Abstract
Background
Chronic kidney disease (CKD) is associated with higher incidence of major surgery. No studies have evaluated the association between preoperative kidney function and postoperative outcomes across a wide spectrum of procedures. We aimed to evaluate the association between CKD and 30-day postoperative outcomes across surgical specialties.
Methods
We selected adult patients undergoing surgery across eight specialties. The primary study endpoint was major complications, defined as death, unplanned reoperation, cardiac complication, or stroke within 30 days following surgery. Secondary outcomes included Clavien-Dindo high-grade complications, as well as cardiac, pulmonary, infectious, and thromboembolic complications. Multivariable regression was performed to evaluate the association between CKD and 30-day postoperative complications, adjusted for baseline characteristics, surgical specialty, and operative time.
Results
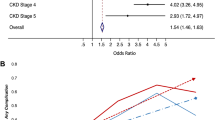
In total, 1,912,682 patients were included. The odds of major complications (adjusted odds ratio [aOR] 2.14 [95% confidence interval (CI): 2.07, 2.21]), death (aOR 3.03 [95% CI: 2.88, 3.19]), unplanned reoperation (aOR 1.57 [95% CI: 1.51, 1.64]), cardiac complication (aOR 3.51 [95% CI: 3.25, 3.80]), and stroke (aOR 1.89 [95% CI: 1.64, 2.17]) were greater for patients with CKD stage 5 vs. stage 1. A similar pattern was observed for the secondary endpoints.
Conclusion
This population-based study demonstrates the negative impact of CKD on operative outcomes across a diverse range of procedures and patients.
Similar content being viewed by others
Background
Glomerular filtration rate (GFR), whether estimated or indirectly measured, is a widely accepted marker of overall renal function [1]. Decreased kidney function for at least 3 months, as indicated by GFR < 60 ml/min/1.73m2 and/or the presence of kidney damage markers, characterizes chronic kidney disease (CKD) [2]. The prevalence of CKD is almost 11% in high-income nations and is the fourteenth leading cause of death worldwide, hence posing a public health challenge [3]. Further, CKD is a well-recognized independent predictor of cardiovascular events, hospitalizations, and all-cause mortality [4, 5].
Chronic kidney disease has been previously demonstrated to influence surgical outcomes, with patients experiencing increased length of stay and requiring intensive postoperative management [6,7,8,9]. Harrison et al. previously reported that CKD is not only associated with a higher incidence of major surgery [10], but also greater odds of death and myocardial infarction following ambulatory non-cardiac operations [11]. However, no studies have evaluated the association between preoperative kidney function and postoperative outcomes across a wide spectrum of common surgical procedures. We postulated that CKD may be independently associated with worse 30-day outcomes following surgery, and that there would be significant variation among specialties. To test this hypothesis, we performed a population-based retrospective cohort study of surgical patients, evaluating the association between CKD and 30-day postoperative outcomes across various specialties.
Materials and methods
Study design and population
This retrospective study utilized data from the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP). Briefly, the ACS-NSQIP is a validated database of prospectively collected 30-day perioperative data from over 600 participating community and academic institutions [12]. This study was deemed exempt by our institutional review board as the ACS-NSQIP contains de-identified data. This study was reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline and the Reporting of Studies Conducted Using Observational Routinely-Collected Health Data (RECORD) statement [13, 14].
Cohort selection
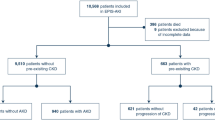
We selected patients aged ≥ 18 years undergoing elective surgery across any of the following eight specialties: gynecology, orthopedics, urology, thoracic, cardiac, general, vascular, and neurosurgery between 2005 and 2021. Using Common Procedural Terminology (CPT) codes (Supplementary Table 1), we identified a representative multiprocedural cohort. Patients with missing age, sex, race, height, weight, preoperative laboratory values, American Society of Anesthesiologists (ASA) physical status class, functional health status, or operative time were excluded.
