Abstract
Backround
Median overall survival (OS) after diagnosis of glioblastoma (GBM) remains 15 months amongst patients receiving aggressive surgical resection, chemotherapy and irradiation. Treatment of patients with a poor preoperative Karnofsky Performance Status Scale (KPSS) is still controversial. Therefore, we retrospectively assessed the outcome after surgical treatment in patients with a KPSS of ≤60%.
Methods
We retrospectively included patients with a de-novo glioblastoma WHO °IV and preoperative KPSS ≤60%, who underwent surgery at two neurosurgical centres between September 2006 and March 2016. We recorded pre- and postoperative tumour volume, pre- and postoperative KPSS, OS, age and MGMT promoter status.
Results
One hundred twenty-three patients (58 females/65 males, mean age 67.4 ± 13.4 years) met the inclusion criteria. Seventy-five of the 123 patients (61%) underwent surgical resection. 48/123 patients (39%) received a biopsy. The median preoperative and postoperative tumour volume of all patients was 33.0 ± 31.3 cm3 (IR 15.0–56.5cm3) and 3.1 ± 23.8 cm3 (IR 0.2–15.0 cm3), respectively. The median KPSS was 60% (range 20–60%) preoperatively and 50% (range 0–80%) postoperatively. Patients who received a biopsy showed a median OS for patients who received a biopsy only was 3.0 months (95% CI 2.0–4.0 months), compared to patients who had a resection and had a median OS of 8 months (95% CI 3.1–12.9 months).
Age (p < 0.001, HR: 1.045 [95% CI 1.022–1.068]), postoperative tumour volume (p = 0.02, HR: 1.016 [95% CI 1.002–1.029]) and MGMT promotor status (p = 0.016, HR: 0.473 [95% CI 0.257–0.871]) were statistically significant in multivariate analysis. In subgroup analyses only age was shown as a significant prognostic factor in multivariate analyses for patients receiving surgery (p < 0.001, HR: 1.046 [95% CI 1.022–1.072]). In the biopsy group no significant prognostic factors were shown in multivariate analysis.
Conclusion
GBM patients with a preoperative KPSS of ≤60% might profit from surgical reduction of tumour burden.
Similar content being viewed by others
Backround
In 1949, Karnofsky and Burchena described their instrument, the Karnofsky Performance Status Scale (KPSS) score, as a numerical scale for quantifying patients’ status in relation to the degree of their independence in daily activities and self-care. Originally, it was used for patients with systemic malignancies and divided them according to their level of activity and medical requirements. Patients are scored into 11 categories from 0 to 100, where, for example, a KPSS of 70% means the patient is able to care for himself but is unable to carry out daily activities [1]. After it had been proven successful in patients with systemic cancer, more and more research groups started to evaluate the KPSS score for brain cancer [2,3,4]. Previously published studies could show a significant correlation between the preoperative KPSS score and the outcome after glioma surgery [5, 6]. In most studies, only patients suffering from a glioblastoma with a KPSS of ≥70% were included [7, 8]. For example, those studies analysed prognostic factors such as tumour size, GTR and adjuvant therapy modalities postoperatively. However, in our clinical daily work, patients with a noticeably lower KPSS are represented as well. It should be noticed that this can be due to clinic symptomology as seizures, acute mental status changes or focal neurologic deficits caused by tumour size and/or location itself. Therefore, the following study intends to show whether it is worthwhile for patients with a KPSS 60% or below to achieve tumour volume reduction.
Methods
This retrospective, non-interventional bicentric study was approved by the medical ethics committee of the Technical University Munich (5625–12) and is in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments [9].
Patient population
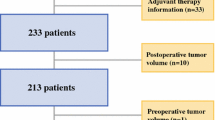
We retrospectively assessed 968 patients with a histologically confirmed glioblastoma WHO IV with a preoperative Karnofsky Performance Status Scale (KPSS) of ≤60%, who were treated surgically between September 2006 and March 2016 in two neurosurgical departments (Fig. 1). According to interdisciplinary neuro-oncological consensus, patients were assigned to surgery with the intent of complete resection or to biopsy to confirm the histopathological diagnosis.
