Abstract
Background
The initial CT blend sign is an imaging marker that has been used to predict haematoma expansion and poor outcomes in patients with small-volume intracerebral haemorrhage (ICH). However, the association of the blend sign with the outcomes of patients undergoing surgery remains unclear. The present study aimed to retrospectively evaluate the influence of the initial CT blend sign on short-term outcomes in patients with hypertensive ICH after stereotactic minimally invasive surgery (sMIS).
Methods
We enrolled 242 patients with spontaneous ICH. The patients were assigned to the blend sign group (91 patients) or non-blend sign (control) group (151 patients) based on the initial CT features. The NIHSS, GCS and mRS were used to assess the effects of sMIS. The rates of severe pulmonary infection and cardiac complications were also compared between the two groups.
Results
Statistically significant differences in the NIHSS and GCS scores were not observed between the blend sign group and the control group. No significant differences in the proportion of patients with good outcomes during the follow-up period were observed between the two groups. A higher rate of re-haemorrhage was noted in the blend sign group. Significant differences in the rates of severe pulmonary infection and cardiac complications were not observed between the two groups.
Conclusions
The initial CT blend sign is not associated with poor outcomes in patients with hypertensive ICH after sMIS. ICH patients with the CT blend sign should undergo sMIS if they are suitable candidates for surgery.
Similar content being viewed by others
Background
Spontaneous ICH is a life-threatening disease associated with high global mortality and morbidity rates worldwide. Various clinical trials on ICH treatment have been performed within the past 10 years to improve patient outcomes [1]. However, although many clinical trials on ICH therapy have been performed, the death rate at 30 days remains as high as 40% worldwide [2]. No therapeutic strategies have been proven to be effective in improving functional outcomes [3]. Open surgery for removing ICH was not more advantageous than medications for patients with ICH in several trials [4]. Although craniotomy can remove ICH effectively, it can result in severe injuries to the brain and severe pulmonary infection [4]. The benefits of open surgical procedures over conservative medications for hypertensive ICH remain disputed [5]. Early neurosurgical procedures for ICH treatment have not been shown to be more beneficial than initial conservative treatment [6]. Brain injuries caused by conventional surgical procedures might counteract the potential advantages of haematoma removal during open surgery [7]. In recent years, sMIS for ICH treatment has been assessed in several clinical trials and has demonstrated promising results [8,9,10]. Minimally invasive punctures and drainage have been shown to cause the least damage to the brain and lead to the shortest operative time [4]. In a recent study, although MIS followed by thrombolysis for ICH treatment did not lead to a higher proportion of patients with good functional outcomes, haematomas that decreased to 15 ml or less led to improved mRS scores at the end of the follow-up period in stabilized patients [4].
Haematoma growth (HE) indicates a very poor outcome. HE may be prevented if physicians can identify patients with high-risk factors in the early stage of ICH [2]. Some imaging markers, such as the blend sign, black hole sign and spot sign on brain CT, have been recognized as predictive factors for HE [2, 8,9,10]. The initial CT blend sign has been reported to be associated with poor outcomes in patients with ICH following the administration of medications [2]. The CT black hole sign and the blend sign can predict postoperative re-haemorrhage after sMIS [11, 12]. However, whether the initial CT blend sign is associated with poor outcomes in patients following sMIS remains unknown. We speculated that the initial CT blend sign is associated with poor outcomes in patients with ICH after sMIS. The present study aimed to retrospectively determine the influence of the initial CT blend sign on outcomes in patients with spontaneous ICH following sMIS.
Methods
The clinical data of patients with ICH who underwent sMIS in the Affiliated Hospital of Guizhou Medical University were collected and analysed. The recruitment period was from January 1, 2018 to June 30, 2019.
