Abstract
Background
Multiple sclerosis (MS) patients often struggle with treatment decisions, in part due to the increasing number of approved disease modifying therapies, each with different characteristics, and also since physicians can struggle to identify which of these characteristics matter most to each individual patient. Decision uncertainty can contribute to late treatment initiation and treatment non-adherence—causes of ‘undertreatment’ in MS. An interactive online patient decision aid that informs patients of their options, considers their individual preferences and goals, and facilitates conversations with their physicians, could improve how patients with relapsing forms of MS make evidence-based treatment decisions.
Objective
To develop and evaluate a prototype patient decision aid (PtDA) for first-line disease modifying therapies for relapsing-remitting multiple sclerosis.
Methods
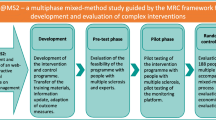
Informed by previous studies and International Patient Decision Aid Standards guidelines, a prototype PtDA was developed for patients with relapsing multiple sclerosis considering first line treatment. Patients with relapsing multiple sclerosis were recruited from the University of British Columbia’s Multiple Sclerosis Clinic to participate in either an online survey or a focus group. Online survey participants completed the PtDA, followed by measures of acceptability, usability, and preparedness for decision-making, and provided general feedback. Focus group participants assessed usability of the revised PtDA. The analysis of qualitative and quantitative data led to improvements of the PtDA prototype.
Results
The prototype PtDA received high ratings for acceptability and usability, and after its use, participants reported high-levels of preparedness for decision-making. Analysis of all qualitative data identified three key themes: the need for credible information; the usefulness of the PtDA; and the importance of normalizing and sharing experiences. Nine content areas were identified for revision. Overall, participants found the PtDA to be a valuable tool for facilitating treatment decisions.
Conclusions
This mixed methods study has led to the development of a PtDA that can support patients with RRMS as they make treatment decisions. Future studies will assess the feasibility of implementation and the impact of the PtDA on both the timely treatment initiation and longer-term adherence.
Similar content being viewed by others
Background
Multiple sclerosis (MS) is a leading cause of non-traumatic neurological disability for young adults [1,2,3]. Approximately 80–85% of MS patients are diagnosed with relapsing-remitting multiple sclerosis (RRMS) and experience clearly defined disease exacerbations that last from a few hours to months [4, 5]. A window of opportunity exists in early RRMS to gain maximal benefit from disease modifying therapies (DMTs) [6, 7]. However, patients often struggle with treatment decisions and delay initiating treatment [6, 8,9,10]. The complexity of treatment decisions is driven by the lack of useful markers to predict the disease course in each individual patient [11] and an increasing number of DMT options, each with unique benefit and side-effect profiles including rare but potentially severe side-effects [12,13,14]. Further complicating the decision, routes of administration, monitoring, cost, availability, and future sequencing considerations vary across DMT options [12,13,14]. Both late treatment initiation and treatment non-adherence are causes of ‘undertreatment’, a persistent issue for patients with MS [8, 15].
Physicians often struggle to accurately assess patient preferences [16] and may rely on their own preferences and cognitive distortions (e.g. overconfidence, tolerance to risk and ambiguity) to make treatment recommendations [17]. These can often be incongruent with individual patient preferences [18,19,20] and may lead to patients being uncertain that the prescribed treatment is the best option for them [21,22,23,24]. In MS, five preference subgroups have been identified. These subgroups are characterized by varying degrees of desire to avoid risk of serious adverse events and improve symptoms, and preferences for specific routes of administration [25]. Given the diversity of preferences, understanding patient preferences may be important to increasing the number of patients that initiate and adhere to therapy long-term [26, 27]. In other chronic diseases, substantial evidence supports the use of patient decision aids (PtDAs) to promote shared decision-making between the patient and their doctors [28]. Shared decision making using PtDAs can help patients feel better informed and have a better understanding of their own values, and may improve assessment of treatment risk and quality of treatment decisions [28].