We retrospectively reviewed pre- and postoperative KPSS, date of initial tumour diagnosis, date of death/last contact, age, sex, adjuvant treatment and histopathological findings from the patients’ medical charts. Also, we performed histopathological analysis according to the WHO criteria of 2016 [10] and quantitatively assessed methylation of the O6-methylguanin-DNA-methyltransferase (MGMT) promoter status. Since the decision regarding adjuvant therapy is made after receipt of the histology in the context of the interdisciplinary neuro-oncological board, depending on the clinical condition, the KPSS was collected approximately 5 days postoperatively.
Then, we calculated the overall survival (OS) from the date of surgery until the date of death or censored for the date of the last patient contact. Only patients with complete magnetic resonance imaging data were included to calculate pre- and postoperative contrast-enhancing tumour volumes. Patients with recurrent tumour or incomplete data were excluded (Table 1).
Imaging
All patients received preoperative and early postoperative MRI (within 72 h after surgery). In centre A, we performed imaging using three different 3 Tesla MRI scanners: Philips Achieva; Philips Ingenia (Philips Medical Systems, The Netherlands B.V.); and Siemens Verio (Siemens Healthcare, Erlangen, Germany). Images included T1w sequences with and without contrast agent, FLAIR (Fluid attenuated inversion recovery) sequences, T2 gradient echo sequences, diffusion-weighted imaging or diffusion-tensor imaging, whereas we calculated isotropic diffusion-weighted images and apparent diffusion coefficient (ADC) maps automatically. Tumour volumes of the contrast-enhancing tumour on pre- and early postoperative MR images using iPlannet® Cranial 3.0.1 were manually segmented by two neurosurgeons (5 and 10 years of experience) and two neuroradiologists (3 years and 6 years of experience).
In centre B, we conducted MR imaging with a 3.0 T MRI scanner (Biograph mMR, Siemens Healthcare, Erlangen, Germany). One neurosurgeon (14 years of experience) and one medical student assessed the volumes of the contrast-enhancing tumour through manual segmentation via iPlannet® Cranial 3.0.1 (iPlannet® 3.0 cranial planning software, Brainlab AG, Munich, Germany). The postoperative tumour volumes of patients who underwent biopsies were considered identical to the preoperative tumour volumes.
Statistical evaluation
We conducted our data analysis using IBM SPSS Statistics Version 24.0 and 26.0 (SPSS Inc., IBM Corp., Armonk, NY, USA). In the descriptive data analysis, we show non-normally distributed data as median and interquartile range (IR), normally distributed variables as mean and standard deviation.
We compared the OS distributions using the Kaplan-Meier estimates (log-rank) and a Cox regression model for multivariate survival analysis. We considered differences with an error probability of less than 0.05 to be statistically significant.
Results
Patients and clinical data
123/968 patients (58 females/65 males) with a mean age of 67.4 ± 13.4 years; (range 21–90 years) met our inclusion criteria: surgical treatment for glioblastoma, preoperative KPSS of ≤60%, preoperative and early postoperative MRI, complete medical documentations with date of initial tumour diagnosis, date of death/last contact, age, sex, adjuvant treatment and histopathological findings. Data are shown for all patients and for the subgroups of patients with biopsy / surgery (Table 1). The median preoperative tumour volume of all patients was 33.0 ± 31.3 cm (IR 15.0–56.5cm3) and the median postoperative tumour volume was 3.1 ± 23.8 cm3 (IR 0.2–15.0 cm3) postoperatively. Complete resection of contrast-enhancing tumours on postoperative MRI was achieved in 24 (19.5%) of all patients. MGMT-methylation status was available in 80 patients (65%), of whom 26 (32.5%) presented with a methylated MGMT-promotor status.
Surgical resection with intent for maximum/complete resection was performed in 75/123 patients (61%) (34/75 females and 41/75 males; mean age 64.4 ± 13.7 years (21–87 years). The median tumour volume was 35.2 cm3 (IR 19.7–65.3 cm3) preoperatively and 0.5 cm3 (IR 0–2.3 cm3) postoperatively. Complete resection of the contrast-enhancing tumour on postoperative MR imaging was seen in 24/75 patients (32%). In this group, we assessed MGMT-methylation status in 52/75 patients (69.3%). We observed methylation of MGMT in 19/52 patients (36.5%) and no methylation of MGMT in 33/52 patients (63.5%).