Study design and participants
Study design
The study was performed retrospectively to determine the relationship between the initial CT blend sign and poor outcomes in patients with ICH after sMIS. The clinical data from patients with ICH were retrieved from medical records. The diagnosis of ICH was confirmed by using a baseline CT scan taken within 1 h of admission. The patients with ICH were assigned to the blend sign group or non-blend sign group (control group) based on the initial CT scan. sMIS was performed within 27 h of admission.
The inclusion criteria were the same as those in our previously published clinical studies [12]. In brief, patients who met the diagnostic criteria for ICH based on non-enhanced CT scans were included. ICH was located in the supratentorial area. The ICH volume was 30ml-50ml. Consent for surgery was obtained from the patients’ authorized representatives.
The exclusion criteria were the same as those in previously published studies [12]. Patients with ICH located in the brainstem and cerebellum were excluded from this study. Patients with secondary ICH due to haemorrhagic transformation from brain infarction were also excluded.
Participants
A total of 710 patients with spontaneous ICH were admitted to our hospital during the study period. Among them, 318 patients with ICH were surgical candidates and underwent sMIS. However, 25 patients with ICH left the hospital within 1 week without medical orders, 21 patients developed ICH in the brainstem, and another 30 patients had ICH with a volume over 50 mL. These 76 patients were excluded from the final analysis (Fig. 1).
Based on the inclusion criteria, 242 consecutive patients who underwent sMIS were included in the present study. The blend sign group included 91 patients, and the non-blend sign group (control group) included 151 patients with spontaneous ICH. The baseline clinical characteristics of the patients are listed in Table 1.
Imaging analysis
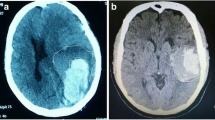
CT scans (General Electric Medical Systems, Milwaukee, WI) were performed using the same parameters as in our previous studies [13]. A neurosurgical expert and a neuroimaging expert independently evaluated the ICH shape features. The haematoma shape was assessed by visual inspection [14]. The blend sign was determined by the criteria proposed in previously published studies [15]. It was composed of two parts with different densities on CT (Fig. 2).
Discrepancies about the blend sign evaluation between the two readers were resolved by discussion. The ICH volume was determined based on the method reported in a previous study [16].
Patient treatment
ICH evacuation
The surgical procedures used to remove ICH were the same as those used in our previous studies [17,18,19]. The skill levels of the surgeons did not affect our results, as the surgeries were performed by experienced surgeons. In brief, the patient was transferred to the operating room after a preoperative CT scan was performed. The coordinates of the ICH area were determined. Local anaesthesia at the puncture point was administered, and the skull was punctured under the guidance of a stereotactic instrument. A puncture-needle set(LY-1-type) was inserted gently into the haematoma. After removing the plastic-needle core, we used a 10-ml syringe to aspirate out the liquid part of the ICH (Fig. 3). Aspiration was stopped if 1/2 of the ICH volume was removed or resistance was encountered. We connected the needle guard to a plastic tube and retained it for drainage of the haematoma. Postoperative CT scans were performed on the first day and the third day after surgery. At any time, if a patient showed deterioration in neurological functions, another CT scan was performed.
Procedures for the stereotactic minimally invasive surgery. A Positioning headframe was fixed on the head firstly and then the patient was transferred for CT scan to figure out the coordinates (a-c). Subsequently the arc frame and guider were fixed to the positioning headframe and a transcranial puncture needle was inserted (d-f). Finally, the liquid part of the ICH was aspirated out (g-h)
Medications
All patients received standard medical management postoperatively according to the guidelines for the treatment of hypertensive ICH [14]. Measures for preventing deep venous thrombosis (DVT), controlling the body temperature and blood glucose level, supporting the patient’s nutritional state and preventing other complications were also taken [13].
Functional outcomes
The modified Rankin Scale (mRS) score was the primary functional outcome. The mRS was administered by neurological experts blinded to the present study. The National Institutes of Health Stroke Scale (NIHSS) scores, the Glasgow Coma Scale (GCS) scores and the ICH volume changes were the secondary outcomes and obtained from medical records. The outcome was considered favourable if the patient achieved a mRS score of 0–3 points. In contrast, a poor outcome was considered if the patient achieved a mRS score of > 3 points [4].