The objectives of this study were to: (1) develop a PtDA for newly diagnosed RRMS patients considering a DMT to manage their condition, and (2) investigate the usability and acceptability of the PtDA in the clinical decision-making process. Although the Cochrane review by Stacey et al. evaluated one German study on the use of an evidence-based patient decision aid on multiple sclerosis immunotherapy [22], various decision support tools have been developed to help patients make treatment decisions in MS [29,30,31,32,33,34,35,36]. However, the decision aid tools tend to focus on only a limited part of the decision (e.g. whether or not to take medication [34]), fail to ask what aspects of treatment matter most to patients, and do not help match these preferences with available DMTs [33,34,35,36]. Both initiating medication and selecting a medication are significant decisions for patients with RRMS and information alone often does not lead to appropriate decision-making [37]. To address this gap, we developed a novel online patient decision aid (RRMS-PtDA) for patients with RRMS who are considering a first-line DMT. Six first-line DMTs available to MS patients in British Columbia, Canada in March 2017 were explored, including: Avonex, Betaseron, Rebif, Copaxone, Aubagio, and Tecfidera. The beta-interferons and Copaxone are modestly effective at preventing relapses and future disability, with only marginal differences in efficacy, tolerability, and short and long-term safety [38]. The oral medications (Aubagio and Tecfidera) may have improved efficacy but also have known and unknown safety concerns. For instance, using Tecfidera carries a risk of developing progressive multifocal leukoencephalopathy (PML) [39]. Information about other treatment options from the MS Society of Canada (e.g. rehabilitation and physical therapy, relapse management therapies, symptom management therapies) were also included. A unique feature of the RRMS-PtDA is its ability to tailor the information provided to match individual patient goals and preferences and to personalize how treatment options are presented.
Methods
Overview
The International Patient Decision Aid Standards (IPDAS) process for systematic development of PtDAs was used to guide the development of this tool [40, 41]. Following a scoping review and assessment of the need for a PtDA for RRMS, a multidisciplinary steering committee was formed. The committee consisted of physician specialists in MS, patients, software developers, and researchers with expertise in decision-making. Ethics was obtained from the Behavioural Research Ethics Board at the University of British Columbia.
Identification of patient preferences and physician perceptions
A previous study used qualitative individual and group interviews to identify the 6 most important aspects of treatment for patients with RRMS: (1) slowing progression of MS; (2) reducing symptoms associated with MS; (3) preventing relapse and MRI changes; (4) minimizing minor side effects; (5) avoiding serious adverse events; and (6) choosing route of administration [25]. The relative importance of each attribute was determined using a stated preference technique known as a discrete choice experiment.
To elicit clinicians’ views on patient decision support needs, a systematic literature review and individual semi-structured interviews with clinicians were conducted. In addition, direct observation of patient-clinician interactions informed development of the PtDA.
Literature review
In collaboration with MS clinicians, a comprehensive literature search for comparative evidence was conducted to provide consistent levels of evidence to present in the PtDA. Of the available evidence, we chose to utilize findings from a review by the Canadian Agency for Drugs and Technologies in Health (CADTH) Therapeutic Review for MS [42]—a comprehensive review of the existing literature, published studies, materials and other information and documentation available to CADTH on treatments for MS. The review included a network meta-analysis that provides indirect estimates on the relative effectiveness of DMTs even where no direct trials exist. Conducted without industry funding and reviewed by health economists, methodologists, and clinical experts in MS, the CADTH report is rigorously peer reviewed, and was identified as providing the most comprehensive, accurate and unbiased evidence. Information from the MS Society of Canada [43] was used to supplement the report as needed.
Assessment of logistical needs for clinical implementation
Qualitative feedback from patients and physicians, a literature review, a review of clinic operations, and best practice recommendations indicated that a PtDA needed to be accessible from multiple locations (e.g., home, or the physician’s office) and easy to update as new treatments and evidence become available. It was also determined that a summary sheet would be needed to provide the results of patients’ preferences and knowledge assessments to physicians prior to patient consultations. Consequently, we developed an online, interactive decision aid using a previously developed open source platform [44].
Initial prototype development
Development of the prototype PtDA was guided by the IPDAS criteria for creating quality PtDAs [40, 41] and supported by the multidisciplinary steering group. Researcher experience with previous PtDAs also informed prototype development.
The initial PtDA was comprised of 5 sections: (1) a history module that collected a brief medical history to provide personalized information on subsequent pages; (2) an information module that presented effectiveness and side effect profiles of the 6 first-line DMTs available to patients in British Columbia, Canada in March 2017; (3) an interactive value clarification module that guided patients to consider which attributes of treatment matter most to them; (4) a decision module that compared the treatment options and suggested a treatment that best fit the patient based on information provided in modules 1 and 2; and (5) a tailored summary screen that described patient health status, preferred treatment choice, and any questions or concerns the patient had for their health care team—the summary could be printed or e-mailed directly to the MS clinic to be included in patient electronic medical records. Videos of a physician explaining the importance and goal of each section were also included as an alternative to reading text only.
The prototype was then reviewed by a team of physicians specializing in the diagnosis and treatment of MS (RC, EM and AT). Physician feedback focused on two areas: evidence on drug effectiveness and side effects; and development of appropriate questions about patient medical history. Several changes to the prototype were made to address the physician feedback.
