Abstract
Background
People with chronic kidney disease (CKD) treated with dialysis are frequently affected by depression. Psychotherapy has been reported to decrease depressive symptoms in various chronic diseases and is a potential treatment option for depression. We aimed to perform a systematic review and meta-analysis to evaluate the effect of psychotherapy on depression in adults with CKD.
Methods
We searched MEDLINE, Embase, Web of Science, and Cochrane for published studies up to October 31, 2023. Two investigators independently reviewed the included studies and extracted relevant data. Randomized controlled trials (RCTs) assessing the impact of interventions that provide psychological, emotional, or social support without the use of pharmacological substances on depressive symptoms in people with CKD were included and summarized. Scores on different tools for depressive assessment and quality of life were pooled.
Results
A total of 19 RCTs published between 2004 and 2023 were included and analyzed. The weighted mean difference (WMD) for all included studies with regard to depression was − 2.32 (95%CI=-3.83, -0.80, P = 0.003). The WMD for Beck Depression Inventory (BDI) score of depression was − 3.27 (95%CI=-7.81, 1.27, P = 0.158) with significant heterogeneity (I2 = 95.1%). Significant WMD was detected for the Hospital Anxiety and Depression Scale (HADS) tool: WMD=-1.90, 95%CI=-2.91, -0.90, P < 0.001. The WMD for all included studies regarding quality of life was 1.21 (95%CI=-0.51, 2.93, P = 0.168). The WMD for Kidney Disease Quality of Life Short Form (KDQOL-SF) score was 4.55 (95%CI = 0.50, 8.60, P = 0.028). The WMD for SF-36 score was 0.02 (95%CI=-10.33, 10.36, P = 0.998). Significant difference on outcomes of S-PRT scale was observed (WMD = 2.42, 95%CI = 1.07, 3.76, P < 0.001).
Conclusions
Psychosocial interventions probably reduce the depression level among CKD patients. Preliminary evidence suggests that psychosocial interventions might be beneficial for the quality of life in CKD patients. Our results provide medical facilities with an evidence-based basis for establishing psychosocial interventions in kidney care settings.
Similar content being viewed by others
Background
Chronic kidney disease (CKD) is diagnosed in the presence of an estimated glomerular filtration rate of < 60 mL/min/1.73 m2 and/or elevated markers of kidney damage for at least 3 months [1]. The worldwide prevalence of CKD was as high as 9.1% in 2017 [2], and the health impacts of CKD are substantial in terms of mortality and morbidity [3]. With a high burden of somatic symptoms of CKD, impaired quality of life, and role impairment, CKD patients who require dialysis treatment were found to be in severe psychological distress [4, 5]. According to a national survey of United States (US) adults, the 12-month prevalence of depressive disorder was 10.4% [6]. Previous studies have shown that depression affects an estimated 300 million people and represents a leading cause of health-related disabilities [7]. Due to the superimposed effect of CKD, the influence of depression might be more severe among CKD patients compared with general populations. A previous meta-analysis found that 19% of CKD patients had anxiety disorders and 43% had anxiety symptoms, [8] and the literature has reported wide variability in the reported prevalence of depression in CKD patients [9, 10]. A previous systematic review and meta-analysis of observational studies showed that the summarized prevalence of depressive symptoms in patients with CKD (Stage 1–5) and transplant recipients were 26.5% and 26.6%, respectively [11].
Psychosocial interventions can be defined as interventions that provide psychological, emotional, or social support without using pharmacological substances. Psychosocial interventions may help reduce distressing symptoms, increase coping strategies, increase social connectedness, assist in strategies to address specific disease-related problems, and decrease anxiety and stress [12]. However, there is currently no uniform treatment standard and way for psychotherapy. The intensity or method, and the level of contact with individual therapists or support workers may vary. Psychosocial interventions may be especially appropriate for patients with CKD, since they avoid potential drug interactions and adverse effects of antidepressant medication. Given that depressive symptoms are associated with reduced treatment adherence, impaired functional capacity, and higher rates of hospitalization [13], several randomized controlled trials (RCTs) have been conducted to explore the association between psychosocial interventions for depression and CKD adults [14, 15].