Complications
Severe complications, including severe pulmonary infection, respiratory failure and heart failure, were recorded and compared. Cardiopulmonary complications did not include the exacerbation of chronic heart failure, respiratory failure, or community-acquired pneumonia.
Postoperative re-haemorrhage was determined based on the criteria used in our previously published studies [12]. An increase in the haematoma volume of > 33% [20] compared to that observed in the previous follow-up CT scan was considered to indicate re-haemorrhage. Hyperdense signals that appeared again after disappearing on the most recent follow-up CT scan was also considered to indicate re-haemorrhage.
Statistical analysis
On the basis of the assumption that 25% of patients in the blend sign group would have a mRS score of 0–3 and 45% of patients in the control group would have a mRS score of 0–3 following sMIS [4], we estimated that the inclusion of 90 patients in each group would provide 81.0% statistical power at an α level of 0.05. The permissible error δ was 0.1.
Commercially available software (SPSS, version 22.0) was used to analyse the differences between the blend sign group and the control group. The categorical data are expressed as proportions, and the continuous variables are expressed as means ± SDs. Student’s t tests (for normally distributed data) or nonparametric tests (for non-normally distributed data) were used to compare the demographic and clinical data as well as the radiological characteristics. P values < 0.05 indicated significant differences. The independent association between the CT blend sign and the outcomes of patients after sMIS was evaluated by binary logistic regression. κ values were calculated to assess the level of interobserver reliability. The κ values were categorized based on the criteria presented in a previous study [12].
Results
The baseline data
A total of 242 patients were included in our study, with ages ranging from 31 to 93 years and an average age of 57.05 ± 12.703 years. The time from onset to the baseline CT scan was 5.0 (2.0–9.7) hours. The mean GCS score at admission was 11 (8–13), and the mean NIHSS score was 16 (14–20). ICH in the basal ganglia area was detected in one hundred eighty-four patients. Thirty-four patients showed ICH in the cerebral lobes, and 24 patients showed ICH in the thalamus.
The 242 patients were assigned to the blend sign group or control group based on their haematoma features. Factors such as age, history of smoking, drinking status, preoperative ICH volume, anticoagulant use, GCS score at admission, NIHSS score at admission, time from onset to admission, time from onset to baseline CT, and time from onset to surgery did not significantly differ between the two groups. Only a higher rate of a history of hypertension was observed in the blend sign group (Table 1).
Discrepancies between the neurosurgical expert and the neuroimaging expert were noted for the scans of 3 patients. The level of interobserver agreement for identifying the CT blend sign was favourable, and the reliability was high between the 2 readers, with a κ value of 0.974 and a 95% confidence interval of 0.94–1.00.
Changes in haematoma volume
The residual ICH volume or the time to remove the drainage tube did not statistically differ between the blend sign group and the control group. No significant differences in the rates of ICH clearance were observed between the two groups (Fig. 4, Table 2). These results indicated that the blend sign had no influence on the removal of ICH by sMIS.
Changes in the GCS and the NIHSS
Both the blend sign group and the control group showed significantly increased GCS scores and decreased NIHSS scores at 1 and 2 weeks after surgery compared with those at admission (Tables 3 and Table 4). However, no significant differences were observed between the two groups at any time point. These findings suggested that the patients from the blend sign group developed the same short-term outcomes as the controls after sMIS.
Complications
Similar rates of severe complications, including pulmonary infection and heart failure, were observed between the blend sign group and the control group (P > 0.05, Table 5). However, a higher rate of re-haemorrhage was noted in the blend sign group (P = 0.049).