Fifty-eight of 75 (77.3%) patients underwent postoperative adjuvant treatment; three of 58 patients (5.1%) underwent monotherapy with temozolomide, 27/58 (46.6%) received radiation therapy only and 28/58 (48.3%) received a combined therapy according to the Stupp regime. The remaining 48 patients (38.7%) (23/48 females, 25/48 males) with a mean age of 72.1 ± 11.6 years (34–90 years) underwent biopsy for tumour histopathological diagnosis. The median tumour volume in these patients was 26.3 ± 30.9 cm3 (IR 8.1–51.7 cm3). MGMT-methylation status was available in 28 patients (58.3%) with 21/28 (75%) unmethylated MGMT promotor status. After confirming histopathological diagnosis of glioblastoma via biopsy, 8/48 (16.7%) received combined radio−/chemotherapy, 3/48 (6.3%) received chemotherapy with temozolomide only, 21/48 (43.7%) received radiotherapy alone and 16/48 (33.3%) did not receive any adjuvant therapy. To show more precisely which patients received adjuvant therapy according to the STUPP regime, we performed a correlation analysis. This showed that younger patients (p = 0.000) received this therapy.
Assuming that the adjuvant therapy could be started 14 days after the operation and lasted approximately 6 weeks, we could suppose the completion of the adjuvant therapy in all but nine patients on the basis of the OS. Unfortunately, the follow-up expired without documentation regarding this information.
Karnofsky performance status scale (KPSS)
The median KPSS of the entire patient cohort was 60% (20–60%) preoperatively and 50% (0–80%) postoperatively. Seventeen patients (22.67%) who had undergone surgical tumour resection had an improved KPSS at time of discharge from the hospital, 25 patients (33.3%) remained unchanged and 33 patients (44.0%) worsened. There was no difference in the median KPSS between patients receiving surgical resection compared to patients receiving biopsy only. In the biopsied group, we recorded a median preoperative KPSS of 60% (range 40–60%) and median postoperative KPSS of 50% (range 0–70%). Patients who were treated by surgical resection showed a median preoperative KPSS of 60% (range 20–60%) and 50% (range 0–80%) postoperatively.
Overall survival (OS)
Median OS was 5.0 months (95% CI 3.0–4.0 months) in the study population (including biopsy group and surgical resection group). Patients who received a biopsy showed a median OS of 3.0 months (95% CI 2.0–4.0 months), whereas patients who underwent surgical resection showed a median OS of 8.0 months (95% CI 3.1–12.9 months).
In order to show a possible survival advantage of the resected patients compared to the biopsied patients, we performed a Cox regression with the parameters: age, postoperative tumour volume, preoperative KPSS and biopsy. This showed no more significance for the biopsied patient group (p = 0.154, 95% CI 0.399–1.156).
At the time of the study, 102/123 patients (82.9%) had died, and 21/123 (17.1%) were still alive or censored for their last date of contact. In-hospital mortality was seen in 3/123 (2.4%). Two of these patients received biopsy and one surgical tumour resection.
Univariate model
Surgical resection compared to biopsy (p < 0.001) and complete resection of the contrast-enhancing tumor part (p = 0.032) showed a significant impact on OS in the univariate analysis using Kaplan-Meier estimates. MGMT-methylation status did not show a significant impact on OS in univariate analysis (p = 0.071) (Figs. 2A-B and 3). Adjuvant therapy regimes also showed a significant prognostic impact, in all patients (p < 0.001) and in the subgroups of patients with biopsy (p = 0.005) and surgery (p < 0.001) (Fig. 4A-C).
Multivariate model
Cox regression, including all treated patients (biopsy group and surgical resection group), showed age at the time of surgery (p < 0.001, HR: 1.045 [95% CI 1.022–1.068]), postoperative tumour volume (p = 0.02, HR: 1.016 [95% CI 1.002–1.029]) and methylation status (p = 0.016, HR: 0.473 [95% CI 0.257–0.871]) as statistical significant predictors of OS. Preoperative tumour volume (p = 0.996, HR: 1.000 [95% CI 0.992–1.009]), preoperative KPSS (p = 0.068, HR: 1.023 [95% CI 0.998–1.049]) and postoperative KPSS (p = 0.237, HR: 0.987 [95% CI 0.965–1.009]) were not significant in the multivariate analysis.
In the subgroup of patients (n = 75) referred for surgery only age was shown as prognostic factor in multivariate analysis: age (p < 0.001, HR: 1.046 [95% CI 1.022–1.072]), postoperative volume (p = 0.701, HR: 0.982 [0.895–1.078]), preoperative KPSS (p = 0.059, HR: 1.026 [0.999–1.054]).