However, the synthesis effect of comprehensive psychotherapy among CKD patients is still unclear and it is debatable whether the psychosocial interventions actually reduce the level of depression. Therefore, the meta-analysis focusing on different psychological interventions including cognitive and behavioral therapies, exercise training, or counselling is needed. In our study, we aimed to collect evidence from RCTs and investigate the effects of psychotherapy (such as cognitive and behavioral therapies, exercise training, and counselling) for depression in adults with CKD.
Methods
Search strategy
This systematic review and meta-analysis were performed and reported in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) checklist [16]. PubMed, Embase, Web of Science, and Cochrane Central Register of Controlled Trials were used for potential studies up to October 31, 2023. A combination of Medical Subject Headings (MeSH) and text terms without language restriction were used: “kidney disease”, “renal disease”, “disease, kidney”, “diseases, kidney”, “kidney diseases”, “renal insufficiency”, “renal insufficiencies”, “kidney insufficiency”, “insufficiency, kidney”, “kidney insufficiencies”, “depression”, “depressive symptoms”, “depressive symptom”, “symptom, depressive”, “emotional depression” and “depression, emotional”. The search strategy can be found in Table S1. Reference lists of review articles, relevant studies, and clinical practice guidelines were also conducted on Google Scholar website for the previous review papers. We confirm that all methods were performed in accordance with the relevant guidelines.
Inclusion and exclusion criteria
The inclusion were:
-
Population: CKD patients aged 18 years or older with (clinical) depression;
-
Intervention: psychosocial interventions (such as cognitive and behavioral therapies, exercise training, counselling and other non-pharmacological treatments) were used and assessed;
-
Control: standard care or usual treatment;
-
Outcome: degree of depression or quality of life;
-
Study design: RCTs.
Studies were excluded if they were:
-
Studies evaluating treatment for other psychiatric disorders, including bipolar affective disorder; necessary data cannot be obtained after contacting the authors of relevant studies and requesting the sharing of unpublished data; duplicate publications (summarize all studies into one ‘record’ in the review); studies published not in English.
Study selection
Two authors independently reviewed study titles and abstracts. Both authors obtained and reviewed full-text articles of studies that were considered to be potentially relevant in order to determine their eligibility. All disagreements during study screening were resolved by a third author. Reasons for exclusion of full texts were collected.
Data extraction
Data extraction was conducted independently by the two authors using a standardized data extraction form using Microsoft Excel (Microsoft Corp.). The type of study design, setting, country, time frame, duration of follow-up, number of participants in each group, inclusion criteria, exclusion criteria, age, sex, details of intervention, and outcomes were collected.
Risk-of-bias and quality of studies assessment
The risk of bias and quality of the included studies were assessed with the revised Cochrane risk-of-bias tool [17]. This is a 5-domain tool including the assessment of bias arising from the randomisation process, bias due to deviations from intended interventions, bias due to deviations from intended interventions, bias in measurement of the outcome, and bias in selection of the reported result; “low concerns”, “high concerns” or “some concerns” was rated for each item in each domain, based on which overall predicted direction of bias was made for each study [17].
Statistical analysis
An overall meta-analysis was performed if there were two or more estimates, irrespective of study design. The weighted mean difference (WMD) of scores assessed before and after intervention from each study was calculated and a random-effect model were used to combine the MDs [18]. Heterogeneity among the outcomes of included studies in this meta-analysis was evaluated using Higgins’s I2. Generally, I2 > 50% could be considered as substantial heterogeneity [19]. Statistical analyses were performed using Stata 15.0 (Stata Corporation, TX, USA).
Publication bias assessment
Potential publication bias was evaluated using the Begg rank correlation [20] and Egger weighted regression methods [21]. The Begg and Egger tests were conducted with Stata 15.0. A two-side P-value of < 0.05 was considered to be significant for all analyses. The publication bias was also evaluated by funnel plots, which provide a useful graphical representation of the presence of bias in the meta-analysis. If the funnel plot is symmetrical, there is no publication bias. Otherwise, the presence of publication bias or other heterogeneity is considered.
Sensitivity analysis
We performed sensitivity analysis to explore the robustness of all pooled effect sizes.
Role of the funding source
The funders of the individuals working on the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Literature search and characteristics of the selected studies
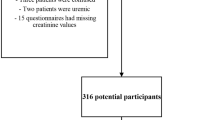
As shown in Fig. 1, from the combination of four database searches, our initial search identified 902 records. After removing duplicates, 808 records remained. We then reviewed the titles, abstracts and full texts, 789 irrelevant articles were excluded. Ultimately, a total of 19 studies [14, 15, 22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38] were finally included in this meta-analysis.
