Abstract
Background
Medulloblastoma is the most common malignant brain tumor in children. To date only few cases of medulloblastoma with hemorrhages have been reported in the literature. Although some studies speculate on the pathogenesis of this anomalous increased vascularization in medulloblastoma, the specific mechanism is still far from clearly understood. A correlation between molecular medulloblastoma subgroups and hemorrhagic features has not been reported, although recent preliminary studies described that WNT-subtype tumors display increased vascularization and hemorrhaging.
Case presentation
Herein, we describe a child with a Wnt-medulloblastoma presenting as cerebellar-vermian hemorrhagic lesion. Brain magnetic resonance imaging (MRI) showed the presence of a midline posterior fossa mass with a cystic hemorrhagic component. The differential diagnosis based on imaging included cavernous hemangioma, arteriovenous malformation and traumatic lesion. At surgery, the tumor appeared richly vascularized as documented by the preoperative angiography.
Conclusions
The case we present showed that Wnt medulloblastoma may be associated with anomalous vascularization. Further studies are needed to elucidate if there is a link between the hypervascularization and the Wnt/β-catenin signaling activation and if this abnormal vasculature might influence drug penetration contributing to good prognosis of this medulloblastoma subgroup.
Similar content being viewed by others
Background
Medulloblastoma is the most common malignant brain tumor in children, representing approximately 25 % of all pediatric brain tumors [1]. Current molecular stratification consists in four subgroups associated with different pathways defined as Wnt, Sonic Hedgehog Homolog (SHH), group 3, and group 4 [2]. Wnt subtype is the rarest subgroup, accounting for 10 % of medulloblastomas [3] and patients with this pathway activation have a very good long-term prognosis [4]. However, the biological effect of Wnt/β-catenin signaling activation and the link with a better prognosis has not been clarified yet. Few cases of medulloblastoma presenting with spontaneous hemorrhage are reported in the literature [5]. Although some studies speculate on the etiology of the anomalous increased vascularization in medulloblastoma [6], a clear pathogenetic role has not been identified. A correlation between specific medulloblastoma subgroup and hemorrhagic features has not been reported [7], however a recent paper demonstrated that Wnt medulloblastoma secretes Wnt antagonists that increase the permeability of the blood–brain barrier (BBB) [8]. Furthermore, a personal communication hypothesized that this aberrant vascular network may be associate with the Wnt subgroup [9]. Herein, we report the case of a child with a Wnt medulloblastoma presenting as a hemorrhagic cerebellar-vermian lesion.
Case presentation
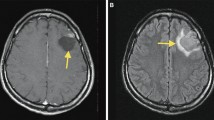
A 7-year-old girl presented to the emergency department of Bambino Gesù Children’s Hospital with a one-month history of headache and vomiting. Neurological examination was normal apart from mild dysmetria at the upper extremities. Multidetector computed tomography (MDCT) reconstruction images show a hyperdense cerebellar-vermian lesion, with fluid-blood levels. This finding was confirmed by brain magnetic resonance imaging (MRI) that showed the presence of a mass with a hyperintense cystic hemorrhagic component, heterogeneous enhancement, poor perilesional edema and absence of obstructive hydrocephalus (Fig. 1A). Spinal MRI and cerebrospinal fluid study were normal. Due to these atypical features a cerebral angiography was performed, showing an intratumoral aneurysm-like formation supplied by a vermian branch of the left posterior inferior cerebellar artery (Fig. 1A). She underwent a midline suboccipital craniotomy with complete resection of the vermian tumor. At surgery, the tumor appeared richly vascularized from vermian branches as documented by the preoperative angiography. Histology (Fig. 1B) showed a diffuse and multinodular proliferation of small undifferentiated cells and immunohistochemistry revealed positivity for synaptophysin and β-catenin (both cytoplasmic and nuclear), all features consistent with the diagnosis of classic medulloblastoma. The tumor was characterized by anomalous vascularization and harbored some clusters of anomalous, thick-walled vascular structures along with numerous variably anastomosing small venous and capillary structures. Gene expression profile of the tumor confirmed a Wnt molecular subgroup (Fig. 2). C-Myc amplification was negative (C-Myc/SPAST and C-Myc/PI4KA). She started therapy as per standard arm of European HIT-SIOP PNET 4 trial which is used for the treatment of standard risk medulloblastoma. According to this protocol, she received radiotherapy (23 Gy to the craniospinal axis and a total of 54 Gy to posterior fossa) given concurrently with weekly vincristine (1.5 mg/m2 i.v.). This treatment was followed by 8 cycles of maintenance-chemotherapy consisting of cisplatin (70 mg/m2 i.v.) and lomustine (75 mg/ m2 orally) on day 1 associated to vincristine (1.5 mg/m2 i.v.) on day 1, 8 and 15. At 22 months after diagnosis she was doing well with no evidence of disease.
