Abstract
Background
Association of leukoencephalopathy and atypical mycobacteriosis has been rarely reported. We present a case that is relevant for its unusual presentation and because it may shed further light on the pathogenic mechanisms underlying reversible encephalopathies.
Case report
We report the case of a Hispanic 64-year-old woman with cognitive decline and extensive leukoencephalopathy. Magnetic resonance imaging revealed white-matter lesions with increased water diffusivity, without blood–brain-barrier disruption. Brain biopsy showed tissue rarefaction with vacuolation, mild inflammation, few reactive astrocytes and decreased aquaporin water-channel expression in the lesions. Six months later, she was diagnosed with atypical mycobacterial pulmonary infection. Brain lesions resolved after antimycobacterial treatment.
Conclusion
We hypothesize leukoencephalopathic changes and vasogenic edema were associated with decreased aquaporin expression. Further studies should clarify if reversible leukoencephalopathy has a causal relationship with decreased aquaporin expression and atypical mycobacterial infection, and mechanisms underlying leukoencephalopathy resolution after antimycobacterial treatment. This article may contribute to the understanding of pathogenic mechanisms underlying magnetic resonance imaging subcortical lesions and edema, which remain incompletely understood.
Similar content being viewed by others
Background
Reversible leukoencephalopathy is characterized by headaches, seizures, vomiting, and confusion, associated with extensive bilateral white-matter subcortical neuroimaging abnormalities, suggestive of vasogenic edema without infarction [1]. Most cases of reversible encephalopathy are referred to as posterior reversible encephalopathy syndrome (PRES), since white matter changes are more prominent in the posterior cerebral regions. Brainstem, cerebellum and other brain regions may also be affected [2]. PRES has been associated with severe hypertension (including eclampsia), renal dysfunction, infection, autoimune disease, and immunosuppressant drug use [1–7]. The hallmark of PRES is reversible cerebral edema due to blood–brain-barrier dysfunction [8]. Pathogenic mechanisms underlying magnetic resonance imaging (MRI) subcortical lesions and edema remains incompletely understood.
Association of brain edema and mycobacteria has previously been reported in the setting of Mycobacterium tuberculosis infection [9–15], and in one patient who presented with acute disseminated encephalomyelitis (ADEM) associated with Mycobacterium intracellulare meningitis, who responded to high dose steroid pulse therapy [16]. In all reported cases of mycobacterial infection associated with edema, underlying mechanisms involved demyelination and inflammation. Association of a non-demyelinating leukoencephalopathy and atypical mycobacteriosis has not, to our knowledge, been previously reported.
We report neuroimaging and brain histology features of a patient with extensive leukoencephalopathy with brain edema and absence of demyelination, that resolved after atypical mycobacterial pulmonary infection treatment.
Case presentation
A Hispanic 64-year-old woman was admitted with headaches, vomiting and confusion. A month earlier, the patient presented with subacute new-onset headaches, nausea, vomiting, gait impairment, and anorexia. There was no history of fever, cough, abdominal pain, previous medical disease or immunosuppressant drug use. Blood pressure was normal. Physical examination was unremarkable. Neurologic examination showed an unsteady gait, without motor weakness or ataxia. Cognitive tests showed a Mini-Mental Status Exam (MMSE) score of 18/30, impaired attention, executive functions, verbal fluency and episodic memory (Table 1).