Outcomes
The primary study endpoint was major complication, defined as death, unplanned reoperation, cardiac complication (myocardial infarction, cardiac arrest requiring cardiopulmonary resuscitation), and stroke within 30 days following surgery [15]. Secondary outcomes included Clavien-Dindo high-grade complication (defined as death, unplanned reoperation, septic shock, pulmonary embolism, cardiac complication, stroke, reintubation, prolonged intubation, coma, or acute renal failure), as well as cardiac, pulmonary (reintubation or prolonged intubation), infectious (pneumonia, urinary tract infection, wound infection, or sepsis), and thromboembolic (deep venous thrombosis or pulmonary embolism) complications.
Exposure and covariates
We calculated estimated GFR, using serum creatinine adjusted for age and sex, with the recommended race-agnostic CKD-EPI Eqs [16, 17]. Estimated GFR was categorized according to the Kidney Disease Improving Global Outcomes CKD 2012 Guidelines, with stage 1: ≥90 mL/min/1.73m2; 2: 60–89 mL/min/1.73m2; 3a: 45–59 mL/min/1.73m2; 3b: 30–44 mL/min/1.73m2; 4: 15–29 mL/min/1.73m2; and 5: <15 mL/min/1.73m2 [2]. The upper bound of age at surgery was capped at 90 years. Body mass index (BMI) was calculated using height and weight, and patients were categorized into underweight, normal, overweight, or obese according to the Centers for Disease Control classification [18].
American Society of Anesthesiologists status, smoking history (within 1 year prior to surgery), steroid use for chronic conditions, functional health status, chronic obstructive pulmonary disease (COPD), diabetes mellitus requiring therapy (oral or insulin), as well as cardiac (congestive heart failure in 30 days before surgery, history of myocardial infarction 6 months prior to surgery, previous cardiac surgery, history of angina 1 month before surgery, or arterial hypertension requiring medication), and neurologic history (transient ischemic attack, stroke, hemiplegia, or quadriplegia) were reported. We reported surgical specialty, preoperative hematocrit and platelet count, as well as operative time and perioperative transfusion of ≥ 1 unit of packed or whole red blood cells.
Statistical analysis
We presented the preoperative characteristics and 30-day postoperative complication rates of all patients, stratified by CKD stage. All data were presented as mean ± standard deviation (SD) for continuous variables, and number (%) for categorical variables. To account for the ordinal nature of CKD stages, we analyzed the trend of each preoperative characteristics and outcomes across CKD stages using the Cochran-Armitage test and ordinal logistic regression analysis for binary and ordinal categorical variables, respectively, and linear regression analysis for continuous variables. We characterized the preoperative profile of patients who experienced 30-day complications, stratified by CKD stage. Multivariable logistic regression analysis was performed for the primary endpoint and its components, as well as the secondary endpoints. Chronic kidney disease stage 1 was used as the reference category. We evaluated the association between CKD and 30-day postoperative complications, adjusted for age, race, BMI, ASA category, cardiac and neurologic history, COPD, diabetes, 12-month smoking status, steroid use for chronic conditions, functional health status, preoperative hematocrit and platelet counts, perioperative transfusion, operative time, and surgical specialty. The models’ predictive margins of CKD stages were estimated and visualized with forest plots. Stratified modeling further examined the association of CKD stages with our primary endpoint, by specialty subgroups. Further, we performed a post-hoc sensitivity analysis by excluding urologic procedures that inherently impact postoperative renal function (i.e. nephrectomy). All tests were two-tailed and p ≤ 0.05 was deemed significant. All analyses were performed with STATA 16.1 (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC). Figures were graphed using GraphPad Prism (GraphPad Software LLC, version 9.0, San Diego, CA, USA).
Results
Variation in baseline characteristics by CKD stage
In total, 1,912,682 patients were included in this analysis (Fig. 1). This cohort was stratified by CKD stage, with 861,721 patients with CKD Stage 1, 752,508 with Stage 2, 167,902 with Stage 3a, 73,616 with Stage 3b, 30,649 with Stage 4, and 26,286 with Stage 5 (Table 1). In terms of demographics, patients with advanced stage CKD tended to be older and underweight (both p for trend < 0.001). These patients demonstrated increased rates of various comorbidities, including a poorer ASA status and increased history of cardiac and neurologic events, COPD, diabetes, tobacco use, and chronic steroid utilization (all p for trend < 0.001). Preoperative platelet counts and hematocrit decreased with advanced CKD stage (both p for trend < 0.001) as well. Overall, functional status was markedly lower in those with worse CKD staging (p for trend < 0.001). Surgery type, as measured by surgical specialty, was markedly different based on CKD staging. Most notably, patients of advanced CKD stage were less likely to undergo gynecologic and orthopedic procedures, but significantly more likely to undergo urologic and vascular operations (p for trend < 0.001).