MRI and histological findings in the hemorrhagic lesion. (A) [CT and MRI findings in the hemorrhagic cerebellar lesion]. Sagittal (a) and Axial (b) MDCT reconstruction images show a hyperdense cerebellar-vermian lesion, with fluid-blood levels (white arrows), confirmed by MRI scan. Axial T2-weighted MRI (c) showed a hemorrhagic cerebellar-vermian lesion, expanding into IV ventricle, with multiple fluid-blood levels (white arrow). Axial-Sagittal T1-weighted MRI, without (d, e) and with (f, g) gadolinium, revealed a solid component inhomogeneously contrast-enhanced (white arrows). Axial gradient-echo sequence (h) showed lack of hypointense hemosiderin rim (cavernous hemangioma classical finding, white arrow). Preoperative left vertebral angiograms (i, l) show hypoplastic vertebral artery terminating as posterior inferior cerebellar artery (vascular variation) and the tumor stain; an aneurysm-like formation (arrow) is seen in the arterial phase. The tumor is fed by the vermian branch of the left posterior inferior cerebellar artery. (B) [Histological findings in hemorrhagic medulloblastoma]. Proliferation of small undifferentiated cells showing a diffuse/multinodular pattern (middle-right), associated to anomalous, thick-walled vascular structures (arrows) (a, H&E, 2.5x). Cells were synaptophysin (b, 20x) and beta-catenin (both cytoplasm and nucleus) positive (c, 20x). Anomalous vascularization was characterized by clusters of anomalous, thick-walled arterial-type vessels (d and f, CD31 20x) along with numerous variably anastomosing small venous and capillary structures (e, CD31, 20x)
Molecular characterization of medulloblastoma. mRNA levels of the indicated genes in medulloblastoma are compared to normal cerebellum as control (CTRLs). Genes are grouped into four molecular subgroups (WNT, SHH, GROUP 3, GROUP 4) as indicated by the four different colors. Relative quantification values are expressed as linear scale arbitrary units
The consensus held in Boston in 2010 supported the existence of four main medulloblastoma subgroups based on the molecular profiling and provided important insights not only in the selection of patients for molecular targeted therapies but also in the outcome prediction [2, 3, 10]. Medulloblastomas with activation of Wnt/β-catenin pathway are rarely metastatic and appear to be a less aggressive variant associated with an excellent prognosis [11]. However, the significance of Wnt activation in medulloblastoma remains to be determined. Several findings suggest that wild type β-catenin has an important physiological role in CNS angiogenesis. During embryogenesis, the Wnt pathway has direct actions on axonal growth through interaction with β-catenin complex [12]. Moreover, it has been demonstrated that Wnt signaling plays an active role in the induction and maintenance of BBB characteristics during embryonic and postnatal development particularly by regulating tight junction proteins expression [13]. Indeed, Wnt/β-catenin signaling is very important for central nervous system (CNS) angiogenesis and conditionally inactivation of β-catenin in the endothelium has been described to alter the development of head vasculature resulting in early embryonic lethality of mice [14, 15]. β-catenin–null animals show vessel fragility, in association to a decrease in intercellular adhesion strength and an increase in paracellular permeability leading to vascular leakage and frequent hemorrhages. The critical role of canonical Wnt signaling in endothelial cells for formation and differentiation of the CNS vasculature has been described also in genetic mouse models [16]. Recently has been reported that G-protein coupled receptor 124 (GPR124) functions as a specific co-stimulator of β-catenin signaling in brain endothelium and its disruption led to defective CNS angiogenesis and blood brain barriergenesis in mice [17]. In mouse embryos, eliminating neuroepithelial Wnt7a and Wnt7b, or endothelial Gpr124 or β-catenin, leads to a reduced CNS angiogenesis with formation of abnormal vascular structures [13, 16, 18] and has been demonstrated that the interaction of Gpr124 with Reck strongly synergize to promote Wnt/β-catenin signaling during brain angiogenesis [19].
Nevertheless, most of the studies correlating Wnt signaling with CNS vasculature anomalies are developmental phenotypes, and the relevance to post-natal development is not clear. Moreover, β-catenin is important in the regulation of vascular endothelial cell-cell adhesions and barrier function by linking the VE-cadherin junction complex to the cytoskeleton and thus vascular anomalies may arise for a non-signaling role [20].
A recent study showed that genetically modified mouse models harboring Wnt-medulloblastoma had more hemorrhagic tumors compared to SHH or group 3 tumors [8]. The authors demonstrated that these effects occur postnatally and reveal that Wnt medulloblastoma secretes Wnt antagonists increasing the permeability of the BBB [8].