Influences of the CT blend sign on the outcome
Fifty patients (54.9%) from the blend sign group showed good outcomes. Among the 151 patients in the control group, 71 (51.8%) displayed good outcomes. No significant differences between the two groups were observed. Among the 128 patients with good outcomes, 50 (39.1%) showed the blend sign on the initial CT scan. The association of the CT blend sign with poor outcomes was determined by performing univariate analysis and binary logistic regression. The univariate analysis demonstrated that the CT blend sign was not statistically significantly associated with functional poor outcomes. Statistical significance (Table 6) was noted in the history of hypertension (P = 0.037), NIHSS score upon admission (P < 0.001), and GCS score upon admission (P < 0.001). These parameters were included in the binary logistic regression model. The final results suggested that the initial NIHSS score and the GCS score were independent predictors of poor functional outcomes in patients with ICH after sMIS (Table 7).
Discussion
Spontaneous ICH accounts for 10–30% of all types of stroke worldwide. HE may predict substantially poor outcomes, and HE may be prevented if patients with high-risk factors could be distinguished in the early stage of ICH [8]. The initial CT blend sign was closely associated with poor HE outcomes in patients treated with medications [9]. The blend sign also predicted postoperative re-haemorrhage in patients with ICH after sMIS [12].
Minimally invasive procedures have been utilized in the treatment of patients with ICH in recent decades. These procedures can remove ICH with minimal iatrogenic injuries to the brain and promote the recovery of neurological functions [17, 21]. Minimally invasive procedures for ICH treatment followed by thrombolysis may be a good therapeutic strategy for ICH [22]. Minimally invasive puncture followed by medication to dissolve the clot led to the least traumatic brain injuries and the shortest operation time [4]. The initial CT blend sign predicted postoperative re-haemorrhage in sMIS-treated patients with ICH [12]. Therefore, we postulated that the blend sign could affect the outcomes of patients with ICH following sMIS. In the present study, the GCS, NIHSS, and mRS scores and postoperative complications were used as indexes to evaluate patient outcomes. However, unexpected results were observed. The GCS score increased, and the NIHSS score decreased significantly at 2 weeks after surgery compared with those at admission. However, no significant differences in the outcome were observed between the blend sign group and the control group. The proportion of patients with favourable outcomes in the blend sign group did not differ from that in the control group. In previous studies, complications after ICH were strongly associated with the outcome [18, 19]. The most common medical complication (15.1%) secondary to ICH was pneumonia [23]. Cardiac complications (5.9%) secondary to ICH also usually occur following an increase in catecholamine levels and elevated levels of brain natriuretic peptide.
In the present study, the patients in the blend sign group and non-blend sign group showed similar rates of severe pulmonary infection and heart failure following sMIS. No significant differences were observed between the two groups, suggesting that the blend sign did not influence the rate of complications following sMIS. The blend sign group showed a higher rate of postoperative re-haemorrhage than that reported in our previously published study [12]. Although the blend sign indicated poor outcomes in patients treated with medications, there was no evidence that the blend sign is associated with poor outcomes in patients after sMIS. sMIS should be performed to treat patients with the initial CT blend sign if the ICH volume is large enough and the patient is a suitable candidate for surgery.
There were some limitations of the present study. The patients were not followed up after discharge. Therefore, we were unable to determine the long-term outcomes. Some patients were discharged from the hospital without medical orders, and the death rate could not be recorded or compared, as no deaths occurred during the hospital stay. The present study was retrospective, and additional randomized prospective studies with larger sample sizes are required in the future.
Conclusions
In conclusion, sMIS can remove intracerebral haematomas effectively. The initial CT blend sign is not associated with poor outcomes in patients with ICH following sMIS. ICH patients with the CT blend sign develop the same outcome as do patients without the CT blend sign after sMIS.
Availability of data and materials
The datasets analysed in the current study are available from the corresponding author upon reasonable request.
Abbreviations
- ICH:
-
Intracerebral haemorrhage
- CT:
-
Computed tomography
- sMIS:
-
Stereotactic minimally invasive surgery
- GCS:
-
Glasgow coma scale
- NIHSS:
-
National Institute of Health Stroke Scale
References
Kim JY, Bae HJ. Spontaneous Intracerebral hemorrhage: management. J Stroke. 2017;19(1):28–39. https://doi.org/10.5853/jos.2016.01935.