Association between postoperative complication rates and CKD stage
Unadjusted postoperative complication rates were significantly increased across patients with advanced CKD staging. Major complications, namely death, unplanned reoperation, cardiac complication and stroke were all significantly more frequent among patients with advanced stage CKD (all p < 0.001). Most notably, while only 1.8% of the total cohort died within 30 days, 12.2% of patients with end stage renal disease (CKD 5) died within the same period. Similarly, reoperations occurred in 13% of patients with CKD 5, compared to 4.2% among the general cohort (Table 2). Regarding secondary endpoints, rates were significantly higher in patients with advanced CKD (4 & 5). Specifically, Clavien-Dindo high-grade complications, bleeding, pulmonary, infectious, and thromboembolic complications were all more common as CKD stage increased (p for trend < 0.001).
Predictive value of CKD for postoperative outcomes
After multivariable logistic regression, the odds of most complications were associated with increasing CKD stage (Figs. 2 and 3). In general, the odds of each complication type increased proportionally with CKD staging. Specifically, the odds of major complications were more than double for CKD stage 5 patients (adjusted odds ratio [aOR] 2.14 [95% confidence interval (CI): 2.07, 2.21]), while odds of death were tripled (aOR 3.03 [95% CI: 2.88, 3.19]), when compared to patients with CKD stage 1. Reoperations were 57% more likely among patients with CKD stage 5 (aOR 1.57 [95% CI :1.51, 1.64]) compared to stage 1. Within the components of the primary outcome, the magnitude of odds was greatest for cardiac complications (CKD stage 5: aOR 3.51 [95% CI: 3.25, 3.80]). Chronic kidney disease stage 4 and 5 were both associated with greater odds of stroke (aOR 1.96 [95% CI: 1.72, 2.23] and aOR 1.89 [95% CI: 1.64, 2.17], respectively), compared to CKD stage 1.
The odds of secondary outcomes for patients with CKD stage 5 were each substantially increased as well. Increasing CKD stage was associated with greater odds of bleeding requiring transfusion (CKD stage 4: aOR 1.81 [95% CI: 1.76, 1.87] and CKD stage 5: aOR 1.19 [95% CI: 1.15, 1.23]). Of note, the risk of a VTE was significantly increased with CKD stages 3a, 3b, and 4, but not stage 5. Subgroup analysis of major complications revealed consistency of results for most specialties, namely cardiac and general surgery, neurosurgery, orthopedics, and urology (Fig. 4). There were notable differences for gynecology, thoracic, and vascular surgery. There was no association between CKD stage 5 and major complications for gynecology. For thoracic and vascular surgery, there was no association between CKD stage 3 and major complications. In sensitivity analysis, overall results remained unchanged for primary and secondary endpoints, after exclusion of CPT codes for nephrectomy (Supplementary Fig. 1). In further subgroup analysis, there was a decrease in the magnitude of the associations between increasing CKD stages and major complications (Supplementary Fig. 2). The only exception was urology, in which there was no longer an association between CKD stage 3 and major complications.
Discussion
This population-based analysis of more than 1.9 million patients provides a comprehensive overview of the effects of CKD on postoperative outcomes, both corroborating previous literature and adding novel insights into the understanding of the importance of CKD. Though it may seem predictable that common patient comorbidities such as CKD can worsen outcomes, this study includes a rigorous statistical analysis, adjusting for numerous confounders, to provide quantifiable risk which can be used to counsel patients on clinical decision-making. Further, this study suggests that CKD is associated with greater odds of nearly all key postoperative adverse outcomes, including cardiovascular events, stroke, pulmonary complications, infectious, and bleeding events. Results remained robust after subgroup and sensitivity analyses.