In the subgroup of patients receiving biopsy (n = 48) no significant prognostic factors were shown in multivariate analysis: age (p = 0.086, HR: 1.028 [95% CI 0.996–1.061]), postoperative volume (p = 0.412, HR: 1.005 [0.993–1.017]), preoperative KPSS (p = 0.598, HR: 0.986 [0.936–1.039]).
Discussion
In this cohort of GBM patients (biopsy group and surgical resection group) with a preoperative KPSS ≤60%, postoperative tumour volume, age at the time of surgery and MGMT-methylation status were significant predictors of OS in the multivariate analysis. In contrast, preoperative tumour volume and KPSS had no significant impact on OS. The subgroup analysis of patients referred for surgery only age was shown as prognostic factor in multivariate analysis, whereas no significant prognostic factors were shown in the subgroups of patients referred for biopsy. In our study population, the significant effect of postoperative tumour volume in univariate analysis correlates with tumour resection compared to the biopsied group. This could explain the lack of significance in the multivariate analysis for this group. Nevertheless, as already understood from other studies, we could also show that extent of resection is an important factor in OS in patients with glioblastoma [11,12,13,14].
In general, patients with poor preoperative KPSS usually do not receive aggressive surgical therapy. Therefore, data on these patients are very limited [15]. In our cohort, 56/123 (45.5%) showed an improved or unchanged postoperative KPSS with a median of 50%. Adjuvant treatment such as radiation therapy or chemotherapy is usually only offered to patients with a KPSS ≥70% [16, 17]. Consequently, these patients are usually considered ineligible for adjuvant oncological treatment even after tumour resection. Malakhov et al. could show that 51.2% of the patients presenting with KPSS< 60% and receiving chemoradiation had improved survival compared to RT alone [18]. However, the majority of our patient cohort (77.6%) who underwent surgical resection received adjuvant therapy. Considering the early postoperative assessment of KPSS in this study, secondary improvement is to be expected. Patients undergoing a biopsy were older (66.6%) with eloquent tumour location than patients, who were selected for surgical tumour resection (41.33%). Only 16.7% of the patients who received a biopsy underwent adjuvant treatment regimes. On the one hand, this is due to the higher age of the biopsy group. Secondly, only 7/48 of these patients showed MGMT-methylation. In accordance with the guidelines, our neuro-oncological interdisciplinary board recommends monotherapy for patients ≥75 years of age, depending on the MGMT-methylation status.
Reduced preoperative KPSS is an important prognostic factor in patients with glioblastoma [19, 20]. Age, comorbidities and neurological deficits have an impact on KPSS and, in conclusion, on OS [20,21,22]. Postoperative deterioration of the performance status scale is usually multifactorial, with the reasons being edema, haemorrhage, postoperative delirium, ischemic events or direct surgical lesions of eloquent brain structures [23].
In our opinion, the KPSS does not offer sufficient information about quality of life and therefore should not be overrated concerning the selection of patients undergoing surgery. For example, patients with preoperative neurological deficits such as hemiparesis due to surrounding edema might have a KPSS of 60% or below and might therefore not be selected for surgical therapy. However, as we know today, the surrounding edema will disappear a few days after surgery, and the patients are able to recover for adjuvant treatment. The KPSS should therefore be considered with care.
The decision for or against aggressive surgical therapy should be made individually by experienced neurosurgeons within the framework of an interdisciplinary neuro-oncology board.
Limitations of the study
This study has limitations. First, the retrospective non-randomized design is the main limitation. Second, molecular status was not available for all patients in our cohort study, as the MGMT-methylation status of patients with glioblastoma is known to be one of the strongest predictors concerning survival prognosis [24, 25].
Conclusion
GBM patients with a preoperative KPSS of ≤60% might profit from surgical reduction of tumour burden. We therefore suggest considering surgical resection even in patients with a KPSS of ≤60% after careful selection based on an interdisciplinary neuro-oncological board decision and counselling of patients and their relatives.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- KPSS:
-
Karnofsky Performance Status Scale
- OS:
-
Overall survival
- GBM:
-
Glioblastoma
- MGMT:
-
O6-methylguanin-DNA-methyltransferase
- EOR:
-
Extent of resection
- HR:
-
Hazard ratio
- IR:
-
Interquartile range
- GTR:
-
Gross tumor resection
- FLAIR:
-
Fluid attenuated inversion recovery
- ADC:
-
Apparent diffusion coefficient
References
Karnofsky DA, Burchenal JH, et al. Experimental observations on the effects of the nitrogen mustards on neoplastic tissues. Cancer Res. 1947;7(1):50.