Study characteristics
The characteristics of the included studies are summarized in Table 1. All studies were RCTs. The studies were published between 2004 and 2023. The participants were from USA (n = 4), UK (n = 2), Brazil (n = 2), China (n = 5), Australia (n = 2), Iran (n = 2), Jordan (n = 1), and Singapore (n = 1). A majority of the participants were hemodialysis patients (9 studies) and comparison group accepted patients-usual care. The sample size ranged from 56 to 124. Table 2 shows tools used to assess depression and quality of life. A series of tools were used to assess depression and quality of life, including Hospital Anxiety and Depression Scale (HADS), Kidney Disease Quality of Life Short Form (KDQOL), the 36/12-item Short Form Health Survey (SF‐36/12), the physical component summary (PCS), and item Self-Perception Relationship Tool (S-PRT-28), et al. The HADS [39] is a self-rating scale consisting of two subscales, HADS-A and HADS-D, with A total of 14 items, of which 7 items rate anxiety (A) and 7 items rate depression (D). Each item was scored on a 4-point scale according to the frequency of symptoms in the last month. Each item was scored from 0 to 3, and the higher the score, the more severe the anxiety or depression symptoms. It is a very brief, easy-to-use screening measure to detect the presence of clinically significant symptoms of anxiety and is designed for use in medical populations [40]. The KDQOL [41] included two parts: kidney disease and dialysis-related quality of life and general health-related quality of life. The KDTA section consists of 43 items, each with a minimum score of 0 and a maximum score of 100, and the score in that domain is the average score of all items in the domain. The SF-36 section is divided into eight dimensions. The SF-36/12 includes eight dimensions, and the total score of the eight dimensions is the total score of the scale, which is more sensitive to change and is often used to assess the effect of treatment. The PCS [42] is derived from a selection of responses to yield a single score between 0 and 100, with a mean of 50 and standard deviation (SD) of 10 in population studies, which reflects “physical health.” The PCS has robust psychometric properties, and has been used in a large number of trials in many different conditions.
Risk of bias and quality of studies
The overall risk of bias and the quality of the included RCTs were acceptable (Fig. 2). Most studies used a randomized and blinded method for including the participants. Risk of bias assessment of included studies was shown in Figure S1, and the GRADE level of evidence was presented in Table S2.
Outcome of depression
A total of 19 studies assessed baseline and follow-up depression scores. The WMD for all included studies was − 2.32 (95%CI=-3.83, -0.80, P = 0.003) (Fig. 3). The WMD for BDI score of depression was − 3.27 (95%CI=-7.81, 1.27, P = 0.158) with significant heterogeneity (I2 = 95.1%) (Figure S2). Significant WMD was detected for the HADS scale: WMD=-1.90, 95%CI=-2.91, -0.90, P < 0.001 (Figure S2). The WMD for HSCL and K10 were − 4.40 (95%CI=-11.49, 2.69, P = 0.224) and − 0.50 (95%CI=-1.27, 0.27, P = 0.205), respectively (Figure S2).
Outcome of quality of life
Sixteen included studies reported results on the assessment of quality of life at baseline and follow-up. The WMD for all included studies was 1.21 (95%CI=-0.51, 2.93, P = 0.168) (Fig. 4). The WMD for KDQOL-SF score was 4.55 (95%CI = 0.50, 8.60, P = 0.028) (Figure S3). The WMD for SF-36 score was 0.02 (95%CI=-10.33, 10.36, P = 0.998) (Figure S3). Significant difference on outcomes of S-PRT scale was observed (WMD = 2.42, 95%CI = 1.07, 3.76, P < 0.001) (Figure S3). There was only one study reported outcomes for each scale including KDQOL-KDCS, PHQ-9, QoL, and SF-12, the respective WMD were 8.00 (95%CI = 0.84, 15.16, P = 0.028), 0 (95%CI=-0.44, 0.44, P = 1.000), 0.02 (95%CI=-10.33, 10.36, P = 0.998), -2.93 (95%CI=-4.35, -1.51, P < 0.001), and 3.60 (95%CI = 0.41, 6.79, P = 0.027) (Figure S3).
