Abstract
Background
Post-stroke depression (PSD) seriously affects the rehabilitation of nerve function and quality of life. However, the pathogenesis of PSD is still not clear. This study aimed to investigate the demographic, clinical, and biochemical factors in patients with PSD.
Methods
Patients with an acute ischemic stroke, who met the inclusion criteria at Shanghai Tenth People’s Hospital from April 2016 to September 2016, were recruited for this study. The stroke severity was assessed using the National Institutes of Health Stroke Scale (NIHSS), and the mental state was assessed using Mini-Mental State Examination (MMSE), Hamilton Depression Scale (HAMD), and Hamilton Anxiety Scale (HAMA) at 1 week of admission. The patients were divided into PSD and non-PSD groups. The demographic and clinical characteristics, as well as the biochemical factors, were compared between the two groups. A logistic regression analysis was performed to identify the risk factors for depression following stroke.
Results
A total of 83 patients with acute ischemic stroke were recruited. Of these, 36 (43.4%) developed depression. The multivariate logistic regression analysis indicated that high NIHSS [odds ratio (OR): 1.84, 95% confidence interval (CI): 1.09–3.12, P = 0.023] and high HAMD scores (OR: 2.38, 95% CI: 1.61–3.50, P < 0.001) were independent risk predictors for PSD and so were lower dopamine level (OR: 0.64, 95% CI: 0.45–0.91, P = 0.014), lower 5-hydroxytryptamine level (OR: 0.99, 95% CI: 0.98–1.00, P = 0.046), higher tumor necrosis factor-α level (OR: 1.05, 95% CI: 1.00–1.09, P = 0.044), and lower nerve growth factor level (OR: 0.06, 95% CI: 0.01–0.67, P = 0.022).
Conclusions
The identification of higher NIHSS scores, higher HAMD scores, lower dopamine level, lower 5-hydroxytryptamine level, higher tumor necrosis factor-α level, and lower nerve growth factor level might be useful for clinicians in recognizing and treating depression in patients after a stroke.
Similar content being viewed by others
Background
Post-stroke depression (PSD) is a common mental disease after stroke onset, mainly manifested as depression, sleep disorders, decreased interest and worthlessness, and even suicidal tendencies, accounting for one third of all patients with stroke [1]. PSD can significantly affect the recovery of neurological function in patients with stroke, significantly reduce the quality of life, and increase mortality [2].
As a result, predicting the occurrence of PSD after primary treatment is important not only for counseling the patients about the disease prognosis but also for applying additional treatment.
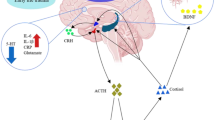
However, it is hard to identify the consistent risk factors from the literature. Large studies have paid much attention to the vascular factors [3, 4] and socioeconomic risk factors [5], but minimal attention has been paid to the biological factors. Intracerebral neurotransmitters have been directly related to PSD, especially monoamine neurotransmitters including noradrenaline (NE), 5-hydroxytryptamine (5-HT), and dopamine (DA), which are important neurotransmitters closely related to human mental activity, especially emotional activity [6]. Plasma concentrations of neurotransmitters seemed to be closely related to intracerebral concentrations [7], suggesting that plasma concentrations could be used to predict the cerebral concentrations using much less invasive procedures [8]. A hypothesis suggests that immune imbalance is implicated in the pathophysiology of PSD and that IL-6 and TNF-α are key cytokines [9]. Interestingly, nerve growth factor (NGF) [10] and calcitonin gene–related peptide (CGRP) [11] have been reported as relevant factors for depression. Besides, the relationship between the lesion site in the brain and PSD is also controversial [12, 13]. Therefore, a better understanding of biological factors associated with PSD is urgently required.
This retrospective study was conducted on Chinese patients to systematically investigate the correlations of depression development 1 week after ischemic stroke with the following factors: intracerebral neurotransmitters, inflammatory cytokines, NGF, CGRP, and lesion site in the brain.
Methods
Study design
This study was approved by the local ethics committee of Shanghai Tenth People’s Hospital. The continuous inpatient electronic medical records at the Department of Neurology, Shanghai Tenth Hospital, were reviewed for an acute cerebral infarction (ACI) between April 2016 and September 2016. After a detailed evaluation with inclusion and exclusion criteria, 83 patients were included in this study (Fig. 1).
