Abstract
Background
Recent studies have focused on immune checkpoint inhibitors. Renal complications associated with the use of immune checkpoint inhibitors are uncommon compared with other immune-related adverse events. Acute interstitial nephritis accounts for most of these renal complications, with nephrotic syndrome quite rare. We herein report a case of nephrotic syndrome associated with immune checkpoint inhibitors that was more severe than that in previous cases. By comparing this case with previous reports, the possible reasons for the particular severity of this case are discussed.
Case presentation
A 75-year-old man developed nephrotic syndrome with acute kidney injury after the first combination therapy of nivolumab and ipilimumab for malignant pleural mesothelioma. The results of a kidney biopsy indicated minimal change disease with mild atherosclerosis, acute interstitial nephritis, and fusion of nearly all podocyte foot processes. Nivolumab and ipilimumab therapy were stopped, and treatment with corticosteroids was initiated. We investigated previously reported cases of nephrotic syndrome using immune checkpoint inhibitors. Seventeen cases of immune checkpoint inhibitor-related nephrotic syndrome, including ours, have been reported. Two of the 17 patients with immune checkpoint inhibitor-related nephrotic syndrome required hemodialysis treatment for acute kidney injury. Unlike many previously reported cases, the present patient was administered two different immune checkpoint inhibitors, which may be one of the reasons for the development of severe nephrotic syndrome.
Conclusions
In addition to previously reported risk factors, immune checkpoint inhibitor combination therapy can exacerbate nephrotic syndrome compared to immune checkpoint inhibitor monotherapy.
Similar content being viewed by others
Background
Recent studies have focused on immune checkpoint inhibitors (ICIs) [1]. In patients with unresectable tumors, ICIs are used as the first-line therapy in a variety of fields [2, 3], which has contributed to substantially improved survival [2]. For example, malignant pleural mesothelioma is a highly aggressive malignancy that is often unresectable at time of the diagnosis, with less than 10% of patients surviving five years or longer [4]. Nivolumab plus ipilimumab have provided significant and clinically meaningful improvements in overall survival versus standard-of-care chemotherapy, which supported its approval in the USA as the first-in-class regimen for previously untreated unresectable malignant pleural mesothelioma [5].
Patients treated with ICIs often develop immune-related adverse events (irAEs), which are immune-mediated secondary effects of ICIs [1].
Renal complications associated with the use of ICIs are uncommon compared with other irAEs [6]. Most cases of renal complications are acute interstitial nephritis, which is said to account for 93% of cases of ICI-related acute kidney injury [7]. In contrast, nephrotic syndrome is extremely rare, as is minimal change disease [2, 6, 8,9,10,11].
We herein report a case of nephrotic syndrome associated with ICIs that was more severe than that in previous cases. By comparing this case with previous reports, the possible reasons for the particular severity of this case are discussed.
Case presentation
A 75-year-old man received first-line nivolumab (treatment dose, 360 mg every 3 weeks) plus ipilimumab (1 mg/kg intravenously every 6 weeks), combination therapy with anti-programmed cell death 1 (anti-PD-1) antibody, and cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) antibody for malignant pleural mesothelioma. His comorbidities included an artificial anus due to perforation of the sigmoid colon and slight proteinuria (urine dipstick test: 30 mg/dl). He did not have any red blood cells, white blood cells, or casts in his urine. His serum albumin, serum creatinine, and hemoglobin A1c values were 3.7 g/dl, 0.82 mg/dl, and 6.0%. He had a history of smoking from 20 to 60 years old. He did not normally take regular medication, but had been taking loxoprofen sodium (60 mg, three times daily), tramadol hydrochloride (37.5 mg, four times daily), and acetaminophen (325 mg, four times daily) for two months for pain caused by the tumor. After the first dose of ICIs (13 days after the initial treatment), he suddenly presented with leg edema, massive proteinuria (19.85 g/day), urinary glucose (0.344 g/day), and hypoalbuminemia (2.0 g/dl). His blood pressure at presentation was 97/48 mmHg. Based on these results, the patient was diagnosed with nephrotic syndrome.
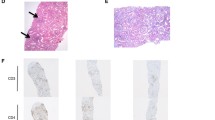
The administration of loxoprofen sodium was discontinued, and a kidney biopsy was performed, and light microscopy revealed 11 glomeruli without focal sclerosis, an increase in the mesangial matrix, or cellularity (Fig. 1a). The renal interstitium exhibited mild fibrosis (Fig. 1b), mild leukocytic infiltration, and slight tubulitis, accompanied by medial hypertrophy and fibroblastic intimal thickening in the interlobular and arcuate arteries. An immunofluorescence analysis yielded negative results. Electron microscopy revealed fusion of nearly all podocyte foot processes without any electron-dense deposits (Fig. 1c). Overall, the biopsy specimens indicated minimal change disease (MCD) with mild atherosclerosis and acute interstitial nephritis (AIN).