Previous studies have not evaluated the association between CKD and postoperative outcomes within a diverse multi-specialty surgical cohort and have instead been limited to specific procedures within surgical specialties, thus limiting generalizability. The association of CKD with complications following major abdominal surgery was previously investigated, with a 29% increase in complications and a sevenfold increase in mortality in hemodialysis patients [6]. Infections were found to be the predominant complication. Similarly, patients with stage 5 CKD undergoing bariatric surgery were found to have nearly doubled risk of readmission and increased length of stay, along with infections and mortality. However, the authors suggest that bariatric surgery could be renoprotective overall, and the absolute rates of complications, while relatively higher, remained low in terms of absolute values [19]. Similarly, with regard to cardiac surgery, CKD has been shown as an independent risk factor of major complications and mortality following aortic repair [20]. Studies have gone as far as finding that simultaneous kidney transplantation may benefit heart transplant outcomes in patients with baseline CKD [21]. CKD in vascular surgery patients has been shown to increase mortality, cardiovascular and cerebrovascular events, bleeds, and AKI along with reduced chances of procedural success [22,23,24].
Orthopedic surgery has similarly shown increased general morbidity, as a single institution study of lumbar arthrodesis patients found increase rates of intensive care unit transfer, delirium, urinary tract infection, and deep vein thrombosis in CKD patients, along with an increase in readmissions although this was not statistically significant [25]. Total hip arthroplasty has also been shown to yield poorer outcomes with hemodialysis, with threefold increased mortality, 50% increased emergency room visits, and 2.7 times increased readmissions, although infection or revision rates were not increased [26]. Overall, the authors remarked that CKD may not increase orthopedic implant-related complications, but general medical morbidity was significantly poorer. In a separate study of diabetic patients receiving humerus fracture fixation surgery, CKD patients were shown to have increased revision, infection, readmission, and mortality risk [27]. Finally, transmetatarsal amputation failure is 100% more likely in CKD patients, while conferring a 182% increased mortality risk [28]. Otolaryngology has paralleled these findings, demonstrating that readmissions occur at fivefold increased rates following parathyroidectomy in CKD patients [29]. Similarly, urology has demonstrated the effects of CKD on nephrectomy outcomes, with one study demonstrating the value of incorporating baseline CKD into a metric predicting postoperative outcomes [30]. There does not appear to be any previous study of the effects of CKD on gynecologic surgery outcomes.
Interestingly, the predictivity of CKD staging for several complications categories, including pulmonary, infectious, thromboembolic, and bleeding events, appeared to slightly decrease between CKD stage 4 to 5, although it still remained significantly greater than that for lower stage CKD patients. One study limited to bariatric surgery patients interestingly found that although increasing CKD stage was associated with postoperative mortality, this effect appeared to be mitigated with stage 4 and 5 CKD [19]. CKD has been classically associated with an increased risk of bleeding, but also a paradoxical thrombotic potential. This is thought to be secondary to delayed clot formation yet increased clot strength and decreased breakdown [31]. Although we found advanced CKD to be associated with an increased risk of bleeding across all stages of CKD, we did not find a similar uniform association for VTE. We believe this finding is due to the higher rates of DVT but not PE among patients with CKD. Large population-based observational studies have found that patients with advanced CKD have greater odds of VTE at 1 year (HR 1.83, 95% CI 1.03–3.25) but not at 30-days (HR 1.64 [95% CI: 0.59, 4.54]) [32]. This could also explain why we failed to show a consistent association between 30-day postoperative VTE and all stages of CKD.
Although NSQIP is a validated database with strict quality control measures, this study has limitations. Namely, data cannot be verified for accuracy and may have inherent biases due to its retrospective nature. Additionally, NSQIP focuses on 30-day outcomes, limiting the ability to study the effects of CKD on long-term postoperative experiences or sequelae of chronically reduced renal function. Nonetheless, this study utilizes a substantially large, diverse cohort, allowing for generalizability to the larger population. Moreover, a wide range of outcomes were studied across surgical specialties, allowing for a comprehensive understanding of the effects of CKD on surgery. Additionally, we do not have information on perioperative anticoagulation or general DVT prophylaxis. Finally, there could still be unmeasured confounding that we failed to adjust, such as preoperative dialysis.