Quantitative usability testing
A web-survey assessed the acceptability, usability, and preliminary usefulness of the prototype PtDA. Faulker et al. [45] suggest using a sample of 10–20 participants in usability studies to identify 80–95% of usability problems. We recruited 18 patients from the University of British Columbia Multiple Sclerosis Clinic (UBC MS Clinic) who were eligible to participate if they: were at least 18 years of age; had a diagnosis of RRMS; could read, speak and understand English, use a computer interface, and provide informed consent; and were treatment naïve or had not used treatment for at least 2 years. This group of patients has been identified to obtain a diverse range of perspectives to identify usability issues. Patients with other forms of MS, such as clinically isolated syndrome and inactive progressive disease were not eligible for this study due to treatment eligibility criteria from local health authorities. Patients meeting inclusion criteria were invited to participate in the study by the research assistant. Those who consented to participating received an email invitation with the link to the survey and decision aid to complete. Participants completed the PtDA followed by the scales below, and open-ended questions on clarity, usability, and perceived need for the PtDA:
System usability scale (SUS)
Developed in 1986, the 10-item SUS uses a 5-point Likert scale (anchored at “Strongly Disagree” and “Strongly Agree”) to assess subjective usability of a product. Scores range from 40 to 100; a score below 68 indicates below average usability, and a score at or above 68 indicates above average usability [46, 47].
Acceptability scale
Developed specifically for PtDAs, the acceptability scale assesses how well information is presented for different topics (e.g. risk factors, treatment etc.) and overall impressions of the decision tool. There is no total score for the Acceptability Scale. Instead, items are analyzed individually [48]. The Acceptability Scale is designed to accommodate any health decision and has been used in many different contexts including atrial fibrillation [49]; prenatal testing [50]; and lung cancer [51].
Preparation for decision making scale (PDMS)
The PDMS assesses patient perception of the usefulness of a PtDA in preparing them to consult with physicians and make a healthcare decision. The 10-items of the PDMS are rated for agreement using a 5-point Likert scale (“Not at all”; “A little”; “Somewhat”; “Quite a bit”; and “A great deal”) [52]. Items are summed to give a score ranging from 0 to 100, with higher scores indicating greater perceived preparedness for decision-making. The PDMS is well validated, with high discriminant validity and both internal and test-retest reliability [53, 54].
Qualitative usability testing
A focus group was conducted to further assess usability and to gain insight on the feedback and findings from the survey. Patients were recruited from the UBC MS Clinic and were eligible to participate if they: had ever received a diagnosis of RRMS; could use a computer interface, read, speak, and understand English, and provide informed consent; and were at least 18 years of age. As the purpose of the focus group was to assess usability, current treatment was not an exclusion criterion. In total, 7 patients participated in the focus group. After providing consent, participants were e-mailed a link to the PtDA one week before the focus group and instructed to complete the PtDA before attending. All participants completed a brief demographic survey. The focus group was 90-min in duration and was led by a facilitator that was not involved in the development of the tool.
Analysis
Descriptive statistics were used to assess participant characteristics as well as scores from the SUS, Acceptability Scale, and PDMS. Where possible, mean scores were compared to the standards identified in the literature (i.e., SUS [46, 47]). All scale scores were interpreted per the scale user manual.
Discussions from the focus group were transcribed and analyzed. Focus group transcripts and the open-ended questions from the survey were analyzed using conventional content analysis [55]. Transcripts were analyzed using inductive coding, conducted in an iterative fashion. Constant comparison was used to maximize parsimony and coherence. Codes were then reviewed by an independent researcher who did not participate in the survey or the focus group. Any coding concerns were resolved by discussion. Lastly, as recommended by Lincoln and Guba [56], member-checking was used to ensure that results were credible and confirmable.
Results
Participant characteristics
In total, 25 individuals with MS participated (n = 18 for the survey; n = 7 for focus groups). Participants ranged from 21 to 70 years of age and reported disease durations of less than 1 year to more than 17 years, indicating a broad representation of disease progression and time since diagnosis. Patient determined disease steps from MS ranged from no limitation to significant limitation requiring bilateral walking support for short distances [57, 58]. As in other cohorts of MS patients [59], the majority of participants were female.
Quantitative prototype testing
Usability
The SUS yielded a mean score of 80.6 (s = 13.7), indicating that the tool had above average usability (Table 1). Analysis of individual items found that 94% of participants rated all items 3 or better on a 5-point scale, indicating consistent usability across features of the PtDA. Importantly, 100% of participants rated the PtDA “easy to use”, “well integrated”, and consistent (Additional file 1: Table S1).