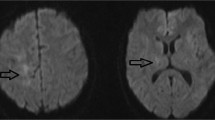
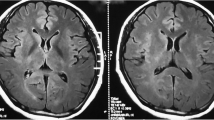
Brain MRI disclosed diffuse and symmetric confluent nonenhancing white matter lesions, that were hyperintense in T2/FLAIR images (Fig. 1a to d). Corresponding apparent diffusion coefficients (ADC) maps suggested vasogenic edema (Fig. 1f). MRI angiography was unremarkable (not shown). Multivoxel spectroscopy, dynamic susceptibility contrast (DSC) perfusion (T2*) and dynamic contrast-enhanced (DCE) permeability (T1) did not disclose relevant abnormalities (Fig. 1e, g and h). Cerebrospinal fluid analysis showed a normal cell count (4 cells/mm3), protein (32 mg/dL) and glucose (80 mg/dL) levels, normal protein electrophoresis values, negative oligoclonal bands and polymerase chain reaction for infectious agents (including tuberculosis). Systemic evaluation was negative for cancer, autoimmune diseases (Anti-nuclear antibodies = negative, Anti-neutrophil cytoplasmic antibodies = negative), and infectious diseases. Thoracic computed tomography (CT) showed nonspecific patchy lung infiltrates. Blood laboratory tests were normal (i.e. Erythrocyte sedimentation rate = 2 mm; C-reactive protein = 1,3 mg/L; Leucocytes = 6,880/mm3). Electroencephalogram EEG showed mild diffuse slowing and brief bursts of diffuse delta waves. The patient underwent two brain biopsies that showed tissue rarefaction with vacuolation, very mild inflammatory cell and macrophage infiltrates, absence of demyelination, malignant cells or granulomas, and no signs of tissue infarction or hemorrhagic changes (Fig. 2a to j). Immunostaining showed scarce CD45+ lymphocytes and CD68+ macrophages, without axonal or myelin damage, with few reactive astrocytes and low aquaporin-4 staining in the lesion compared to the normal surrounding areas. Aquaporin-1 staining was also reduced in the lesion, less extensively than aquaporin-4.
Serial brain magnetic resonance imaging studies of a patient with reversible leukoencephalopathy. Brain magnetic resonance imaging (MRI). Initial FLAIR images (a-d) show diffuse symmetrical confluent hyperintensities involving cerebral white matter, extending to the brainstem and cerebellar white matter. Note mass effect evidenced by sulci, fissure and ventricle effacement (more remarkable considering patient’s age - 64 years old). Corresponding white matter MR spectroscopy (e) (multivoxel, TE = 135 ms) demonstrates no definite metabolic changes. Apparent diffusion coefficients (ADC) map (f) demonstrates diffusion facilitation, signaling vasogenic edema. There was no contrast enhancement (not shown) or significant changes appreciated in color maps proportional to relative cerebral blood volume (rCBV) (g) obtained from a dynamic susceptibility contrast (DSC) perfusion (T2*) study. Color maps proportional to wash in rate (h) obtained from a dynamic contrast-enhanced (DCE) permeability (T1) sequence were also unremarkable. Images F-H are in the same level as D. After treatment for atypical mycobacteriosis, white matter changes disappeared, as shown in (FLAIR) images (i-l) obtained two years after the initial exam (arrows in J and L point to biopsy sites, partially characterized in these images)
Brain biopsy results of a patient with reversible leukoencephalopathy. a to j: Brain biopsy results of the patient with reversible leukoencephalopathy prior to atypical mycobacteria treatment shows (a) mild tissue rarefaction with vacuolation, very sparse perivascular inflammatory infiltrates, and (b) no evidence of demyelination. c–d Presence of relatively few glial fibrillary acidic protein (GFAP) positive astrocytes with reduced aquaporin-4 expression in the lesion compared to the surrounding area. Scale bar = 100 μm. High magnification (400x) on D shows aquaporin-4 on the membrane of reactive astrocytes. Scale bar = 10 μm. e Aquaporin-1 expression is also reduced in the lesion, but in less extensively than aquaporin-4. f–g Myelin sheath is preserved with no loss of myelin basic protein (MBP) and myelin associated glycoprotein (MAG). h No signs of neuronal or axonal damage. i–j Few lymphocytes (CD45+) and macrophages (CD68+) are found in the perivascular space, while immunoglobulin and complement C9neo deposition are absent (not shown). Scale bar = 50 μm. (Magnification a–d = 100x; e–j = 200x)
The patient was treated initially with intravenous methylprednisolone (1 g/day for three days), followed by oral dexamethasone (10 mg/day) for six months. Clinical and neurologic status and brain MRI remained unchanged. Activities of daily living were impaired, with a Functional Activity Questionnaire (FAQ) score of 25 and MMSE score of 18. Whole body positron emission tomography-computed tomography obtained at this point revealed a hypermetabolic right pulmonary mass. Lesion histology showed granulomas containing Mycobacterium abscessus. The patient was treated with levofloxacin, clarithromycin and amycacin. Steroids were tapered and discontinued. A year later, cognitive functions and functional status were improved (MMSE = 21; FAQ score = 10) (Table 1), and brain MRI disclosed remarkable resolution of white matter changes (Fig. 1i–l).