The findings of this study may be utilized to better approximate fitness for surgery and hence inform shared decision-making amongst providers and their patients. Namely, this study can inform the incorporation of CKD within novel risk stratification tools which predict the likelihood of specific adverse events following an operation. Identification of CKD in a patient undergoing surgery may also permit improved intraoperative and postoperative surveillance or prophylactic techniques to mitigate risk of specific complications.
Conclusions
In summary, this population-based study indicates the negative impacts of CKD on operative outcomes across a diverse range of procedures and patients. As CKD increases in prevalence, further research is necessary to better elucidate the effects of reduced renal function on surgical safety and patient experiences. An improved understanding of the multifactorial effects of CKD can facilitate shared decision-making and improve patient-centered outcomes across surgical specialties.
Data availability
The datasets generated and/or analysed during the current study are available in the American College of Surgeons National Surgical Quality Improvement Program repository. The American College of Surgeons National Surgical Quality Improvement Program and the hospitals participating in the ACS NSQIP are the source of the data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
Abbreviations
- GFR:
-
Glomerular filtration rate
- CKD:
-
Chronic Kidney Disease
- ACS-NSQIP:
-
American College of Surgeons National Surgical Quality Improvement Program
- RECORD:
-
Reporting of Studies Conducted Using Observational Routinely Collected Health Data
- CPT:
-
Common Procedural Terminology
- ASA:
-
American Society of Anesthesiologists
- BMI:
-
Body mass index
- COPD:
-
Chronic obstructive pulmonary disease
- SD:
-
Standard deviation
- VTE:
-
Venous Thromboembolism
- CI:
-
Confidence interval
- DVT:
-
Deep venous thrombosis
References
Levey AS, Coresh J, Tighiouart H, Greene T, Inker LA. Measured and estimated glomerular filtration rate: current status and future directions. Nat Rev Nephrol. 2020;16:51–64. https://doi.org/10.1038/s41581-019-0191-y
Stevens PE, Levin A. Kidney disease: improving global outcomes chronic kidney Disease Guideline Development Work Group, M. evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158:825–30. https://doi.org/10.7326/0003-4819-158-11-201306040-00007
Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet. 2017;389:1238–52. https://doi.org/10.1016/S0140-6736(16)32064-5
Fox CS, Matsushita K, Woodward M, Bilo HJ, Chalmers J, Heerspink HJ, Lee BJ, Perkins RM, Rossing P, Sairenchi T, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet. 2012;380:1662–73. https://doi.org/10.1016/S0140-6736(12)61350-6
Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–305. https://doi.org/10.1056/NEJMoa041031
Cloyd JM, Ma Y, Morton JM, Kurella Tamura M, Poultsides GA, Visser BC. Does chronic kidney disease affect outcomes after major abdominal surgery? Results from the National Surgical Quality Improvement Program. J Gastrointest Surg. 2014;18:605–12. https://doi.org/10.1007/s11605-013-2390-3
Karambelkar AD, Chawla LS, Busse LW. The perioperative management of the patient with chronic kidney disease. In Chronic Renal Disease; Elsevier: 2020; pp. 1291–1307.