Acceptability
All items of the Acceptability Scale were rated positively. The majority of patients reported that the PtDA presented the right amount of information (72%; n = 13), with a subset of patients reporting that the PtDA presented slightly less information than desired (22%; n = 4). Likewise, most patients reported that information was presented in a balanced manner (67%; n = 12); however, one third reported that the PtDA was slightly or clearly biased towards taking treatment (n = 6). Half of patients reported that the information presented aligned with the information they had received from other health professionals (n = 9), however 39% of patients (n = 7) reported some discrepancies between the PtDA and information received from other health professionals, and 11% (n = 2) reported no alignment between the two sources of information (Table 1, Additional file 1: Table S2). Patients rated the clarity of the information highly; 75% of content areas were rated as having “fair” clarity or above by all respondents. Two items—information on funding and authors, and references—were each rated “poor” clarity by 1 participant (6%) (Additional file 1: Table S3).
Preparation for decision-making
Analysis of PDMS scores found a mean PDMS rating of 76.3 (s = 15.3), indicating a high level of perceived preparedness to make a treatment decision after completing the PtDA (Table 1). See Additional file 1: Table S4 for participant ratings of individual items.
Qualitative assessment of the PtDA
Content analysis of the focus group transcript and open-ended text responses identified three overarching themes: (1) need for information; (2) usefulness of the PtDA; and (3) importance of normalizing and sharing experiences.
Theme 1: need for information that the PtDA provides
Focus group participants who had previously made treatment decisions before the PtDA was available reported that the physician generally provided information orally. Upon receiving a diagnosis and learning of treatment options, participants were not provided with information booklets or other informational materials that could be taken home. As a result, several participants reported that early appointments were difficult: the quantity of information was overwhelming; and it was a challenge to keep track of the questions they had asked or wanted to ask. These participants also reported trying to find information online but found that it was hard to find trustworthy information. Participants who were more recently diagnosed reported difficulty compiling and comparing the information they found in a meaningful way. Feedback and discussion emphasized that participants wanted credible information that they could reflect on after their initial appointment with a neurologist.
“I still think like with my neurologist, it [getting information] was largely in person…my girlfriend was trying to keep up with her [the neurologist] and I think I probably remember one sentence that she said so it was all kind of overwhelming.”
“Well the problem was that at that time too, what you would see online, you couldn’t believe a lot of it because there was a lot of stuff [like], “Send us this money and we’ll cure you” sort of thing where that I don’t think quite exists like it used to.”
Theme 2: usefulness of the decision aid
The feedback on the usefulness of the PtDA was overwhelmingly positive. Focus group and open-ended responses suggested that the tool filled a need; it provided credible information, in an accessible manner, at a time when patients are feeling overwhelmed. Participants reflected on the impact that this tool would have had or did have on their decision-making process.
“The decision aid cut my research in half.”
“So, at our first meeting, we probably spent way too much time talking about the different treatments. I didn’t have anything like this, this little table. That would have been like a godsend.”
Theme 3: importance of normalizing and sharing experiences
Focus group participants also reported that the PtDA may provide a unique opportunity to normalize and share experiences with those newly diagnosed with MS. Participants suggested replacing existing videos of the physician describing the importance of each section with patients sharing their experiences of living with the disease and strategies they tried. This opportunity was particularly important because participants reported having limited contact with people with lived experience of MS, and instead were often inundated with suggestions from family and friends. Importantly, for focus group participants this normalization was not about the disease symptoms but rather about the emotional challenges that can arise when receiving a diagnosis.
“[It would be helpful to say] this decision doesn’t have to be made today. It is okay to take some time.”
“And I think that, you know, you sort of touched on this, that overwhelming. It’s normal to feel overwhelmed with all this information.”
Modifications
Based on the feedback from the quantitative and qualitative work, eight content areas were identified for improvement. These included: clarity; content; content presentation; video content; hyperlinks; functionality; value elicitation method; and summary. For most content areas, participant feedback was largely consistent and specific—for example, 5 out of 7 focus group patients suggested the ability to download a 1-page reference chart comparing all medications. Illustrative quotations, and changes made to address feedback are listed in Table 2 and screenshots from the revised PtDA are shown in Fig. 1.
Discussion
This study used an iterative approach that engaged all relevant stakeholders to develop and assess a PtDA for RRMS. We utilized a mixed-methods approach and found that the PtDA was both highly usable and acceptable. Despite high initial usability and acceptability ratings, patient feedback was used to further refine the RRMS-PtDA. Of note, participants perceived the information presented in the PtDA to be unbiased and credible, two characteristics which are known to be critical for creating effective PtDAs [60]. In addition to high usability and acceptability ratings, treatment-naïve patients reported high levels of preparedness to make a decision after completing the PtDA.