Conclusions
This report illustrates a case of leukoencephalopathy associated with atypical pulmonary mycobacteriosis. Although we cannot establish a cause-effect relationship of atypical mycobacteriosis and leukoencephalopathy, lack of central nervous system (CNS) granulomas and caseous necrosis, lack neurological worsening following steroid therapy, and improvement after antimycobacterial treatment suggest a remote effect of the lung infection, causing the CNS disorder.
Initial chest CT had initially shown nonspecific patchy lung infiltrates, that could, retrospectively, indicate early stage atypical mycobacterial infection. Additionally, the patient presented remarkable, albeit partial, neurological improvement after antimycobacterial treatment. There was no response to steroid therapy, brain pathology studies did not disclose inflammatory activity, and there was absence of intra-thecal antibody production, rendering the possibility of an adaptative immune mechanism (i.e. antibody or cell-mediated immune responses) extremely unlikely. Steroid therapy may have contributed to worsening of the mycobacterial lung infection.
Mycobacteria are known to be highly immunogenic: mycobacteria containing compounds are used in mouse models of ADEM and Multiple Sclerosis, through activation of adaptative immunity [17, 18]. Mycobacteria also activate the innate immune system, with production of cytokines and inflammation mediators, such as nitric oxide [19].
Mechanism of brain involvement in this case can be inferred from imaging and pathology findings of tissue edema with scarce reactive astrocytes, and reduced aquaporin-4 and aquaporin-1 expression in the lesion, compared to surrounding areas. Considering the lack of evidence of adaptative immune response in the brain, we speculate that activation of an innate-immune response in the lung either by the mycobacteria or through a host mediated response may have exerted a remote effect on aquaporin expression in the brain, leading to interstitial white matter edema. Alternatively, but less likely, antimycobacterial agents may have exerted a direct action reverting white matter lesions [20].
Few studies have evaluated pathological findings in reversible encephalopathies [1, 2, 8, 21], and some studies suggest the pathogenic role of cellular channel dysfunction. Reversible leukoencephalopathy has been described in few anti-aquaporin-4 antibody positive neuromyelitis optica (NMO) cases following immunotherapeutic interventions [3]. Lesion reversibility in these cases suggests that immune-mediated tissue destruction associated with blood–brain barrier (BBB) disruption may not be the main underlying mechanism.
It is conceivable that vasogenic edema noted on diffusion-weighted MRI sequences in reversible leukoencephalopathy cases represents parenchymal excess water content, caused by impaired water influx control, independent from BBB disruption. In our case, water influx control was probably impaired due to astrocyte aquaporin dysfunction, in a mechanism akin to that hypothesized in brain edema associated with NMO [3]. Additionally, experimentally induced acute hypertension in rabbits led to exogenous markers leakage in arterioles and capillaries through channels (often sigmoid-shaped) and cytoplasm and by transendothelial pinocytosis, causing brain-barrier disruption and edema [22]. These findings suggest impaired water channel function as a possible mechanism underlying reversible leukoencephalopathy.
We are not aware of previous non-demyelinating reversible leukoencephalopathy cases that improved after atypical mycobacteriosis treatment. We found vacuolated white matter lesions with paucity of reactive astrogliosis and decreased aquaporin water-channel expression. A causal relationship between mycobacteriosis and interstitial edema remains speculative. Alternatively, unexpected drug effects may have contributed to brain changes resolution. Elucidating pathogenic mechanisms underlying reversible leukoencephalopathies may lead to improved therapeutic strategies to treat this condition.
Consent
Written informed consent was obtained from the patient for publication of this Case Report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Abbreviations
- ADEM:
-
Acute disseminated encephalomyelitis
- ADC:
-
Apparent diffusion coefficients
- BBB:
-
Blood–brain barrier
- CNS:
-
Central nervous system
- CT:
-
Computed tomography
- DCE:
-
Dynamic contrast-enhanced
- DSC:
-
Dynamic susceptibility contrast
- FLAIR:
-
Fluid attenuation inversion recovery
- FAQ:
-
Functional Activity Questionnaire
- MRI:
-
Magnetic resonance imaging
- MMSE:
-
Mini-Mental Status Exam
- NMO:
-
Neuromyelitis optica
- PRES:
-
Posterior reversible encephalopathy syndrome
- rCBV:
-
Relative cerebral blood volume
References
Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:494–500.