Inclusion criteria
Patients who met all of the following inclusion criteria were eligible for the study:
-
1)
Patients who fully understood the purpose of this study, expressed voluntary participation, agreed to sign informed consent, and were willing to bear the relevant risks.
-
2)
Patients who met the criteria proposed at the Fourth Cerebrovascular Disease Conference held by the Chinese Medical Association and were diagnosed with ACI by computed tomography (CT) or magnetic resonance imaging (MRI)
-
3)
Patients who were admitted to hospital within 24 h after stroke onset.
Exclusion criteria
-
1)
Presence of intracranial hemorrhage or subdural hematoma evidenced by cranial CT scan
-
2)
Presence of depression-positive mental disorder within the previous 6 months
-
3)
Presence of disturbance of consciousness or serious cognitive dysfunction
-
4)
Presence of complete aphasia, sensory aphasia, or apraxia
-
5)
Presence of severe infectious diseases such as respiratory system infections, urinary system infections, and gastrointestinal infections; severe heart failure; liver and kidney disease; blood disorder; immune disease; thyroid disease; epilepsy; or cancer
-
6)
Severe condition with a life expectancy of less than 1 week
-
7)
Pregnant or lactating women
-
8)
Dependence on alcohol, tobacco, or other substances
-
9)
Presence of autoimmune diseases or mental retardation.
At admission, demographic data and history of conventional vascular risk factors were recorded.
Testing indexes of plasma concentrations
Venous blood samples were collected the next morning after admission for basic biochemical tests, fasting and postprandial blood glucose, glycosylated hemoglobin (HbAlc), low-density lipoprotein (LDL), high-density lipoprotein (HDL), and thyroid function. Samples were marked with a unique study number only. Some of the fasting blood was centrifuged, and the serum was stored at −80°C. The concentrations of norepinephrine (NE), 5-HT, dopamine (DA), CGRP, and NGF were detected using enzyme-linked immunosorbent assay.
Detailed assessment and grouping
Stroke severity was assessed using the National Institutes of Health Stroke Scale (NIHSS) [14] at the time of admission. The mental state was assessed using Mini-Mental State Examination (MMSE), Hamilton Depression Scale (HAMD), and Hamilton Anxiety Scale (HAMA) by trained neurologists at 1 week of admission.
CT brain scans were obtained routinely in the emergency room, and it was also possible that the patient was sent to the inpatient Neurology department directly before the cranial CT if the symptoms and signs were quite typical, thus in this situation, cranial CT was performed in Neurology department immediately after admission. Cranial MRI was performed within 24–72 h after admission to assess the site of the brain infarct.
Patients with HAMD scores greater than or equal to 8 were included in the PSD group [15]. Patients with HAMD scores less than 8 were enrolled in the non-PSD group. Finally, the 2 groups included 36 and 47 patients, respectively.
Statistical analysis
The Kolmogorov–Smirnov test was used to determine whether the metrological data followed the normal distribution. Continuous variables, which followed a normal distribution, were expressed as mean ± standard deviation (x ± s). Patients with PSD and without PSD were compared using the independent two-sample t test or Mann–Whitney U test. The chi-square/Fisher’s exact test was used for categorical variables. Variables having a P value less than 0.1 in the univariate analysis were selected and evaluated using multivariate logistic regression models with the conditional forward selection method to minimize confounding and examine their independent contributions of what we adjusted for. All statistical assessments were two tailed, and a P value less than 0.05 was considered statistically significant. Statistical analyses were performed using SPSS 22.0 statistical software (SPSS Inc., IL, USA).
Results
General patient characteristics
A total of 210 patients presented to the Neurology department at the Shanghai Tenth Hospital for ACI between April 2016 and September 2016. Eighty-three of them were found to fulfill the criteria for further analysis.
The average age was 69.4 years (range 50–86 years); 51.8% were men (Table 1). Fifty-nine patients (71.1%) had a history of hypertension, 38 (45.8%) had diabetes mellitus, and 13 (15.7%) had atrial fibrillation.