Importantly, participants reported that the PtDA addressed a pressing need for consistent, accessible information in a single location. Furthermore, several focus group participants reported that the PtDA would have decreased the extent to which they felt overwhelmed in initial neurological consultations. With an increasing number of DMT options becoming available, the PtDA helps overcome information overload by ordering options such that those that best match patient’s preferences are shown first [61]. Notably, this does not prevent patients from reviewing all treatment options, but nudges patients towards spending their cognitive effort on the DMTs that are the most likely candidates.
The RRMS-PtDA was designed to facilitate shared decision-making (SDM) between patients and physicians; however, not all patients want to share that decision, and instead some prefer to have the physician make treatment decisions [62]. The Control Preferences Scale, which assesses patient preferences for SDM, is embedded in the RRMS-PtDA, and patient SDM preferences are included in the summary page. By asking about SDM preferences, the RRMS-PtDA ensures that the process is always collaborative as even those who prefer to defer entirely to the treating physician can state their preference and this preference will guide future neurological consultations.
The results of the present study are bolstered by a mixed-methods approach, as well as the engagements of diverse participants with varying disease durations and patient reported disability. The strengths of this study should be considered in the context of its limitations. First, because the PDMS was not administered before and after completion of the PtDA, it is unclear if high PDMS scores are a result of the PtDA itself or simply a characteristic of study participants. Future studies should include pre- post- measures to determine the impact of the PtDA on preparedness. Additionally, the PtDA was developed for first-line treatment of RRMS with DMTs. Consequently, the results from the prototype testing may not apply to individuals who are already receiving a DMT, considering second line therapies or experiencing progressive MS. Although the PtDA utilized a highly credible information source [34], this may not reflect individual physicians’ interpretation of the same data or take into account their clinical experience with different therapies. The goal of a PtDA is to enhance the patient-physician encounter by providing basic information to help frame physician recommendations and enable more consultation time to be spent addressing the patient’s concerns and questions rather than explaining treatment options. Moreover, the PtDA was created specifically for patients at the UBC MS Clinic and may not be generalizable to other patient groups. The PtDA would need to be tailored to each clinic/region, where different treatments may be available, different clinical pathways in place, and patients may have different preferences for attributes of treatment in MS. We also deliberately sought a heterogenous group of participants so that we would obtain a diverse range of perspectives to identify usability issues. For the survey, we had limited ability to recruit just a narrower group and thought that including a group that was not on treatment for at least 2 years would be useful. Lastly, although the PtDA was highly rated, the present study did not assess feasibility.
Conclusions
We have developed an RRMS-PtDA for first-line DMTs. The PtDA lays the foundation for and promotes SDM by both assessing patient preferences for SDM and by providing clear, credible information on treatments. Thus, the PtDA allows physician consultations to focus on patient priorities, preferences, and questions rather than information provision. The PtDA, available at www.msdecisionaid.com by contacting the research investigator, can be adapted for other jurisdictions and languages, and updated as new treatments and evidence emerges. Future studies will determine how best to implement the PtDA in routine practice, and the effect the PtDA has on treatment initiation and adherence.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CADTH:
-
Canadian agency for drugs and technologies in health
- DMT:
-
Disease modifying therapy
- IPDAS:
-
International patient decision aid standards
- MS:
-
Multiple sclerosis
- PtDA:
-
Patient decision aid
- RRMS:
-
Relapsing-remitting multiple sclerosis
- RRMS-PtDA:
-
Relapsing-remitting multiple sclerosis patient decision aid
- SDM:
-
Shared decision-making
- UBC:
-
University of British Columbia
References
Beck CA, Metz LM, Svenson LW, Patten SB. Regional variation of multiple sclerosis prevalence in Canada. Mult Scler. 2005;11(5):516–9.
Canadian Institute for Health Information. The burden of neurological diseases, disorders and injuries in Canada. Ottawa: CIHI; 2007.
Evans C, Beland S-G, Kulaga S, Wolfson C, Kingwell E, Marriott J, et al. Incidence and prevalence of multiple sclerosis in the Americas: a systematic review. Neuroepidemiology. 2013;40(3):195–210.
Milo R, Miller A. Revised diagnostic criteria of multiple sclerosis. Autoimmun Rev. 2014;13(4):518–24.
Multiple Sclerosis Society of Canada. What is MS? Available from: https://mssociety.ca/about-ms/what-is-ms. Accessed 15 July 2018.