Lee VH, Wijdicks EM, Manno EM, Rabinstein AA. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol. 2008;65:205–10.
Magaña SM, Matiello M, Pittock SJ, McKeon A, Lennon VA, Rabinstein AA, et al. Posterior reversible encephalopathy syndrome in neuromyelitis optica spectrum disorders. Neurology. 2009;72(8):712–7.
Mak A, Chan BP, Yeh IB, Ho RC, Boey ML, Feng PH, et al. Neuropsychiatric lupus and reversible posterior leucoencephalopathy syndrome: a challenging clinical dilemma. Rheumatology. 2008;47:256–62.
Bartynski WS, Boardman JF, Zeigler ZR, Shadduck RK, Lister J. Posterior reversible encephalopathy syndrome in infection, sepsis, and shock. AJNR Am J Neuroradiol. 2006;27:2179–90.
Guerriero S, Ciraci L, Centoducati T, Pignatelli F, Lamargese V, Salvati A, et al. Bilateral Visual Loss as Presenting Symptom of Posterior Reversible Encephalopathy Syndrome in a Patient with HIV/Tuberculosis Coinfection: A Case Report. Case Rep Ophthalmol Med. 2012; doi: 10.1155/2012/850176
Viehman JA, Khalil D, Barhoma C, Hanna RM. Mycobacterium avium-intracellulare otomastoiditis in a young AIDS patient: case report and review of the literature. Hiv/Aids. 2013;5:61–6.
Feske SK. Posterior reversible encephalopathy syndrome: a review. Semin Neurol. 2011;31:202–15.
Dastur DK, Udani PM. The pathology and pathogenesis of tuberculous encephalopathy. Acta Neuropathol. 1966;6:311–26.
Dastur DK. The pathology and pathogenesis of tuberculous encephalopathy and myeloradiculopathy: a comparison with allergic encephalomyelitis. Childs Nerv Syst. 1986;2:13–9.
Udani PM, Dastur DK. Tuberculous encephalopathy with and without meningitis. Clinical features and pathological correlations. J Neurol Sci. 1970;10:541–61.
Lammie GA, Hewlett RH, Schoeman JF, Donald PR. Tuberculous encephalopathy: a reappraisal. Acta Neuropathol. 2007;113(3):227–34.
Kim HJ, Shim KW, Lee MK, Park MS, Kim SH, Kim EY, et al. Tuberculous encephalopathy without meningitis: pathology and brain MRI findings. Eur Neurol. 2011;65(3):156–9.
Char G, Morgan OS. Tuberculous encephalopathy. A rare complication of pulmonary tuberculosis. West Indian Med J. 2000;49(1):70–2.
Chetty KG, Kim RC, Mahutte CK. Acute hemorrhagic leukoencephalitis during treatment for disseminated tuberculosis in a patient with AIDS. Int J Tuberc Lung Dis. 1997;1(6):579–81.
Okada H, Yoshioka K. Acute Disseminated Encephalomyelitis Associated with Meningitis due to Mycobacterium intracellulare. Inter Med. 2010;49:2113–6.
Wolf NA, Amouzegar TK, Swanborg RH. Synergistic interaction between Toll-like receptor agonists is required for induction of experimental autoimmune encephalomyelitis in Lewis rats. J Neuroimmunol. 2007;185(1–2):115–22.
Zorzella-Pezavento SF, Guerino CP, Chiuso-Minicucci F, França TG, Ishikawa LL, Masson AP, et al. BCG and BCG/DNAhsp65 Vaccinations Promote Protective Effects without Deleterious Consequences for Experimental Autoimmune Encephalomyelitis. Clin Dev Immunol. 2013;2013:721383.
Lee J, Sandor M, Heninger E, Fabry Z. Mycobacteria-induced suppression of autoimmunity in the central nervous system. J Neuroimmune Pharmacol. 2010;5(2):210–9.
Dalhoff A, Shalit I. Immunomodulatory effects of quinolones. Lancet Infect Dis. 2003;3:359–71.
Chester EM, Agamanolis DP, Banker BQ, Victor M. Hypertensive encephalopathy: a clinicopathologic study of 20 cases. Neurology. 1978;28:928–39.