Publication bias
Because only 2 studies were included in the analysis of SPRT, Egger’s test could not performed. There was no statistically significant publication bias according to both Begg’s test (P > 0.05) and Egger’s test (P > 0.05) in the analyses of other parameters (Figures S4-S8) (Table S3). Nevertheless, funnel plots for pooled results of included studies on changes of BDI, KDQOL-SF, and SF-36 are graphically asymmetrical, indicating potential bias in the included studies despite of the insignificant results on Begg’s test and Egger’s test.
Sensitivity analysis
We conducted a sensitive analysis to assess the dependability and coherence of the final outcome. Our goal was to test the robustness of our conclusions by systematically omitting one study at a time and re-evaluating the combined effect size of the remaining dataset. This meticulous sensitivity assessment method confirmed that the overall results remained stable even with the removal of any specific study. Therefore, it is evident that no single study had a disproportionate impact on the overall results (Figures S9-S13).
Discussion
In our current study, we systematically reviewed and summarized the effect of psychotherapy on depression in adults with CKD. In our study, by pooling 19 articles, psychotherapy could effectively remit depression for CKD and significantly improve the quality of life of people with CKD.
Depression is the most common psychological problem in patients undergoing dialysis and about 25% of dialysis patients were found major depression [11, 45, 46], which may lead to lower adherence to dialysis prescriptions, lower health-related quality of life, and even poorer clinical outcomes [47,48,49,50]. Depression was reported to be caused by medications, reduction of physical function, or restriction of daily dietary [51]. Without an optimal screening tool for the assessment of depression, several tools including the Beck Depression Inventory (BDI), Patient Health Questionnaire, and Center for Epidemiologic Studies Depression Scale are widely used for the measurement of depression in CKD people.
Psychosocial interventions, which include counselling, social group support, cognitive-behavioral therapy, relaxation or visualization techniques, exercise, education, or individual social support including by telephone, are defined as interventions that can provide psychological, emotional, or social support without using pharmacological substances and have been demonstrated to be effective for people with depression [52]. A previous meta-analysis of the effects of psychosocial interventions on social functioning in depression and schizophrenia among general population found that psychosocial interventions delivered in outpatients and primary care settings are effective in improving social functioning in people with depression and should be incorporated into efforts to scale up services [52]. In our study, we also found a positive effect of psychosocial interventions on CKD. Of note, in all included studies ot the current meta-analysis, patients in the control group (standard care or usual treatment) also received treatment, the baseline treatment for intervention group and control were equal except for the psychosocial approaches used in the group. Subsequently, results of this study highlighted the significance of specialized psycosocial interventions and certified mental health professionals in the CKD treating team. Very few studies have assessed the effect of psychosocial interventions on people with CKD and few meta‐analyses have been published before. Our findings are consistent with the findings of previous meta‐analysis of published RCTs evaluating psychosocial interventions for anxiety symptoms in individuals with CKD [53]. Our study highlighted what constitutes a psychosocial intervention on CKD, of which, “psychosocial” was widely used to describe the interventions as behavioral, educational, psychological, or social. At the same time, complementary therapies such as physical and mental intervention also play an important role in patients with CKD and dialysis. Chu et al. found that music and psychotherapy reduced anxiety symptoms by 8.06–43.5% and 36.1–41.1%, respectively, and psychotherapy reduced depressive symptoms by 56.8% [53]. While we also found there was significant heterogeneity across the included RCTs and the potential sources of the heterogeneity could be the type of interventions, follow-up duration, or baseline characteristics of CKD patients. These potential heterogeneity sources could not fully taken into account in our study owing to the limited number of studies in each subgroup under different assessment tools. Meanwhile, each included study had unique research background, such as the healthcare policy, cultural factors, and study country, which lead to an inevitable heterogeneity of our study.