Frohman EM, Havrdova E, Lublin F, Barkhof F, Achiron A, Sharief MK, et al. Most patients with multiple sclerosis or a clinically isolated demyelinating syndrome should be treated at the time of diagnosis. Arch Neurol. 2006;63:614–9.
Giovannoni G, Butzkueven H, Dhib-Jalbut S, et al. Brain health: time matters in multiple sclerosis. UK: Oxford PharmaGenesis; 2015.
Noyes K, Weinstock-Guttman B. Impact of diagnosis and early treatment on the course of multiple sclerosis. Am J Manag Care. 2013;19:s321–31.
Comi G, Filippi M, Barkhof F, Durelli L, Edan G, Fernández O, et al. Effect of early interferon treatment on conversion to definite multiple sclerosis: a randomized study. Lancet. 2001;357:1576–82.
Neuhaus M, Calabrese P, Annoni JM. Decision-making in multiple sclerosis patients: a systematic review. Mult Scler Int. 2018;7835952.
Tilling K, Lawton M, Robertson N, Tremlett H, Zhu F, Harding K, et al. Modelling disease progression in relapsing-remitting onsent multiple sclerosis using multilevel models applied to longitudinal data from two natural history cohorts and one treated cohort. Health Technol Assess. 2016;20(81):1–48.
Heesen C, Solari A, Giordano A, Kasper J, Köpke S. Decisions on multiple sclerosis immunotherapy: new treatment complexities urge patient engagement. J Neurol Sci. 2011;306:192–7.
Wingerchuk DM, Carter JL. Multiple sclerosis: current and emerging disease-modifying therapies and treatment strategies. Mayo Clin Proc. 2014;89:225–40.
Trojano M, Tintore M, Montalban X, Hillert J, Kalincik T, Iaffaldano P, et al. Treatment decisions in multiple sclerosis – insights from real-world observational studies. Nat Rev Neurol. 2017;13:105–18.
Hansen K, Schüssel K, Kieble M, Werning J, Schulz M, Friis R, et al. Adherence to disease modifying drugs among patients with multiple sclerosis in Germany: a retrospective cohort study. PLoS One. 2015;10:e0133279.
Mulley A, Trimble C, Elwyn G. Stop the silent misdiagnosis: patients’ preferences matter. BMJ. 2012;345:e6572.
Saposnik G, Sempere AP, Raptis R, Prefasi D, Selchen D, Maurino J. Decision making under uncertainty, therapeutic inertia, and physicians’ risk preferences in the management of multiple sclerosis (DIScUTIR MS). BMC Neurol. 2016;16:58.
Harrison M, Milbers K, Hudson M, Bansback N. Do patients and health care providers have discordant preferences about which aspects of treatments matter most? Evidence from a systematic review of discrete choice experiments. BMJ Open. 2017;7:e014719.
Rothwell PM, McDowell Z, Wong CK, Dorman PJ. Doctors and patients don’t agree: cross sectional study of patients’ and doctors’ perceptions and assessments of disability in multiple sclerosis. BMJ. 1997;314:1580–3.
Palace J. Partnership and consent in MS treatment choice. J Neurol Sci. 2013;335:5–8.
Lejbkowicz I, Caspi O, Miller A. Participatory medicine and patient empowerment towards personalized healthcare in multiple sclerosis. Expert Rev Neurother. 2012;12:343–52.
Heesen C, Kasper J, Köpke S, Richter T, Segal J, Mühlhauser I. Informed shared decision making in multiple sclerosis—inevitable or impossible? J Neurol Sci. 2007;259:109–17.
Reynolds MW, Stephen R, Seaman C, Rajagopalan K. Persistence and adherence to disease modifying drugs among patients with multiple sclerosis. Curr Med Res Opin. 2010;26:663–74.
Saunders C, Caon C, Smrtka J, Shoemaker J. Factors that influence adherence and strategies to maintain adherence to injected therapies for patients with multiple sclerosis. J Neurosci Nurs. 2010;42:S10–8.
Lynd LD, Traboulsee A, Marra CA, Mittmann N, Evans C, Li KH, Carter M, Hategekimana C. Quantitative analysis of multiple sclerosis patients’ preferences for drug treatment: a best-worst scaling study. Ther Adv Neurol Disord. 2016;9:287–96.
Haskard Zolnierek KB, DiMatteo MR. Physician communication and patient adherence to treatment: a meta-analysis. Med Care. 2009;47:826–34.
Caster O, Edwards IR. Quantitative benefit-risk assessment of methylprednisolone in multiple sclerosis relapses. BMC Neurol. 2015;15:206.