We report a girl with a Wnt medulloblastoma presenting an anomalous vascularization. Differential diagnosis based on MRI imaging appearance includes cavernous hemangioma, arteriovenous malformation and traumatic lesion. Angiographic opacification of dilated arteriolar vessels with slow flow and lack of early venous drainage confirmed an aberrant tumor vascular network. This patient is doing well at 22 months after diagnosis. Medulloblastoma can present heterogeneous features on MRI with variable enhancement patterns, cystic areas, hemorrhage and calcification [21]. Although intratumoral bleeding can be found in brain tumors, only few cases of medulloblastoma with spontaneous hemorrhages have been reported in the literature [5, 22]. Park et al. showed that the incidence of spontaneous hemorrhage was 5.6 % in patients with primary or recurrent medulloblastoma [23]. A study evaluating vascular regulatory expression profiles across medulloblastoma subgroups documented an upregulation of proangiogenic factors in SHH subgroup [6]. Despite specific mechanisms driving aberrant vascularization and hemorrhage in medulloblastoma remain not completely elucidated, new researches revealed that medulloblastoma genotype dictates tumor vessels phenotype [8].
Conclusions
We reported a case of Wnt/β-catenin medulloblastoma associated with an anomalous vascularization. This may support the evidence that Wnt medulloblastomas may be associated to aberrant vascular network contributing to a better drug penetration and therefore to their excellent prognosis [8]. This finding should be further investigated in a large patient cohort in order to elucidate the vascular microenvironment in Wnt medulloblastoma subgroup.
Abbreviations
SHH, sonic hedgehog homolog; MDTC, multidetector computed tomography; MRI, magnetic resonance imaging; BBB, blood–brain barrier; CNS, central nervous system
References
Farwell JR, Dohrmann GJ, Flannery JT. Central nervous system tumors in children. Cancer. 1977;40:3123–32.
Northcott PA, Korshunov A, Witt H, Hielscher T, Eberhart CG, Mack S, Bouffet E, Clifford SC, Hawkins CE, French P, Rutka JT, Pfister S, Taylor MD. Medulloblastoma comprises four distinct molecular variants. J Clin Oncol. 2011;29:1408–14.
Kool M, Korshunov A, Remke M, Jones DT, Schlanstein M, Northcott PA, Cho YJ, Koster J, Schouten-van Meeteren A, van Vuurden D, Clifford SC, Pietsch T, von Bueren AO, Rutkowski S, McCabe M, Collins VP, Bäcklund ML, Haberler C, Bourdeaut F, Delattre O, Doz F, Ellison DW, Gilbertson RJ, Pomeroy SL, Taylor MD, Lichter P, Pfister SM. Molecular subgroups of medulloblastoma: an international meta-analysis of transcriptome, genetic aberrations, and clinical data of, WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol. 2012;123:473–84.
Ellison DW, Dalton J, Kocak M, Nicholson SL, Fraga C, Neale G, Kenney AM, Brat DJ, Perry A, Yong WH, Taylor RE, Bailey S, Clifford SC, Gilbertson RJ. Medulloblastoma: clinicopathological correlates of SHH, WNT, and non-SHH/WNT molecular subgroups. Acta Neuropathol. 2011;121:381–96.
Menekse G, Gezercan Y, Demirturk P, Uysal I, Okten AI. Fatal cerebellar hemorrhage as an initial presentation of medulloblastoma in a child. J Pediatr Neurosci. 2015;10:287–9.
D’Asti E, Kool M, Pfister SM, Rak J. Coagulation and angiogenic gene expression profiles are defined by molecular subgroups of medulloblastoma: evidence for growth factor-thrombin cross-talk. J Thromb Haemost. 2014;12:1838–49.
Perreault S, Ramaswamy V, Achrol AS, Chao K, Liu TT, Shih D, Remke M, Schubert S, Bouffet E, Fisher PG, Partap S, Vogel H, Taylor MD, Cho YJ, Yeom KW. MRI surrogates for molecular subgroups of medulloblastoma. AJNR Am J Neuroradiol. 2014;35:1263–9.
Phoenix TN, Patmore DM, Boop S, Boulos N, Jacus MO, Patel YT, Roussel MF, Finkelstein D, Goumnerova L, Perreault S, Wadhwa E, Cho YJ, Stewart CF, Gilbertson RJ. Medulloblastoma Genotype Dictates Blood Brain Barrier Phenotype. Cancer Cell. 2016;29:508–22.
Perreault S, Chao K, Ramaswamy V, Shih D, Remke M, Luu B, Schubert S, Fisher P, Partap S, Vogel H, Taylor M, Goumnerova L, Cho YJ. Medulloblastoma in the operative theater: are they playing according to their subtypes? Abstracts of papers, WFNO-SNO 2013. Neuro Oncol. 2013;15(3):iii165–iii172.