Thirty-six (43.4%) of the 83 enrolled patients with ACI were diagnosed as having PSD during their hospitalization. In general, patients with PSD were more likely to present with higher NIHSS scores (median, 3 vs 1), higher HAMD scores (median, 8 vs 0), higher HAMA scores (median, 6.5 vs 0), and lower MMSE scores (median, 25 vs 29) than did patients without PSD (Table 1), indicating that the neurological deficits were more serious in the PSD group. PSD was more likely to occur in patients with frontal lesions (41.7% vs 19.15%) and parietal lesions (16.7% vs 14.9%).
Tests for biochemical indicators
Several laboratory tests were conducted to examine the differences in biochemical indicators between the two groups. As a result, patients with PSD were more likely to present with lower TT3 levels (average 1.1 vs 1.3 μg/L), but no significant difference in thyroid-stimulating hormone (TSH) levels was reported (Table 2).
This study also found that the monoamine neurotransmitters were significantly lower and the levels of IL-6 and TNF-α were significantly higher in the PSD group than in the non-PSD group (Table 2). Moreover, patients with PSD were more likely to present with lower NGF levels (average 6.5 vs 8.1 ng/L); no significant difference in CGRP levels was found (Table 2).
Logistic regression analysis
Univariate logistic regression identified the following demographic and clinical characteristics associated with PSD: frontal lesions [odds ratio (OR) 3.02; P = 0.028], NIHSS scores (OR 2.08 per 1-point increase in NIHSS scores; P < 0.001), HAMD scores (OR 2.63 per 1-point increase in HAMD scores; P < 0.001), HAMA scores (OR 2.10 per 1-point increase in HAMA scores; P < 0.001), and MMSE scores (OR 0.77 per 1-point decrease in MMSE scores; P = 0.001)]. In multivariable logistic regression, only NIHSS scores (OR 1.84 per 1-point increase in NIHSS scores; P = 0.023) and HAMD scores (OR 2.38 per 1-point increase in HAMD scores; P < 0.001) were independent demographic and clinical predictors of PSD (P < 0.05) (Table 3).
Univariate logistic regression identified the following biochemical indicators associated with PSD: TT3 (OR 0.01; P < 0.001), NE (OR 0.99; P < 0.001), DA (OR 0.85; P < 0.001), 5-HT (OR 0.99; P < 0.001), IL-6 (OR 3.23; P < 0.001), TNF-α (OR 1.03; P < 0.001), and NGF (OR 0.55; P < 0.001). In multivariable logistic regression, only DA (OR 0.64; P = 0.014), 5-HT (OR 0.99; P = 0.046), TNF-α (OR 1.05; P = 0.044), and NGF (OR 0.06; P = 0.022) were independent biochemical predictors of PSD (P < 0.05) (Table 4).
The multivariable logistic regression equation was logit(P) = 1/2(− 6.36 + 0.61 × NIHSS + 0.87 × HAMD + 46.25–0.45 × DA – 0.01 × 5-HT + 0.04 × TNF-α – 2.8 × NGF).
Discussion
This study investigated the risk factors associated with PSD, such as demographic factors, clinical characteristics, and biochemical factors. NIHSS scores, MMSE scores, HAMA scores, HAMD scores, monoamine neurotransmitters, inflammatory cytokines, NGF, and the lesion site in the brain were found to be related to PSD, whereas CGRP was not related to PSD. Importantly, the study found that NIHSS and HAMD scores were demographic and clinical characteristics independently associated with PSD. Moreover, DA, 5-HT, TNF-α, and NGF levels were biochemical indicators independently associated with PSD.
As shown in Tables 1 and 3, significant differences were found in NIHSS, HAMD, HAMA, and MMSE scores between the two groups, indicating that neurological deficits were more serious in the PSD group. Other studies also showed the same results [16, 17]. Further, a significant difference was found in frontal and parietal lesions between the two groups. Likewise, a study showed the involvement of subcutaneous pathway in the frontal lobe, especially the caudate nucleus, globus pallidus, internal capsule knee, and left superior hemisphere [12]; however, other studies showed no significant correlation between the lesion site and PSD [13, 18]. Unlike the present study, Vahid-Ansari et al. developed a preclinical model of PSD in mice by inducing a unilateral ischemic lesion in the medial prefrontal cortex after stroke [19]. The results regarding the value of lesion site in predicting disease outcome are still controversial. Therefore, retrospective trials with larger series of patients with PSD are warranted to demonstrate the value of various lesion sites.