Chen S, Zhao B, Wang W, Shi L, Reis C, Zhang J. Predictors of hematoma expansion predictors after intracerebral hemorrhage. Oncotarget. 2017;8(51):89348–63. https://doi.org/10.18632/oncotarget.19366.
Wilkinson DA, Pandey AS, Thompson BG, Keep RF, Hua Y, Xi G. Injury mechanisms in acute intracerebral hemorrhage. Neuropharmacology. 2018;134(Pt B):240–8. https://doi.org/10.1016/j.neuropharm.2017.09.033.
Hanley DF, Thompson RE, Rosenblum M, Yenokyan G, Lane K, McBee, et al. Efficacy and safety of minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (MISTIE III): a randomised, controlled, open-label, blinded endpoint phase 3 trial. Lancet. 2019;393(10175):1021–32. https://doi.org/10.1016/S0140-6736(19)30195-3.
Kim HT, Lee JM, Koh EJ, Choi HY. Surgery versus conservative treatment for spontaneous Supratentorial Intracerebral hemorrhage in spot sign positive patients. J Korean Neurosurg Soc. 2015;58:309–15.
Mendelow AD, Gregson BA, Fernandes, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the international surgical trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet. 2005;365(9457):387–97. https://doi.org/10.1016/S0140-6736(05)70233-6.
Labib MA, Shah M, Kassam, et al. The safety and feasibility of image-guided BrainPath-mediated Transsulcul hematoma evacuation: a multicenter study. Neurosurgery. 2017;80:515–24.
Huang YW, Yang MF. Combining investigation of imaging markers (island sign and blend sign) and clinical factors in predicting hematoma expansion of Intracerebral hemorrhage in the basal ganglia. World Neurosurg. 2018;120:e1000–10. https://doi.org/10.1016/j.wneu.2018.08.214.
Li Q, Yang WS, Wang XC, Cao D, Zhu D, Lv FJ, et al. Blend sign predicts poor outcome in patients with intracerebral hemorrhage. PLoS One. 2017;12(8):e0183082. https://doi.org/10.1371/journal.pone.0183082.
Yu Z, Zheng J, Guo R, Ma L, Li M, Wang X, et al. Performance of blend sign in predicting hematoma expansion in intracerebral hemorrhage: a meta-analysis. Clin Neurol Neurosurg. 2017;163:84–9. https://doi.org/10.1016/j.clineuro.2017.10.017.
Shen Z, Wang L, Wu G, Li Q, Ren S, Mao Y. Computed tomographic black hole sign predicts postoperative Rehemorrhage in patients with spontaneous intracranial hemorrhage following stereotactic minimally invasive surgery. World Neurosurg. 2018;120:e153–60. https://doi.org/10.1016/j.wneu.2018.07.256.
Wu G, Shen Z, Wang L, Sun S, Luo J, Mao Y. Post-operative re-bleeding in patients with hypertensive ICH is closely associated with the CT blend sign. BMC Neurol. 2017;17(1):131. https://doi.org/10.1186/s12883-017-0910-6.
Wang L, Zhang L, Mao Y, Li Y, Wu G, Li Q. Regular-shaped hematomas predict a favorable outcome in patients with hypertensive Intracerebral hemorrhage following stereotactic minimally invasive surgery. Neurocrit Care. 2021;34(1):259–70. https://doi.org/10.1007/s12028-020-00996-2.
Hemphill JC 3rd, Greenberg SM, et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46(7):2032–60. https://doi.org/10.1161/STR.0000000000000069.
Li Q, Zhang G, Huang YJ, Dong MX, Lv FJ, Wei X, et al. Blend sign on computed tomography: novel and reliable predictor for early hematoma growth in patients with Intracerebral hemorrhage. Stroke. 2015;46(8):2119–23. https://doi.org/10.1161/STROKEAHA.115.009185.