Taylor MD, Northcott PA, Korshunov A, Remke M, Cho YJ, Clifford SC, Eberhart CG, Parsons DW, Rutkowski S, Gajjar A, Ellison DW, Lichter P, Gilbertson RJ, Pomeroy SL, Kool M, Pfister SM. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 2012;123:465–72.
Clifford SC, Lusher ME, Lindsey JC, Langdon JA, Gilbertson RJ, Straughton D, Ellison DW. Wnt/Wingless pathway activation and chromosome 6 loss characterize a distinct molecular sub-group of medulloblastomas associated with a favorable prognosis. Cell Cycle. 2006;5:2666–70.
Arévalo JC, Chao MV. Axonal growth: where neurotrophins meet Wnts. Curr Opin Cell Biol. 2005;17:112–5.
Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, Reis M, Felici A, Wolburg H, Fruttiger M, Taketo MM, von Melchner H, Plate KH, Gerhardt H, Dejana E. Wnt/beta-catenin signaling controls development of the blood–brain barrier. J Cell Biol. 2008;183:409–17.
Cattelino A, Liebner S, Gallini R, Zanetti A, Balconi G, Corsi A, Bianco P, Wolburg H, Moore R, Oreda B, Kemler R, Dejana E. The conditional inactivation of the beta-catenin gene in endothelial cells causes a defective vascular pattern and increased vascular fragility. J Cell Biol. 2003;162:1111–22.
Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci U S A. 2009;106:641–6.
Stenman JM, Rajagopal J, Carroll TJ, Ishibashi M, McMahon J, McMahon AP. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science. 2008;322:1247–50.
Posokhova E, Shukla A, Seaman S, Volate S, Hilton MB, Wu B, Morris H, Swing DA, Zhou M, Zudaire E, Rubin JS, St Croix B. GPR124 functions as a WNT7-specific coactivator of canonical β-catenin signaling. Cell Rep. 2015;10:123–30.
Cullen M, Elzarrad MK, Seaman S, Zudaire E, Stevens J, Yang MY, Li X, Chaudhary A, Xu L, Hilton MB, Logsdon D, Hsiao E, Stein EV, Cuttitta F, Haines DC, Nagashima K, Tessarollo L, St Croix B. GPR124, an orphan G protein-coupled receptor, is required for CNS-specific vascularization and establishmentof the blood–brain barrier. Proc Natl Acad Sci U S A. 2011;108(14):5759–64.
Vanhollebeke B, Stone OA, Bostaille N, Cho C, Zhou Y, Maquet E, Gauquier A, Cabochette P, Fukuhara S, Mochizuki N, Nathans J, Stainier DY. Tip cell-specific requirement for an atypical Gpr124- and Reck-dependent Wnt/β-catenin pathway during brain angiogenesis. Elife. 2015;8:4.
Guo M, Breslin JW, Wu MH, Gottardi CJ, Yuan SY. VE-cadherin and beta-catenin binding dynamics during histamine-induced endothelial hyperpermeability. Am J Physiol Cell Physiol. 2008;294:C977–84.
Yeom KW, Mobley BC, Lober RM, Andre JB, Partap S, Vogel H, Barnes PD. Distinctive MRI features of pediatric medulloblastoma subtypes. AJR Am J Roentgenol. 2013;200:895–903.
Tachibana O, Oki H, Hayashi Y, Nonomura A, Yamashima T, Yamashita J. Repetitive intratumoral hemorrhage in medulloblastoma. A case report. Surg Neurol. 1990;33:378–83.
Park TS, Hoffman HJ, Hendrick EB, Humphreys RP, Becker LE. Medulloblastoma: clinical presentation and management. Experience at the hospital for sick children, toronto, 1950–1980. J Neurosurg. 1983;58:543–52.
Acknowledgements
We thank the child’s parents, who gave their informed consent for publication.
Funding
No funding to declare.
Availability of data and materials
The data supporting our findings will not be shared since is not ethically appropriate (underlying data pose privacy concerns and might reveal the identity of participant).
Authors’ contributions
AM, AC and FL designed the case report; AC, AM, VAD, GSC, FDC, EM, AM and ADG. analyzed data; and AP, EF, ADG wrote the paper. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Written informed consent for publication of this Case Report and any accompanying images was obtained from patient’s parents. A copy of the written consent is available for review to the Editor of this journal.
Ethics approval and consent to participate
This clinical study was approved by the local ethical committee of Bambino Gesù Children’s Hospital.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Di Giannatale, A., Carai, A., Cacchione, A. et al. Anomalous vascularization in a Wnt medulloblastoma: a case report. BMC Neurol 16, 103 (2016). https://doi.org/10.1186/s12883-016-0632-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-016-0632-1