Hansson HA, Johansson B, Blomstrand C. Ultrastructural studies on cerebrovascular permeability in acute hypertension. Acta Neuropathol. 1975;32:187–98.
Acknowledgements
This study was partially supported by KAKENHI (22229008) of The Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan, and by the Health and Labor Sciences Research Grant on Intractable Diseases (Neuroimmunological Diseases) from the Ministry of Health, Labor and Welfare of Japan. The funding sources had no role in the design, collection, analysis, or interpretation of the study, nor in the writing of the article or decision to submit.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
Dr. Oliveira has no conflicts of interest to disclose. Dr. Castro has no conflicts of interest to disclose. Dr. Sato is an associated editor of the Arquivos de Neuropsiquiatria (official journal of the Brazilian Academy of Neurology), receives scholarship from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan, has received research support from Ichiro Kanehara Foundation (2011), and speaker honorarium from Novartis. Dr. Soares-Neto has no conflicts of interest to disclose. Dr. Lucato has no conflicts of interest to disclose. Dr. Callegaro has no conflicts of interest to disclose. Dr. Medeiros has no conflicts of interest to disclose. Prof. Misu has received speaker honoraria from Bayer Schering Pharma, Biogen Idec Japan, Mitsubishi Tanabe Pharma Corporation, Asahi Kasei Medical Co., and Astellas Pharma Inc. and has received research support from Bayer Schering Pharma, Biogen Idec Japan, Asahi Kasei Kuraray Medical Co., The Chemo-Sero-Therapeutic Research Institute, Teva Pharmaceutical K.K., Mitsubishi Tanabe Pharma Corporation, Teijin Pharma, and Grants-in-Aid for Scientific Research from the Ministry of Education, Science and Technology, and the Ministry of Health, Labor and Welfare of Japan. Prof. Fujihara serves on scientific advisory boards for Bayer Schering Pharma, Biogen Idec, Mitsubishi Tanabe Pharma Corporation, Novartis Pharma, Chugai Pharmaceutical, Ono Pharmaceutical, Nihon Pharmaceutical, Merck Serono, Alexion Pharmaceuticals, Medimmune and Medical Review; has received funding for travel and speaker honoraria from Bayer Schering Pharma, Biogen Idec, Eisai Inc., Mitsubishi Tanabe Pharma Corporation, Novartis Pharma, Astellas Pharma Inc., Takeda Pharmaceutical Company Limited, Asahi Kasei Medical Co., Daiichi Sankyo, and Nihon Pharmaceutical; serve as an editorial board member of Clinical and Experimental Neuroimmunology (2009-present) and a advisory board member of Sri Lanka journal of Neurology; has received research support from Bayer Schering Pharma, Biogen Idec Japan, Asahi Kasei Medical, The Chemo-Sero-Therapeutic Research Institute, Teva Pharmaceutical, Mitsubishi Tanabe Pharma, Teijin Pharma, Chugai Pharmaceutical, Ono Pharmaceutical, Nihon Pharmaceutical, and Genzyme Japan; is funded as the secondary investigator (#22229008, 2010–2015) by the Grants-in-Aid for Scientific Research from the Ministry of Education, Science and Technology of Japan and as the secondary investigator by the Grants-in-Aid for Scientific Research from the Ministry of Health, Welfare and Labor of Japan (2010-present). Prof. Nitrini has no conflicts of interest to disclose.
Authors’ contributions
MCBO = conception and study design, data analysis and drafting the manuscript; LHMC = study design, data analysis, and drafting the manuscript; DKS = study conception, data analysis and drafting the manuscript; HRSN = data analysis, and drafting the manuscript; LTL = data analysis and review of the manuscript; DC = conception of the study, data analysis and review of the manuscript; RSSM = data analysis and review of the manuscript; TM = design of the study, data analysis, and drafting the manuscript; KF = study conception, data analysis and review of the manuscript; RN = study conception, data analysis and review of the manuscript. All authors read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Oliveira, M.C.B., Sato, D.K., Soares-Neto, H.R. et al. Leukoencephalopathy resolution after atypical mycobacterial treatment: a case report. BMC Neurol 15, 159 (2015). https://doi.org/10.1186/s12883-015-0415-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12883-015-0415-0