Kozlowski L, Bielawska K, Zhymaila A, Malyszko J. Chronic kidney Disease Prevalence in patients with Colorectal Cancer undergoing surgery. Diagnostics (Basel). 2022;12. https://doi.org/10.3390/diagnostics12092137
Pizano A, Scott CK, Porras-Colon J, Driessen AL, Miller RT, Timaran CH, Modrall JG, Tsai S, Kirkwood ML, Ramanan B. Chronic kidney disease impacts outcomes after abdominal aortic aneurysm repair. J Vasc Surg. 2022. https://doi.org/10.1016/j.jvs.2022.09.003
Harrison TG, Ruzycki SM, James MT, Ronksley PE, Zarnke KB, Tonelli M, Manns BJ, McCaughey D, Schneider P, Dixon E, et al. Estimated GFR and incidence of major surgery: a Population-based Cohort Study. Am J Kidney Dis. 2021;77(e361):365–75. https://doi.org/10.1053/j.ajkd.2020.08.009
Harrison TG, Hemmelgarn BR, James MT, Manns BJ, Tonelli M, Brindle ME, McCaughey D, Ruzycki SM, Zarnke KB, Wick J, et al. Association of kidney function with Major postoperative events after non-cardiac ambulatory surgeries: a Population-based Cohort Study. Ann Surg. 2021. https://doi.org/10.1097/SLA.0000000000005040
American College of Surgeons National Surgical Quality Improvement Program (ACS NSQIP). Frequently Asked Questions. Available online: https://www.facs.org/quality-programs/data-and-registries/acs-nsqip/faq/ (accessed on February 13, 2022).
Benchimol EI, Smeeth L, Guttmann A, Harron K, Moher D, Petersen I, Sorensen HT, von Elm E, Langan SM, Committee RW. The REporting of studies conducted using Observational routinely-collected health data (RECORD) statement. PLoS Med. 2015;12:e1001885. https://doi.org/10.1371/journal.pmed.1001885
von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, Initiative S. The strengthening the reporting of Observational studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573–7. https://doi.org/10.7326/0003-4819-147-8-200710160-00010
Wallis CJ, Bjarnason G, Byrne J, Cheung DC, Hoffman A, Kulkarni GS, Nathens AB, Nam RK, Satkunasivam R. Morbidity and mortality of radical nephrectomy for patients with disseminated Cancer: an analysis of the National Surgical Quality Improvement Program Database. Urology. 2016;95:95–102. https://doi.org/10.1016/j.urology.2016.04.055
Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, Crews DC, Doria A, Estrella MM, Froissart M, et al. New Creatinine- and cystatin C-Based equations to Estimate GFR without Race. N Engl J Med. 2021;385:1737–49. https://doi.org/10.1056/NEJMoa2102953
Delgado C, Baweja M, Crews DC, Eneanya ND, Gadegbeku CA, Inker LA, Mendu ML, Miller WG, Moxey-Mims MM, Roberts GV, et al. A Unifying Approach for GFR Estimation: recommendations of the NKF-ASN Task Force on reassessing the inclusion of race in diagnosing kidney disease. Am J Kidney Dis. 2022;79(e261):268–88. https://doi.org/10.1053/j.ajkd.2021.08.003
Weir CB, Jan ABMI. Classification Percentile And Cut Off Points. In StatPearls; Treasure Island (FL), 2022.
Carvalho Silveira F, Martin WP, Maranga G, le Roux CW, Ren-Fielding CJ. The impact of CKD on Perioperative Risk and Mortality after bariatric surgery. Kidney360. 2021;2:236–44. https://doi.org/10.34067/KID.0004832020
Paajanen P, Karkkainen JM, Tenorio ER, Mendes BC, Oderich GS. Effect of patient frailty status on outcomes of fenestrated-branched endovascular aortic repair for complex abdominal and thoracoabdominal aortic aneurysms. J Vasc Surg. 2022;76(e1172):1170–9. https://doi.org/10.1016/j.jvs.2022.05.008
Atkins J, Hess NR, Fu S, Read JM, Hajj JM, Ramu B, Silverman DN, Inampudi C, Vanbakel AB, Hashmi ZA, et al. Outcomes in patients with LVADs undergoing simultaneous heart-kidney transplantation. J Card Fail. 2022;28:1584–92. https://doi.org/10.1016/j.cardfail.2022.04.016