Monoamine neurotransmitters include mainly noradrenaline (NE), 5-HT, and DA. The somata of these neurons are located in the brainstem, and the axons reach the frontal cortex through the thalamus and basal ganglia. If any of the aforementioned locations are damaged, the levels of monoamine neurotransmitters decrease, resulting in depression [20]. Likewise, as shown in Table 2, it was found that NE, 5-HT, and DA levels were significantly lower in the PSD group than in the non-PSD group, supporting the hypotheses of monoamine neurotransmitters. Similarly, reduced DA concentrations in ischemic striatum have been demonstrated in a mouse model of chronic PSD [21]. In addition to monoamine neurotransmitters, a low plasma glutamate has also been reported to be associated with early-onset PSD recently [22].
This study found the levels of IL-6 and TNF-α significantly higher in the PSD group than in the non-PSD group. Inflammatory cytokines are implicated in the pathogenesis of PSD. Spalletta et al. [23] believed that increased inflammatory cytokines after stroke induced damage in the marginal zone by activating indoleamine-2,3-dioxygenase, leading to 5-HT depletion in the secondary edge system. Besides, inflammatory cytokines can also affect the protective cytokines and some neurotransmitters in the brain, thus indirectly promoting the occurrence of PSD. Moreover, some studies have shown the overexpression of inflammatory cytokines in patients with cerebral ischemic stroke [24, 25]. Similarly, IL-6, IL-10, TNF-α, and interferon-γ levels increased to different degrees in patients with PSD, corresponding well with the result shown in the patients with PSD in the present study [9, 26]. Interestingly, increased serum IL-18 levels were also suggested as a biomarker for PSD [27].
NGF, a secretory protein first found in neurotrophic factors, inhibits apoptosis and promotes survival, growth, and differentiation of neurons [28]. Some studies have suggested increased NGF expression in cerebral ischemia [29, 30]. This study found the serum NGF levels to be significantly lower in the PSD group than in the non-PSD group. Likewise, several studies have found the NGF levels to be significantly lower in the severe depression group than in the normal control group [31, 32]. Other studies showed that elevated serum NGF levels could significantly ameliorate the depression symptoms and improve the quality of life [33, 34]. Unlike the present study, several studies suggested no correlation between depression and NGF [35, 36]. The differences in patient groups and experimental design might account for the difference in results.
Further, CGRP, an active peptide of 37 amino acids widely distributed in the nervous and cardiovascular system, has potent vasodilator and neuroprotective effects [37]. The CGRP synthesis was known to increase when nerve damage or inflammatory responses occurred [38]. The present study found that CGRP levels were higher in the PSD group than in the non-PSD group, but with no statistically significant difference. Unlike the present study, Shao et al. showed that CGRP immunoreactivity (CGRP-ir) concentration in the cerebrospinal fluid and hippocampus increased in rats with PSD and the administration of CGRP into the ischemic rats increased depression-like behaviors in a dose-dependent manner [11]. A study also found that CGRP antagonists could significantly ameliorate the depression symptoms [39]. The difference in sample size might account for the diversity in results.
Only 83 patients were examined in the present study and the stroke severity of sample was not high. Hence, the results must be confirmed by conducting large-sample studies. The reason for this minimally affected sample was the strict inclusion and exclusion criteria. It is common that patients with higher NIHSS always have aphasia with different degrees of severity, and old-age patients have multiple-system and multiple-organ disorders, and cognition dysfunction, which were all part of the exclusion criteria. However, the preliminary risk factor model provided more confidence to take action for individuals with higher stroke severities. Besides, a potential limitation of this study was the inherent differences between the participants in this clinical trial and the general population of stroke survivors. Another limitation was the absence of medical assessment record of the previous mental states before the stroke onset because the depression history was sometimes not objective for depressive patients without illness perception/cognition. Furthermore, the ideal time for testing the levels of plasma parameters in patients with acute stroke still needs further longitudinal studies. Nevertheless, the results were noteworthy because this novel study systematically investigated the correlations of depression after ischemic stroke with the following factors: neurotransmitters, inflammatory cytokines, NGF, CGRP, and lesion site in the brain.