Given the lack of consistency in how psychosocial interventions were defined, our findings suggest that psychosocial interventions should be defined in the context of both psychological and social components. However, due to the time requirements of dialysis for CKD cases and the feeling of fatigue after dialysis, many patients may be reluctant to participate in a time-intensive psychosocial intervention. That would be a major concern that impedes the practice of psychosocial interventions. A previous meta-analysis [54] focusing on psychotherapies across general population in different age groups reported the small but statistically significant effect sizes of psychotherapies in general population. According to the results of our study, there were significant in decreasing the levels of depression and improvement in quality of life after psychotherapy intervention compared with baseline. However, the clinically relevant could be limited due to the WMDs were also small. In the clinical application process, the significant positive effect might not be observed straightly. Compared with conventional treatment, psychological intervention did have a positive effect on CKD patients, even if it was statistically significant. Future studies could carry out psychological interventions in CKD patients with different disease stages to discover that in the practical clinical applications the intervention was the most effective among which subgroups of CKD patients. In addition, it is not possible to definitively establish the impact of psychosocial interventions on major depression, anxiety, withdrawal from dialysis, or death from any cause. The potential adverse events of treatment are largely unknown. Feasible, effective, and acceptable interventions are needed and may be sustainable for implementation within clinical settings.
There were several limitations in our study. First, several important individual pieces of information were not provided, thus we couldn’t perform a more accurate analysis of clinical characteristics. Meanwhile, due to insufficient information, we cannot deploy more subgroup analyses, especially on medication use, more relevant RCTs are warranted to investigate the impact of medication on the effects of psychosocial interventions for depression in CKD adults. Second, although a comprehensive search strategy was independently performed by 2 investigators in 4 databases with cross reviewing, we cannot guarantee that all relevant studies have been included in our analysis. Third, the funnel plots for included studies on changes of BDI, KDQOL-SF, and SF-36 are indeed asymmetrical, but no publication bias between studies was shown based on the results of Begg’s test and Egger’s test, which suggest the limitations to these methods. The asymmetry of the funnel plot may be due to other source of heterogeneity across enrolled studies, which is proved by the high I2 values. Fourth, depressive outcomes and quality of life were assessed using various tools. Depression was deemed as a continuous outcome either as major (or severe) depression or depression (end of treatment) and the severity of depressive symptoms was assessed as a dichotomous outcome. Standardization of outcome reporting in future psychosocial intervention trials is therefore needed.
Conclusions
Our study suggests that psychosocial interventions are promising for improving psychological well-being in adults with CKD. Psychosocial interventions, such as cognitive and behavioral therapies, exercise training, and counselling, may reduce depression and improve quality of life in these patients. In future, researchers investigating psychosocial treatments should consider the use of standardized interventions.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Abbreviations
- RCTs:
-
Randomized controlled trials
- SD:
-
Standard deviation
- WMD:
-
Weighted mean difference
- CKD:
-
Chronic kidney disease
- MeSH:
-
Medical Subject Heading
- HADS:
-
Hospital Anxiety and Depression Scale
- KDQOL:
-
Kidney Disease Quality of Life Short Form
- BDI:
-
Beck Depression Inventory
References
Inker LA, Astor BC, Fox CH, Isakova T, Lash JP, Peralta CA, Kurella Tamura M, Feldman HI. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. 2014;63(5):713–35.
Saginala K, Barsouk A, Aluru JS, Rawla P, Padala SA, Barsouk A. Epidemiology of bladder cancer. Med Sci (Basel). 2020;8(1):15.
Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet. 2017;389(10075):1238–52.
Hamler TC, Miller VJ, Petrakovitz S. Chronic kidney disease and older African American adults: how embodiment influences self-management. Geriatr (Basel). 2018;3(3):52.
Tong A, Sainsbury P, Chadban S, Walker RG, Harris DC, Carter SM, Hall B, Hawley C, Craig JC. Patients’ experiences and perspectives of living with CKD. Am J Kidney Dis. 2009;53(4):689–700.
Hasin DS, Sarvet AL, Meyers JL, Saha TD, Ruan WJ, Stohl M, Grant BF. Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiat. 2018;75(4):336–46.
Giannakopoulou O, Lin K, Meng X, Su M-H, Kuo P-H, Peterson RE, Awasthi S, Moscati A, Coleman JRI, Bass N, et al. The genetic architecture of depression in individuals of east Asian ancestry: a genome-wide association study. JAMA Psychiat. 2021;78(11):1258–69.
Huang CW, Wee PH, Low LL, Koong YLA, Htay H, Fan Q, Foo WYM, Seng JJB. Prevalence and risk factors for elevated anxiety symptoms and anxiety disorders in chronic kidney disease: a systematic review and meta-analysis. Gen Hosp Psychiatry. 2021;69:27–40.