Qi Y, He J, Pan M, Yan J. Impact of impaired renal function on outcomes of chronic total occlusion undergoing revascularization: a systemic review and meta-analysis. Int Urol Nephrol. 2022;54:3179–91. https://doi.org/10.1007/s11255-022-03192-7
Borrelli S, Garofalo C, Gabbai FB, Liberti ME, Chiodini P, Simeon V, De Nicola L, Minutolo R. Sex difference in cardiovascular risk in patients with chronic kidney disease: pooled analysis of four cohort studies. Nephrol Dial Transpl. 2023. https://doi.org/10.1093/ndt/gfad036
Lacquaniti A, Campo S, Falliti G, Caruso D, Gargano R, Giunta E, Monardo P. Free light chains, high mobility Group Box 1, and Mortality in Hemodialysis patients. J Clin Med. 2022;11. https://doi.org/10.3390/jcm11236904
Adogwa O, Elsamadicy AA, Sergesketter A, Oyeyemi D, Galan D, Vuong VD, Khalid S, Cheng J, Bagley CA, Karikari IO. The impact of chronic kidney disease on postoperative outcomes in patients undergoing lumbar decompression and Fusion. World Neurosurg. 2018;110:e266–70. https://doi.org/10.1016/j.wneu.2017.10.147
Hoggard TM, Chen DQ, Quinlan ND, Bell JE, Werner BC, Cui Q. Outcomes following total hip arthroplasty for osteonecrosis of the femoral head in patients on Hemodialysis. J Bone Joint Surg Am. 2022;104:90–4. https://doi.org/10.2106/JBJS.20.00352
Chen CT, Lin SJ, Kuo LT, Chen TH, Hsu WH, Chen CL, Yu PA, Peng KT, Tsai YH. Effect of chronic kidney disease on outcomes following proximal humerus fragility fracture surgery in diabetic patients: a nationwide population-based cohort study. PLoS ONE. 2021;16:e0258393. https://doi.org/10.1371/journal.pone.0258393
Ahn J, Raspovic KM, Liu GT, Lavery LA, La Fontaine J, Nakonezny PA, Wukich DK. Renal function as a predictor of early transmetatarsal amputation failure. Foot Ankle Spec. 2019;12:439–51. https://doi.org/10.1177/1938640018816371
Ferrandino R, Roof S, Ma Y, Chan L, Poojary P, Saha A, Chauhan K, Coca SG, Nadkarni GN, Teng MS. Unplanned 30-Day readmissions after parathyroidectomy in patients with chronic kidney disease: a nationwide analysis. Otolaryngol Head Neck Surg. 2017;157:955–65. https://doi.org/10.1177/0194599817721154
Choudhary GR, Jena R, Likhiteswer P, Gupta P, Pandey H, Yadav T, Madduri VKS, Singh M. Patient REnal and Tumor Attribute score (PRETA score): a comprehensive renal nephrometry score for use in patients with renal masses planned for minimally invasive nephron sparing surgery. J Robot Surg. 2022;16:1463–70. https://doi.org/10.1007/s11701-022-01389-7
Nunns GR, Moore EE, Chapman MP, Moore HB, Stettler GR, Peltz E, Burlew CC, Silliman CC, Banerjee A, Sauaia A. The hypercoagulability paradox of chronic kidney disease: the role of fibrinogen. Am J Surg. 2017;214:1215–8. https://doi.org/10.1016/j.amjsurg.2017.08.039
Parikh AM, Spencer FA, Lessard D, Emery C, Baylin A, Linkletter C, Goldberg RJ. Venous thromboembolism in patients with reduced estimated GFR: a population-based perspective. Am J Kidney Dis. 2011;58:746–55. https://doi.org/10.1053/j.ajkd.2011.06.021
Acknowledgements
None.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, C.R. and R.S.; Methodology, C.R.; Software, E.H. AND J.X.; Validation, E.H.; Formal analysis, M.G.; Writing—original draft preparation, C.R. and Y.B.S.; Writing—review and editing, Z.M., S.H., B.J.M, N.E., Z.K., A.J., C.J.D.W., and R.S.; Supervision, R.S. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was deemed exempt by our institutional review board as the database used contains de-identified data.
Clinical trial number
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Riveros, C., Ranganathan, S., Shah, Y.B. et al. Association of chronic kidney disease with postoperative outcomes: a national surgical quality improvement program (NSQIP) multi-specialty surgical cohort analysis. BMC Nephrol 25, 305 (2024). https://doi.org/10.1186/s12882-024-03753-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-024-03753-1