Kidney biopsy findings. (a) Periodic acid Schiff (PAS) staining did not show focal sclerosis or increase in mesangial matrix or cellularity. Bar = 50 μm. (b) Masson-Trichrome (MT) staining showed medial hypertrophy and fibroblastic intimal thickening (arrows) of the arch arteries. Bar = 100 μm. (c) Representative electron micrograph obtained from a kidney biopsy. Extensive podocyte foot process fusion was revealed (arrows), without any electron-dense deposits. Bar = 2 μm
Nivolumab and ipilimumab therapy were stopped, and treatment with corticosteroids (prednisone, 1 mg/kg) was initiated. However, diuretic medicines did not work well, resulting in the need for hemodialysis initiation, and excess fluid was removed. His urinary protein and glucose levels gradually decreased with continued treatment and prednisone was tapered. Hemodialysis was discontinued because his urine output gradually increased 35 days after initiating hemodialysis.
Since discharge, his serum creatinine and urine albumin-to-creatinine ratio was 1.06 mg/dl and 1.06 g/gCr. The patient has continued to attend outpatient visits in good spirits, and prednisone tapering is ongoing (Fig. 2). Regarding the malignant pleural mesothelioma, the patient will be monitored to see if it grows, without re-administration of ICIs. If it grows, other chemotherapy will be administered.
Discussion and conclusions
We encountered a case of nephrotic syndrome with MCD caused by an ICI. One proposed mechanism concerning how ICIs lead to podocyte injury is remote production of a “permeability factor” that may cause release of cytokines that then promote podocyte foot-process effacement [8]. Although MCD secondary to cancer is well-recognized, there is evidence that the present case was related to therapy rather than to the malignancy itself. First, nephrotic syndrome developed soon after administration of the first ICIs in this case. In previous reports, approximately 30% of ICI-related acute kidney injury occurred within 5 weeks, with a median time from administration to the onset of 14 weeks [7]. Second, in cases of MCD secondary to cancer, the course of proteinuria typically parallels that of the underlying malignancy, with MCD remitting after successful cancer treatment [12]. Computed tomography, performed when the patient had severe renal failure requiring dialysis, showed that his tumor had shrunk dramatically. These findings indicated that MCD is caused by ICIs. Furthermore, some studies showed an association between the occurrence of irAEs and the survival in patients treated with ICIs [1, 13, 14], which may explain why the present case with severe nephrotic syndrome showed dramatic tumor shrinkage. In addition to MCD, this patient also presented with AIN, albeit in a mild form. Loxoprofen sodium, similar to ICIs, is known to potentially induce MCD and AIN. Although the risk of non-steroidal anti-inflammatory drugs (NSAIDs) related nephrotic syndrome persists for up to 2 years after discontinuation of NSAIDs, it is particularly high within the first 2 weeks of exposure [15]. The patient had been taking loxoprofen sodium for 2 months prior to the onset of nephrotic syndrome. The diagnosis of nephrotic syndrome was attributed to ICIs, considering the timeline between drug administration and the onset of the disease.
We investigated previously reported cases of nephrotic syndrome using ICIs. PubMed was searched using the following two formulas: (1) (“Antibodies, Monoclonal, Humanized”[MeSH Terms] OR “immune checkpoint inhibitors”[MeSH Terms]) AND (“Nephritis”[MeSH Terms] OR “Nephrosis”[MeSH Terms]), and (2) (PD-1 OR CTLA-4 OR “immune checkpoint inhibitors”) AND (“minimal change disease” OR nephritis); 444 and 424 articles, respectively, were identified using the search strategies. We checked all titles and abstracts of the articles to identify pertinent articles, and then examined the contents of the remaining papers to determine their applicability to this investigation.
Our survey identified 17 cases of ICI-related nephrotic syndrome, including our own (Table 1) [8,9,10,11, 16,17,18,19,20,21,22,23,24]. The ratio of males (14) to females (3) was 4.7:1. The patients’ age ranged from 40 to 75 years, with an average age of 62.1 years old. Lung adenocarcinoma was the underlying condition in five cases, malignant melanoma in five, malignant pleural mesothelioma in two, renal cell carcinoma in two, Hodgkin lymphoma in two, and chondroma in one. Pembrolizumab was the most commonly used ICI, with multiple ICIs used in two cases. The pathological types of nephrotic syndrome were as follows: MCD in 7 (41.1%), membranous nephropathy in 3 (17.6%), focal segmental glomerulosclerosis in 2 (11.8%), IgA nephropathy in 1 (5.9%), AA-type amyloidosis in 1 (5.9%), pauci-immune glomerulonephritis with crescents in 1 (5.9%), and not performed in 1 (5.9%). Most cases developed nephrotic syndrome within a few months of initial treatment, but some occurred more than a year later. As the first treatment, most patients were administered high-dose prednisone (approximately 1 mg/day), and in 1 case [16], only the ICI was discontinued. The patient was observed; however, there was no improvement, and prednisone was eventually initiated. Most patients did not require dialysis, with dialysis being necessary in only 2 cases (11.8%).