Overall, it is speculated that the inflammatory response can aggravate the injury in ischemic regions and, meanwhile, lead to 5-HT depletion, increase in DA level, and inhibition of NGF expression, thereby promoting the development of PSD. However, the relationship between inflammatory response with PSD and ideal optimum plasma biomarkers still needs further investigation. The findings of this study might be helpful in preventing PSD and ensuring the adequacy of treatment. All stroke survivors should be screened early for depression. It is critical that patients with PSD are provided with appropriate treatment. Also, larger studies with longer follow-up should be conducted in the future.
Conclusions
The risk factors for PSD were identified as higher NIHSS scores, higher HAMD scores, lower DA level, lower 5-HT level, higher tumor necrosis factor-α level, and lower NGF level. These results might be useful for clinicians in recognizing and treating depression in patients after a stroke.
Abbreviations
- 2hPBG:
-
2-h postprandial blood glucose
- 5-HT:
-
5-hydroxytryptamine
- ACI:
-
Acute cerebral infarction
- CGRP:
-
Calcitonin gene–related peptide
- CGRP-ir:
-
CGRP immunoreactivity
- CI:
-
Confidence interval
- CT:
-
Computed tomography
- DA:
-
Dopamine
- FBG:
-
Fasting blood glucose
- HAMA:
-
Hamilton anxiety scale
- HAMD:
-
Hamilton depression scale
- HbAlc:
-
Glycosylated hemoglobin
- HDL:
-
High-density lipoprotein
- IL-6:
-
Interleukin 6
- IQR:
-
Interquartile range
- LDL:
-
Low-density lipoprotein
- MMSE:
-
Mini-mental state examination
- MRI:
-
Magnetic resonance imaging
- NE:
-
Noradrenaline
- NGF:
-
Nerve growth factor
- NIHSS:
-
National institutes of health stroke scale
- OR:
-
Odds ratio
- PSD:
-
Post-stroke depression
- SD:
-
Standard deviation
- TNF-α:
-
Tumor necrosis factor-α
- TSH:
-
Thyroid-stimulating hormone
- TT3:
-
Total triiodothyronine
- TT4:
-
Total thyroxine
References
Hackett ML, Pickles K. Part I: frequency of depression after stroke: an updated systematic review and meta-analysis of observational studies. Int J Stroke. 2014;9(8):1017–25.
Stern-Nezer S, Eyngorn I, Mlynash M, Snider RW, Venkatsubramanian C, Wijman CAC, Buckwalter MS. Depression one year after hemorrhagic stroke is associated with late worsening of outcomes. NeuroRehabilitation. 2017;41(1):179–87.
Arauz A, Rodriguez-Agudelo Y, Sosa AL, Chavez M, Paz F, Gonzalez M, Coral J, Diaz-Olavarrieta C, Roman GC. Vascular cognitive disorders and depression after first-ever stroke: the Fogarty-Mexico stroke cohort. Cerebrovasc Dis. 2014;38(4):284–9.
Zhang Y, He JR, Liang HB, Lu WJ, Yang GY, Liu JR, Zeng LL. Diabetes mellitus is associated with late-onset post-stroke depression. J Affect Disord. 2017;221:222–6.
Liu R, Yue Y, Jiang H, Lu J, Wu A, Geng D, Wang J, Lu J, Li S, Tang H, et al. A risk prediction model for post-stroke depression in Chinese stroke survivors based on clinical and socio-psychological features. Oncotarget. 2017;8(38):62891–9.
Parker G, Brotchie H. Mood effects of the amino acids tryptophan and tyrosine: ‘Food for Thought’ III. Acta Psychiatr Scand. 2011;124(6):417–26.
Gao HQ, Zhu HY, Zhang YQ, Wang LX. Reduction of cerebrospinal fluid and plasma serotonin in patients with post-stroke depression: a preliminary report. Clin Invest Med. 2008;31(6):E351–6.
Lang D, Ude C, Wurglics M, Schubert-Zsilavecz M, Klein J. Brain permeability of bilobalide as probed by microdialysis before and after middle cerebral artery occlusion in mice. J Pharm Pharm Sci. 2010;13(4):607–14.
Su JA, Chou SY, Tsai CS, Hung TH. Cytokine changes in the pathophysiology of poststroke depression. Gen Hosp Psychiatry. 2012;34(1):35–9.
