T, Hotta D, Nomura R, Nakagaki Y, et al. Stabilization of symptomatic carotid atherosclerotic plaques by statins: a clinico-pathological analysis. Heart Vessel. 2018;33(11):1311–24.
Acknowledgments
We gratefully appreciate all the participants and staff for their contributions.
Funding
The study was supported by grants from the Beijing Natural Science Foundation (Z200016).
Author information
Authors and Affiliations
Contributions
Concept and design: FC, XZ (Xihai Zhao), XG, XZ (Xingquan Zhao), and YJ. Drafting of the manuscript: FC, DM, and XZ (Xingquan Zhao). Critical revision of the manuscript for important intellectual content: XZ (Xingquan Zhao) and YJ. Provision of study material or patients: DM, BS, and YJ. Collection and assembly of data: FC, DM, AW, XZ (Xingquan Zhao), BS, YJ, and XG. Check and approve of clinical definitions: XZ (Xihai Zhao) and XZ (Xingquan Zhao). Data analysis: FC and AW. Data interpretation: FC, AW, BS, XZ (Xingquan Zhao), and XG. Administrative, technical, or material support: XZ (Xihai Zhao), BS, and XZ (Xingquan Zhao). Supervision: XZ (Xingquan Zhao) and YJ. Final approval of manuscript: all authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The ethics committee of Beijing Tiantan Hospital approved this study. All study subjects had signed informed consent. We confirm that all methods in our study were performed in accordance with the principles of the Declaration of Helsinki and relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Che, F., Mi, D., Wang, A. et al. Extracranial carotid plaque hemorrhage predicts ipsilateral stroke recurrence in patients with carotid atherosclerosis – a study based on high-resolution vessel wall imaging MRI. BMC Neurol 22, 237 (2022). https://doi.org/10.1186/s12883-022-02758-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-022-02758-3