The present patient had fusion of nearly all podocyte foot processes and a more severe condition than previous cases, and required dialysis. The main factors leading to nephrotic syndrome with acute kidney injury in the general population include but are not limited to old age, hypertension, arterial/arteriolar lesions, and a serum albumin level < 2.0 g/dl [25]. Old age and arterial/arteriolar lesions were present in the present case. He had proteinuria at baseline because of nephrosclerotic changes due to arterial/arteriolar lesions. The urinary protein in this case reflected the risk of acute kidney injury. In addition, this case showed renal glycosuria, indicating complication with tubular dysfunction [26]. Although the pathogenesis of tubular dysfunction in nephrotic syndrome remains unclear, it is hypothesized that increased tubular protein reabsorption leads to a massive influx of protein into the tubular lumen, which in turn directly damages tubular epithelial cells [26]. The detection of tubular dysfunction coincides with worsening of the renal function and more proteinuria than in cases without tubular dysfunction [26]. AIN may also have been involved in acute kidney injury in this case. In this sense, the present case was a severe one. Furthermore, unlike many previously reported cases, our case was administered two different ICIs, which can cause nephrotic syndrome to become severe; the fact that combination therapy induces multiple concurrent irAEs more often than monotherapy [27] supports this hypothesis.
In summary, we encountered a case in which combination ICI therapy (anti-PD-1 and CTLA-4 antibodies) led to severe nephrotic syndrome with acute kidney injury. In addition to previously reported risk factors, such as advanced age and atherosclerosis, ICI combination therapy can increase the risk of nephrotic syndrome compared to ICI monotherapy.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- AIN:
-
acute interstitial nephritis
- anti-PD-1:
-
anti-programmed cell death 1
- CTLA-4:
-
cytotoxic T-lymphocyte-associated antigen 4
- ICIs:
-
immune checkpoint inhibitor
- irAE:
-
immune-related adverse event
- MCD:
-
minimal change disease
- non-steroidal anti-inflammatory drugs:
-
NSAIDs
References
Kfoury M, Najean M, Lappara A, Voisin A-L, Champiat S, Michot J-M, et al. Analysis of the association between prospectively collected immune-related adverse events and survival in patients with solid tumor treated with immune-checkpoint blockers, taking into account immortal-time bias. Cancer Treat Rev. 2022;110:102452.
Bickel A, Koneth I, Enzler-Tschudy A, Neuweiler J, Flatz L, Früh M. Pembrolizumab-associated minimal change disease in a patient with malignant pleural mesothelioma. BMC Cancer. 2016;16:656.
Reck M, Remon J, Hellmann MD. First-line immunotherapy for non-small-cell Lung Cancer. J Clin Oncol. 2022;40:586–97.
Quhal F, Mori K, Bruchbacher A, Resch I, Mostafaei H, Pradere B, et al. First-line immunotherapy-based combinations for metastatic renal cell carcinoma: a systematic review and network Meta-analysis. Eur Urol Oncol. 2021;4:755–65.
Van Gerwen M, Alpert N, Wolf A, Ohri N, Lewis E, Rosenzweig KE, et al. Prognostic factors of survival in patients with malignant pleural mesothelioma: an analysis of the National Cancer Database. Carcinogenesis. 2019;40:529–36.
Baas P, Scherpereel A, Nowak AK, Fujimoto N, Peters S, Tsao AS, et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet. 2021;397:375–86.
Cortazar FB, Kibbelaar ZA, Glezerman IG, Abudayyeh A, Mamlouk O, Motwani SS, et al. Clinical features and outcomes of Immune Checkpoint inhibitor-Associated AKI: a Multicenter Study. J Am Soc Nephrol. 2020;31:435–46.
Kitchlu A, Fingrut W, Avila-Casado C, Chan CT, Crump M, Hogg D, et al. Nephrotic syndrome with Cancer immunotherapies: a report of 2 cases. Am J Kidney Dis. 2017;70:581–5.
Gao B, Lin N, Wang S, Wang Y. Minimal change disease associated with anti-PD1 immunotherapy: a case report. BMC Nephrol. 2018;19:156.
Glutsch V, Grän F, Weber J, Gesierich A, Goebeler M, Schilling B. Response to combined ipilimumab and nivolumab after development of a nephrotic syndrome related to PD-1 monotherapy. J Immunother Cancer. 2019;7:181.