Stark AMSW, Mehdorn HM. Outcome evaluation in glioblastoma patients using different ranking scores: KPS, GOS, mRS and MRC. Eur J Cancer Care (Engl). 2010;1(19):39–44.
Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011;115(1):3–8. https://doi.org/10.3171/2011.2.JNS10998.
Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95(2):190–8. https://doi.org/10.3171/jns.2001.95.2.0190.
Chambless LB, Kistka HM, Parker SL, Hassam-Malani L, McGirt MJ, Thompson RC. The relative value of postoperative versus preoperative Karnofsky performance scale scores as a predictor of survival after surgical resection of glioblastoma multiforme. J Neuro-Oncol. 2015;121(2):359–64. https://doi.org/10.1007/s11060-014-1640-x.
Laws ER, Parney IF, Huang W, Anderson F, Morris AM, Asher A, et al. Survival following surgery and prognostic factors for recently diagnosed malignant glioma: data from the Glioma outcomes project. J Neurosurg. 2003;99(3):467–73. https://doi.org/10.3171/jns.2003.99.3.0467.
Palmer JD, Bhamidipati D, Song A, Eldredge-Hindy HB, Siglin J, Dan TD, et al. Bevacizumab and re-irradiation for recurrent high grade gliomas: does sequence matter? J Neuro-Oncol. 2018;140(3):623–8. https://doi.org/10.1007/s11060-018-2989-z.
Ening G, Huynh MT, Schmieder K, Brenke C. Repeat-surgery at Glioblastoma recurrence, when and why to operate? Clin Neurol Neurosurg. 2015;136:89–94. https://doi.org/10.1016/j.clineuro.2015.05.024.
General Assembly of the World Medical A. World medical association declaration of helsinki: ethical principles for medical research involving human subjects. J Am Coll Dent. 2014;81(3):14–8.
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–20. https://doi.org/10.1007/s00401-016-1545-1.
Li YM, Suki D, Hess K, Sawaya R. The influence of maximum safe resection of glioblastoma on survival in 1229 patients: can we do better than gross-total resection? J Neurosurg. 2016;124(4):977–88. https://doi.org/10.3171/2015.5.JNS142087.
Grabowski MM, Recinos PF, Nowacki AS, Schroeder JL, Angelov L, Barnett GH, et al. Residual tumor volume versus extent of resection: predictors of survival after surgery for glioblastoma. J Neurosurg. 2014;121(5):1115–23. https://doi.org/10.3171/2014.7.JNS132449.
Chaichana KL, Martinez-Gutierrez JC, De la Garza-Ramos R, Weingart JD, Olivi A, Gallia GL, et al. Factors associated with survival for patients with glioblastoma with poor pre-operative functional status. J Clin Neurosci. 2013;20(6):818–23. https://doi.org/10.1016/j.jocn.2012.07.016.
Bette S, Barz M, Wiestler B, Huber T, Gerhardt J, Buchmann N, et al. Prognostic value of tumor volume in Glioblastoma patients: size also matters for patients with incomplete resection. Ann Surg Oncol. 2018;25(2):558–64. https://doi.org/10.1245/s10434-017-6253-0.
Uzuka T, Aoki H, Natsumeda M, Takahashi H, Fujii Y. Effectiveness of maximal safe resection for glioblastoma including elderly and low Karnofsky performance status patients: retrospective review at a single institute. Neurol Med Chir (Tokyo). 2012;52(8):570–6. https://doi.org/10.2176/nmc.52.570.
Pretanvil JA, Salinas IQ, Piccioni DE. Glioblastoma in the elderly: treatment patterns and survival. CNS Oncol. 2017;6(1):19–28. https://doi.org/10.2217/cns-2016-0023.
Ironside S, Das S, Sahgal A, Moroney C, Mainprize T, Perry JR. Optimal Therapies for Newly Diagnosed Elderly Patients with Glioblastoma. Curr Treat Options Oncol. 2017;18(11):66. https://doi.org/10.1007/s11864-017-0508-7.