Xiong P, Zeng Y, Wan J, Xiaohan DH, Tan D, Lu J, Xu F, Li HY, Zhu Z, Ma M. The role of NGF and IL-2 serum level in assisting the diagnosis in first episode schizophrenia. Psychiatry Res. 2011;189(1):72–6.
Shao B, Zhou YL, Wang H, Lin YS. The role of calcitonin gene-related peptide in post-stroke depression in chronic mild stress-treated ischemic rats. Physiol Behav. 2015;139:224–30.
Vataja R, Pohjasvaara T, Leppävuori A, Mäntylä R, Aronen HJ, Salonen O, Kaste M, Erkinjuntti T. Magnetic resonance imaging correlates of depression after ischemic stroke. Arch Gen Psychiatry. 2001;58(10):925–31.
Karakus K, Kunt R, Memis CO, Kunt DA, Dogan B, Ozdemiroglu F, Sevincok L. The factors related to early-onset depression after first stroke. Psychogeriatrics. 2017; doi: 10.1111/psyg.12266.
Lyden P, Brott T, Tilley B, Welch KM, Mascha EJ, Levine S, Haley EC, Grotta J, Marler J. Improved reliability of the NIH stroke scale using video training. NINDS TPA Stroke Study Group. Stroke. 1994;25(11):2220–6.
Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62.
Yang S, Hua P, Shang X, Cui Z, Zhong S, Gong G, Humphreys GW. A significant risk factor for poststroke depression: the depression-related subnetwork. J Psychiatry Neurosci. 2015;40(4):259–68.
Zhang L, Sui R, Zhang L, Zhang Z. Morphological and metabolic alteration of cerebellum in patients with post-stroke depression. Cell Physiol Biochem. 2016;40(3–4):420–30.
Berg A, Palomaki H, Lehtihalmes M, Lonnqvist J, Kaste M. Poststroke depression: an 18-month follow-up. Stroke. 2003;34(1):138–43.
Vahid-Ansari F, Lagace DC, Albert PR. Persistent post-stroke depression in mice following unilateral medial prefrontal cortical stroke. Transl Psychiatry. 2016;6(8):e863.
Santos M, Gold G, Kovari E, Herrmann FR, Hof PR, Bouras C, Giannakopoulos P. Neuropathological analysis of lacunes and microvascular lesions in late-onset depression. Neuropathol Appl Neurobiol. 2010;36(7):661–72.
Kronenberg G, Balkaya M, Prinz V, Gertz K, Ji S, Kirste I, Heuser I, Kampmann B, Hellmann-Regen J, Gass P, et al. Exofocal dopaminergic degeneration as antidepressant target in mouse model of poststroke depression. Biol Psychiatry. 2012;72(4):273–81.
Geng LY, Qian FY, Qian JF, Zhang ZJ. The combination of plasma glutamate and physical impairment after acute stroke as a potential indicator for the early-onset post-stroke depression. J Psychosom Res. 2017;96:35–41.
Spalletta G, Bossu P, Ciaramella A, Bria P, Caltagirone C, Robinson RG. The etiology of poststroke depression: a review of the literature and a new hypothesis involving inflammatory cytokines. Mol Psychiatry. 2006;11(11):984–91.
Li F, Pendy JT Jr, Ding JN, Peng C, Li X, Shen J, Wang S, Geng X. Exercise rehabilitation immediately following ischemic stroke exacerbates inflammatory injury. Neurol Res. 2017;39(6):530–537.
Yang B, Hamilton JA, Valenzuela KS, Bogaerts A, Xi X, Aronowski J, Mays RW, Savitz SI. Multipotent adult progenitor cells enhance recovery after stroke by modulating the immune response from the spleen. Stem Cells. 2017;35(5):1290–1302.
Spalletta G, Cravello L, Imperiale F, Salani F, Bossu P, Picchetto L, Cao M, Rasura M, Pazzelli F, Orzi F, et al. Neuropsychiatric symptoms and interleukin-6 serum levels in acute stroke. J Neuropsychiatry Clin Neurosci. 2013;25(4):255–63.
Yang L, Zhang Z, Sun D, Xu Z, Zhang X, Li L. The serum interleukin-18 is a potential marker for development of post-stroke depression. Neurol Res. 2010;32(4):340–6.