Saito S, Kadota T, Gochi M, Takagi M, Kuwano K. Re-administration of pembrolizumab with prednisolone after pembrolizumab-induced nephrotic syndrome. Eur J Cancer. 2020;126:74–7.
Murakami N, Motwani S, Riella LV. Renal complications of immune checkpoint blockade. Curr Probl Cancer. 2017;41:100–10.
Eggermont AMM, Kicinski M, Blank CU, Mandala M, Long GV, Atkinson V, et al. Association between Immune-related adverse events and recurrence-free survival among patients with stage III Melanoma Randomized to Receive Pembrolizumab or Placebo: a secondary analysis of a Randomized Clinical Trial. JAMA Oncol. 2020;6:519–27.
Maillet D, Corbaux P, Stelmes JJ, Dalle S, Locatelli-Sanchez M, Perier-Muzet M, et al. Association between immune-related adverse events and long-term survival outcomes in patients treated with immune checkpoint inhibitors. Eur J Cancer. 2020;132:61–70.
Bakhriansyah M, Souverein PC, van den Hoogen MWF, de Boer A, Klungel OH. Risk of nephrotic syndrome for non-steroidal anti-inflammatory drug users. Clin J Am Soc Nephrol. 2019;14:1355–62.
Audard V, Larousserie F, Grimbert P, Abtahi M, Sotto J-J, Delmer A, et al. Minimal change nephrotic syndrome and classical Hodgkin’s lymphoma: report of 21 cases and review of the literature. Kidney Int. 2006;69:2251–60.
Fadel F, El Karoui K, Knebelmann B. Anti-CTLA4 antibody-induced lupus nephritis. N Engl J Med. 2009;361:211–2.
Daanen RA, Maas RJH, Koornstra RHT, Steenbergen EJ, van Herpen CML, Willemsen AECAB. Nivolumab-associated nephrotic syndrome in a patient with renal cell carcinoma: a Case Report. J Immunother. 2017;40:345–8.
Mamlouk O, Selamet U, Machado S, Abdelrahim M, Glass WF, Tchakarov A, et al. Nephrotoxicity of immune checkpoint inhibitors beyond tubulointerstitial nephritis: single-center experience. J Immunother Cancer. 2019;7:2.
Gallan AJ, Alexander E, Reid P, Kutuby F, Chang A, Henriksen KJ. Renal vasculitis and pauci-immune Glomerulonephritis Associated with Immune Checkpoint inhibitors. Am J Kidney Dis. 2019;74:853–6.
Ishibuchi K, Iwakura T, Kaneko M, Fukasawa H, Furuya R. Pembrolizumab-associated nephrotic syndrome recovered from transient hemodialysis in a patient with lung cancer. CEN Case Rep. 2020;9:215–9.
Chen M, Zhang L, Zhong W, Zheng K, Ye W, Wang M. Case Report: THSD7A-Positive Membranous Nephropathy caused by Tislelizumab in a Lung Cancer patient. Front Immunol. 2021;12:619147.
Kim DW, Jeon H, Kim S, Lee W, Kim HJ, Rhee H, et al. Pembrolizumab-induced focal segmental glomerulosclerosis: a case report. Medicine. 2021;100:e27546.
Wakabayashi K, Yamamoto S, Hara S, Okawara M, Teramoto K, Ikeda N, et al. Nivolumab-induced membranous nephropathy in a patient with stage IV lung adenocarcinoma. CEN Case Rep. 2022;11:171–6.
Meyrier A, Niaudet P. Acute kidney injury complicating nephrotic syndrome of minimal change disease. Kidney Int. 2018;94:861–9.
Praga M, Andres A, Hernandez E, Montoyo C, Mazuecos A, Campo C, et al. Tubular dysfunction in nephrotic syndrome: incidence and prognostic implications. Nephrol Dial Transpl. 1991;6:683–8.
Wang DY, Salem J-E, Cohen JV, Chandra S, Menzer C, Ye F, et al. Fatal toxic effects Associated with Immune Checkpoint inhibitors: a systematic review and Meta-analysis. JAMA Oncol. 2018;4:1721–8.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
RS, KK, HS, AF, KT, MY, FT, DT, KO, YS, and TM acquired the clinical data. RS, KK, and KD analyzed the patients’ clinical course and interpreted the data. RS and KK wrote a draft of the manuscript, and KO, TM, and KD critically revised the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for the publication of this case report. A copy of the written consent form is available for review by the editor of this journal.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Saiki, R., Katayama, K., Saiki, H. et al. Nephrotic syndrome with acute kidney injury due to combination therapy of immune checkpoint inhibitors: a case report and review of the literature. BMC Nephrol 25, 51 (2024). https://doi.org/10.1186/s12882-024-03494-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-024-03494-1