Stacey D, Légaré F, Lewis K, Barry MJ, Bennett CL, Eden KB, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;(4):CD001431.
Köpke S, Kasper J, Mühlhauser I, Nübling M, Heesen C. Patient education program to enhance decision autonomy in multiple sclerosis relapse management: a randomized-controlled trial. Mult Scler. 2009;15:96–104.
Solari A, Martinelli V, Trojano M, Lugaresi A, Granella F, Giordano A, et al. An information aid for newly diagnosed multiple sclerosis patients improves disease knowledge and satisfaction with care. Mult Scler. 2010;16:1393–405.
Kasper J, Köpke S, Mühlhauser I, Heesen C. Evidence-based patient information about treatment of multiple sclerosis—a phase one study on comprehension and emotional responses. Patient Educ Couns. 2006;62:56–63.
Prunty MC, Sharpe L, Butow P, fulcher G. The motherhood choice: a decision aid for women with multiple sclerosis. Patient Educ Couns. 2008;71:108–15.
Kasper J, Köpke S, Mühlhauser I, Nübling M, Heesen C. Informed shared decision making about immunotherapy for patients with multiple sclerosis (ISDIMS): a randomized controlled trial. Eur J Neurol. 2008;15:1345–52.
Healthwise. Multiple sclerosis: should I start taking medicines for MS? https://www.healthlinkbc.ca/health-topics/tf2571 (2017). Accessed 01 May 2018.
Multiple Sclerosis Society of Canada. Exploring your options: considering the risks and benefits of MS medications. 2015. https://mssociety.ca/uploads/files/1909-msexploringoptionsbro2015-eng-v2-web.pdf. Accessed 01 May 2018.
Multiple Sclerosis Trust. MS Decisions aid. https://www.mstrust.org.uk/understanding-ms/ms-symptoms-and-treatments/ms-decisions/decision-aid (n.d.). Accessed 15 Apr 2018.
Witteman HO, Scherer LD, Gavaruzzi T, Pieterse AH, Fuhrel-Forbis A, Chipenda Dansokho S, et al. Design features of explicit values clarification methods: a systematic review. Med Decis Mak. 2016;36:453–71.
Reder AT, Ebers GC, Traboulsee A, Li D, Langdon D, Goodin DS, et al. Cross-sectional study assessing long-term safety of interferon-beta-1b for relapsing-remitting MS. Neurology. 2010;74(23):1877–85.
Rosenkranz T, Novas M, Terborg C. PML in a patient with lymphocytopenia treated with dimethyl fumarate. N Engl J Med. 2015;372(15):1476–8.
Elwyn G, O’Connor AM, Stacey D, Volk R, Edwards A, Coulter A, et al. Developing a quality criteria framework for patient decision aids: online international Delphi consensus process. BMJ. 2006;333:417.
Coulter A, Stilwell D, Kryworuchko J, Mullen PD, Ng CJ, van der Weijden T. A systematic development process for patient decision aids. BMC Med Inform Decis Mak. 2013;13(S2).
Canadian Agency for Drugs and Technologies in health. CADTH therapeutic review. Comparative clinical and cost-effectiveness of drug therapies for relapsing-remitting multiple sclerosis. 2013. http://www.cadth.ca/media/pdf/TR0004_RRMS_ScienceReport_e.pdf. Accessed 02 Feb 2017.
Multiple Sclerosis Society of Canada. https://mssociety.ca (2018). Accessed 15 Apr 2018.
Dynamic Computer Interactive Decision Application (DCIDA). https://www.dcida.ubc.ca (n.d.). Accessed 02 Feb 2017.
Faulkner L. Beyond the five-user assumption: benefits of increased sample sizes in usability testing. Behav Res Methods Instrum Comput. 2003;35(3):379–83.
Brooke J. SUS: a ‘quick and dirty’ usability scale. Usability Eval Ind. 1996;189:4–7.
Bangor A, Kortum P, Miller J. Determining what individual SUS scores mean: adding an adjective rating scale. J Usability Stud. 2009;4:114–23.
O’Connor AM, Cranney A. User manual – acceptability. Ottawa: Ottawa Hospital Research Institute; 1996. https://decisionaid.ohri.ca/docs/develop/user_manuals/UM_acceptability.pdf. Accessed 05 May 2018
Man-Son Hing M, Laupacis A, O’Connor A, Wells G, Lemelin J, Wood W, Dermer M. Warfarin for atrial fibrillation. The patient’s perspective. Arch Intern Med. 1996;156:1841–8.