Ducassou S, Prouzet-Mauleon V, Deau MC. Brunet de la grange P, Cardinaud B, Soueidan H, Quelen C, Brousset P, Pasquet JM, Moreau-Gaudry F et al: MYB-GATA1 fusion promotes basophilic leukaemia: involvement of IL33 and nerve growth factor receptors. J Pathol. 2017;242(3):347–57.
Chen C, Zhang W, Lou BD, Pan J, Cao Y, Zhong F, Zhou WJ, Wu J. Effect of Electroacupuncture stimulation of acupoints of the pericardium meridian on serum NGF and Nogo-a contents and cerebral NGF and Nogo-a expression in cerebral ischemia rats. Zhen Ci Yan Jiu. 2015;40(2):94–8.
Dmitrieva VG, Stavchansky VV, Povarova OV, Skvortsova VI, Limborska SA. Dergunova LV: [effects of ischemia on the expression of neurotrophins and their receptors in rat brain structures outside the lesion site, including on the opposite hemisphere]. Mol Biol. 2016;50(5):775–84.
Wiener CD, de Mello FS, Pedrotti Moreira F, Bittencourt G, de Oliveira JF, Lopez Molina M, Jansen K, de Mattos Souza LD, Rizzato Lara D, Portela LV, et al. Serum levels of nerve growth factor (NGF) in patients with major depression disorder and suicide risk. J Affect Disord. 2015;184:245–8.
Oglodek EA, Just MJ, Szromek AR, Araszkiewicz A. Melatonin and neurotrophins NT-3, BDNF, NGF in patients with varying levels of depression severity. Pharmacological Rep. 2016;68(5):945–51.
Filho CB, Jesse CR, Donato F, Giacomeli R, Del Fabbro L, da Silva AM, de Gomes MG, Goes AT, Boeira SP, Prigol M, et al. Chronic unpredictable mild stress decreases BDNF and NGF levels and Na(+),K(+)-ATPase activity in the hippocampus and prefrontal cortex of mice: antidepressant effect of chrysin. Neuroscience. 2015;289:367–80.
Kaihola H, Olivier J, Poromaa IS, Akerud H. The effect of antenatal depression and selective serotonin reuptake inhibitor treatment on nerve growth factor signaling in human placenta. PLoS One. 2015;10(1):e0116459.
Kheirouri S, Noorazar SG, Alizadeh M, Dana-Alamdari L. Elevated brain-derived Neurotrophic factor correlates negatively with severity and duration of major depressive episodes. Cogn Behav Neurol. 2016;29(1):24–31.
Cirulli F, Alleva E. The NGF saga: from animal models of psychosocial stress to stress-related psychopathology. Front Neuroendocrinol. 2009;30(3):379–95.
Hind WH, Tufarelli C, Neophytou M, Anderson SI, England TJ, O'Sullivan SE. Endocannabinoids modulate human blood-brain barrier permeability in vitro. Br J Pharmacol. 2015;172(12):3015–27.
Muzzi M, Buonvicino D, De Cesaris F, Chiarugi A. Acute and chronic triptan exposure neither alters rodent cerebral blood flow nor worsens ischemic brain injury. Neuroscience. 2017;340:1–7.
Jiao J, Opal MD, Dulawa SC. Gestational environment programs adult depression-like behavior through methylation of the calcitonin gene-related peptide gene. Mol Psychiatry. 2013;18(12):1273–80.
Acknowledgments
The authors thank the patients for their commitment to this research and Professor Kyle Stuart for the English improvement.
Funding
This study was supported by the International Exchange Program for Graduate Students, Tongji University (No. 2016020033), and the National Natural Science Foundation of China (81771131).
Availability of data and materials
All data are available without restriction from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
GM and YZ conceived and designed the study. YT and XL performed the experiments. LL and XL analyzed and prepared the manuscript. XM and GM analyzed the data and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the local ethics committee of Shanghai Tenth People’s Hospital. All the participants agreed to participate.
Consent for publication
Consent for publication was obtained from all the participants.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Meng, G., Ma, X., Li, L. et al. Predictors of early-onset post-ischemic stroke depression: a cross-sectional study. BMC Neurol 17, 199 (2017). https://doi.org/10.1186/s12883-017-0980-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-017-0980-5