Malakhov N, Lee A, Garay E, Becker DJ, Schreiber D. Patterns of care and outcomes for glioblastoma in patients with poor performance status. J Clin Neurosci. 2018;52:66–70. https://doi.org/10.1016/j.jocn.2018.03.006.
Sacko A, Hou MM, Temgoua M, Alkhafaji A, Marantidou A, Belin C, et al. Evolution of the Karnosky performance status throughout life in glioblastoma patients. J Neuro-Oncol. 2015;122(3):567–73. https://doi.org/10.1007/s11060-015-1749-6.
Stark AM, Stepper W, Mehdorn HM. Outcome evaluation in glioblastoma patients using different ranking scores: KPS, GOS, mRS and MRC. Eur J Cancer Care (Engl). 2010;19(1):39–44. https://doi.org/10.1111/j.1365-2354.2008.00956.x.
Arvold ND, Reardon DA. Treatment options and outcomes for glioblastoma in the elderly patient. Clin Interv Aging. 2014;9:357–67. https://doi.org/10.2147/CIA.S44259.
Chang SM, Parney IF, McDermott M, Barker FG, Schmidt MH, Huang W, et al. Perioperative complications and neurological outcomes of first and second craniotomies among patients enrolled in the Glioma Outcome Project. J Neurosurg. 2003;98(6):1175–81. https://doi.org/10.3171/jns.2003.98.6.1175.
Gempt J, Forschler A, Buchmann N, Pape H, Ryang YM, Krieg SM, et al. Postoperative ischemic changes following resection of newly diagnosed and recurrent gliomas and their clinical relevance. J Neurosurg. 2013;118(4):801–8. https://doi.org/10.3171/2012.12.JNS12125.
Felsberg J, Rapp M, Loeser S, Fimmers R, Stummer W, Goeppert M, et al. Prognostic significance of molecular markers and extent of resection in primary glioblastoma patients. Clin Cancer Res. 2009;15(21):6683–93. https://doi.org/10.1158/1078-0432.CCR-08-2801.
Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. https://doi.org/10.1056/NEJMoa043331.
Acknowledgements
No acknowledgements required.
Informed consent
All patients sign a generally valid declaration of consent for participation in retrospective studies upon admission.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Conceptualization: JG2, MB, JG1, MS. Methodology: MB, SB. Formal analysis and investigation: MB, JG1, AKA, FB, IG-T. Writing- original draft preparation: MB, JG1. Writing- review and editing: IJ, Y-MR, JG2, BM, FS-G, BW, TH. Funding acquisition: no funding. Resources: no other resources. Supervision: BM, JG2, SEC. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This retrospective, non-interventional bicentric study was approved by the local medical ethics committee, Technical University Munich, School of Medicine, (No. 5625–12) and is in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments [9].
All patients sign a generally valid declaration of consent for participation in retrospective studies upon admission.
Consent for publication
With the consent for participation, all patients also give their permission for the results obtained to be published.
Competing interests
JG, BM and SB work as consultants for Brainlab (Brainlab AG, Munich).
YMR receives financial research grants from BrainLAB, Carl Zeiss Medical, DepuySynthes, Icotec, Medtronic, Silony, Spineart and Ulrich Medical. Furthermore, YMR works as a consultant for BrainLAB and Icotec.
TH worked as a medical consultant for Brainlab AG (Munich, Germany) until 2016 and is head of scientific collaborations at Smart Reporting GmbH (Munich, Germany)—all unrelated to the present study.
In addition, BM works as a consultant for Medtronic, Spineart, Icotec, Relievant and Depuy/Synthes. In these firms, BM acts as a member of the advisory board. Furthermore, BM reports a financial relationship with Medtronic, Ulrich Medical, Brainlab, Spineart, Icotec, Relievant and Depuy/Synthes. He received personal fees and research grants for clinical studies from Medtronic, Ulrich Medical, Brainlab, Icotec and Relievant. All this occurred independently of the submitted work. BM holds the royalties/patent for Spineart.
All named potential conflicts of interest are unrelated to this study. All other authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Barz, M., Gerhardt, J., Bette, S. et al. Prognostic value of tumour volume in patients with a poor Karnofsky performance status scale – a bicentric retrospective study. BMC Neurol 21, 446 (2021). https://doi.org/10.1186/s12883-021-02424-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-021-02424-0