Won SY, Zagorcic A, Dubinski D, Quick-Weller J, Herrmann E, Seifert V, et al. Excellent accuracy of ABC/2 volume formula compared to computer-assisted volumetric analysis of subdural hematomas. PLoS One. 2018;13:e0199809.
Li G, Qin X, Pen G, Wu W, Yang J, Yang Q. Effect of minimally invasive aspiration in treatment of massive intracerebral hemorrhage. In: Li G, Qin X, Pen G, Wu W, Yang J, Yang Q, editors. Acta Neurochirurgica supplement; 2011. p. 381.
Putaala J, Lehto M, Meretoja A, Silvennoinen K, Curtze S, Kääriäinen J, et al. In-hospital cardiac complications after intracerebral hemorrhage. Int J Stroke. 2014;9(6):741–6. https://doi.org/10.1111/ijs.12180.
Shibazaki K, Kimura K, Sakai K, Aoki J, Sakamoto Y. Plasma brain natriuretic peptide is elevated in the acute phase of intracerebral hemorrhage. J Clin Neurosci. 2014;21(2):221–4. https://doi.org/10.1016/j.jocn.2013.02.035.
Brott T, Broderick J, Kothari R, Barsan W, Tomsick T, Sauerbeck L, et al. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke. 1997;28(1):1–5. https://doi.org/10.1161/01.STR.28.1.1.
Wartenberg KE, Mayer SA. Ultra-early hemostatic therapy for Intracerebral hemorrhage: future directions. Front Neurol Neurosci. 2015;37:107. https://doi.org/10.1159/000437117.
Hanley DF, Thompson RE, Muschelli J, Rosenblum M, McBee N, Lane K, et al. Safety and efficacy of minimally invasive surgery plus alteplase in intracerebral haemorrhage evacuation (MISTIE): a randomised, controlled, open-label, phase 2 trial. Lancet Neurol. 2016;15(12):1228–37. https://doi.org/10.1016/S1474-4422(16)30234-4.
Stein M, Hamann GF, Misselwitz B, Uhl E, Kolodziej M, Reinges MH. In-hospital mortality and complication rates in surgically and conservatively treated patients with spontaneous Intracerebral hemorrhage in Central Europe: a population-based study. World Neurosurg. 2016;88:306–10. https://doi.org/10.1016/j.wneu.2015.11.075.
Acknowledgements
We are grateful for the help provided by the Imaging Department of the Affiliated Hospital of Guizhou Medical University for the computed tomography image analysis. We also wish to thank all the postgraduates who were involved in this study for their hard work.
Funding
This research was supported by the Natural Science Foundation of China (81971126/H0906) and Medical Speciality and Community Project Construction in Baoshan District –Neurorehabilitation Speciality (BSZK-2018-A01) as well as the High-level Overseas Talents Innovation and Entrepreneurship Merit-based Funding Projects [(2020) 05]. The funding body did not take part in the design of the study; data collection, analysis, or interpretation; or writing of the manuscript.
Author information
Authors and Affiliations
Contributions
GW, LW and JL conceived the study, helped design the study, coordinated the study and drafted the manuscript. XY, LZ, YL and YM conducted the clinical study. YZ and LW performed the statistical analyses and revised the manuscript. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing of interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
This retrospective study (including the use of the clinical data and the personal patient data) was approved by the Ethics Committee of the Affiliated Hospital of Guizhou Medical University. All the patients’ authorized representatives and the patients who had the ability to communicate with the doctors agreed to undergo minimally invasive surgery. Written informed consent was obtained.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, X., Zhu, Y., Zhang, L. et al. The initial CT blend sign is not associated with poor patient outcomes after stereotactic minimally invasive surgery. BMC Neurol 21, 160 (2021). https://doi.org/10.1186/s12883-021-02181-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-021-02181-0