Drake ER, Engler-Todd L, O’Connor AM, Surh LC, Hunter A. Development and evaluation of a decision aid about prenatal testing for women of advanced maternal age. J Genet Couns. 1999;8:217–33.
Fiset V, O’Connor AM, Evans W, Graham I, Degreasse C, Logan J. Development and evaluation of a decision aid for patients with stage IV non-small cell lung cancer. Health Expect. 2000;3:125–36.
Graham ID, O’Connor AM. User manual – preparation for decision making scale. Ottawa: Ottawa Hospital Research Institute; 1995. https://decisionaid.ohri.ca/docs/develop/user_manuals/UM_prepdm.pdf. Accessed 01 May 2018
Bennett C, Graham ID, Kristjansson E, Kearing SA, Clay KF, O’Connor AM. Validation of a preparation for decision making scale. Patient Educ Couns. 2010;78:130–3.
O’Connor AM, Jacobsen MJ, Elmslie T, Jolly E, Wells G, Bunn H, et al. Simple vs complex patient decision aids: is more necessarily better? Med Decis Mak. 2000;20:496.
Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15:1277–88.
Lincoln YS, Guba EG. Naturalistic inquiry (volume 75). Newbury Park: Sage Publications; 1985.
Vollmer TL, Ni WJ, Stanton S, Hadjimichael O. The NARCOMS patient registry: a resource for investigators. Int J MS Care. 1999;1:28–34.
NARCOMS: Multiple Sclerosis patient registry. https://www.narcoms.org (2017). Accessed 15 Apr 2018.
Orton SM, Herrera BM, Yee IM, Valdar W, Ramagopalan SV, Sadovnick AD, Ebers GC. Sex ratio of multiple sclerosis in Canada: a longitudinal study. Lancet Neurol. 2006;5:932–6.
O'Donnell S, Cranney A, Jacobsen MJ, Graham ID, O'Connor AM, Tugwell P. Understanding and overcoming the barriers of implementing patient decision aids in clinical practice. J Eval Clin Pract. 2006;12:174–81.
Bansback N, Li LC, Lynd L, Bryan S. Development and preliminary user testing of the DCIDA (dynamic computer interactive decision application) for ‘nudging’ patients towards high quality decisions. BMC Med Inform Decis Mak. 2014;14:62.
Cofield SS, Thomas N, Tyry T, Fox RJ, Salter A. Shared decision making and autonomy among US participants with multiple sclerosis in the NARCOMS registry. Int J MS Care. 2017;19:303–12.
Acknowledgements
We thank all the patients involved in the study.
Funding
Vancouver and UBC Hospital Foundation. The funding agency had no role in the concept, design, conduct or interpretation of the results. BC SUPPORT Unit through partnership with CHEOS.
Author information
Authors and Affiliations
Contributions
NB contributed to the conception and design of the study, participated in the analysis and interpretation of data, drafted the initial version of the manuscript. JAC contributed to the acquisition, and analysis and interpretation of data, and drafted the initial version of the manuscript. RC contributed to the conception and design of the study. RM contributed to the acquisition, and analysis and interpretation of data, drafted the initial version of the manuscript. EL and AS contributed to the acquisition of data. ML contributed to the analysis and interpretation of data. LDL contributed to the conception and design of the study. AT contributed to the conception and design of the study. All authors critically commented on the manuscript and approved the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the University of British Columbia Behavioural Research Ethics Board (H16–00965). All participants provided written consent prior to participating.
Consent for publication
Not applicable; all participants have provided consent that any feedback they provided may be used in publications without being identified.
Competing interests
RC has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with Biogen, Genzyme, Teva, Novartis, EMD Serono. RC has received research support from MedImmune, Teva. EL has received personal compensation for consultancy with EMD Serono and Hoffmann-La Roche. LL has served on advisory boards from AstraZeneca, Novartis, Boehringer Ingelheim, Teva, and Pfizer. AT has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with Biogen, Chugai, F. Hoffmann-La Roche Ltd., Novartis, Sanofi Genzyme and Teva. AT has received research support from F. Hoffmann-La Roche Ltd.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Table S1. Participants’ ratings on the System Usability Scale (N = 18). Table S2. Participants’ ratings on acceptability and usability. Table S3. Participants’ ratings on the clarity of the information presented in each section. Table S4. Participants’ ratings on the Preparation for Decision Making Scale (N = 18). (DOCX 76 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Bansback, N., Chiu, J.A., Carruthers, R. et al. Development and usability testing of a patient decision aid for newly diagnosed relapsing multiple sclerosis patients. BMC Neurol 19, 173 (2019). https://doi.org/10.1186/s12883-019-1382-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-019-1382-7