Alsuwaida A, Alwahhabi F. The diagnostic utility of self-reporting questionnaire (SRQ) as a screening tool for major depression in hemodialysis patients. Saudi J Kidney Dis Transpl. 2006;17(4):503–10.
Arapaslan B, Soykan A, Soykan C, Kumbasar H. Cross-sectional assessment of psychiatric disorders in renal transplantation patients in Turkey: a preliminary study. Transpl Proc. 2004;36(5):1419–21.
Palmer S, Vecchio M, Craig JC, Tonelli M, Johnson DW, Nicolucci A, Pellegrini F, Saglimbene V, Logroscino G, Fishbane S, et al. Prevalence of depression in chronic kidney disease: systematic review and meta-analysis of observational studies. Kidney Int. 2013;84(1):179–91.
Garcia-Llana H, Remor E, Del Peso G, Selgas R. The role of depression, anxiety, stress and adherence to treatment in dialysis patients health-related quality of life: a systematic review of the literature. Nefrologia. 2014;34(5):637–57.
Hedayati SS, Minhajuddin AT, Afshar M, Toto RD, Trivedi MH, Rush AJ. Association between major depressive episodes in patients with chronic kidney disease and initiation of dialysis, hospitalization, or death. JAMA. 2010;303(19):1946–53.
Morais EM, Moreira PR, Winkelmann ER. Movie watching during dialysis sessions reduces depression and anxiety and improves quality of life: a randomized clinical trial. Complement Ther Med. 2020;52:102488.
Rodrigue JR, Mandelbrot DA, Pavlakis M. A psychological intervention to improve quality of life and reduce psychological distress in adults awaiting kidney transplantation. Nephrol Dial Transplant. 2011;26(2):709–15.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (Clin Res ed). 2021;372:n71.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (Clin Res ed). 2019;366:l4898.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clin Res ed). 2003;327(7414):557–60.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34.
Chan KY, Yip T, Yap DY, Sham MK, Wong YC, Lau VW, Li CW, Cheng BH, Lo WK, Chan TM. Enhanced psychosocial support for caregiver burden for patients with chronic kidney failure choosing not to be treated by dialysis or transplantation: a pilot randomized controlled trial. Am J Kidney Dis. 2016;67(4):585–92.
Cukor D, Coplan J, Brown C, Friedman S, Newville H, Safier M, Spielman LA, Peterson RA, Kimmel PL. Anxiety disorders in adults treated by hemodialysis: a single-center study. Am J Kidney Dis. 2008;52(1):128–36.
Dingwall KM, Sweet M, Cass A, Hughes JT, Kavanagh D, Howard K, Barzi F, Brown S, Sajiv C, Majoni SW, et al. Effectiveness of wellbeing intervention for chronic kidney disease (WICKD): results of a randomised controlled trial. BMC Nephrol. 2021;22(1):136.
Duarte PS, Miyazaki MC, Blay SL, Sesso R. Cognitive-behavioral group therapy is an effective treatment for major depression in hemodialysis patients. Kidney Int. 2009;76(4):414–21.
Hare J, Clark-Carter D, Forshaw M. A randomized controlled trial to evaluate the effectiveness of a cognitive behavioural group approach to improve patient adherence to peritoneal dialysis fluid restrictions: a pilot study. Nephrol Dial Transplant. 2014;29(3):555–64.
Jenkins ZM, Tan EJ, O’Flaherty E, Knowles S, Thompson DR, Ski CF, Rossell SL, Coco C, Ierino FL, Gock H, et al. A psychosocial intervention for individuals with advanced chronic kidney disease: a feasibility randomized controlled trial. Nephrol (Carlton). 2021;26(5):442–53.
Moattari M, Ebrahimi M, Sharifi N, Rouzbeh J. The effect of empowerment on the self-efficacy, quality of life and clinical and laboratory indicators of patients treated with hemodialysis: a randomized controlled trial. Health Qual Life Outcomes. 2012;10:115.
Sharp J, Wild MR, Gumley AI, Deighan CJ. A cognitive behavioral group approach to enhance adherence to hemodialysis fluid restrictions: a randomized controlled trial. Am J Kidney Dis. 2005;45(6):1046–57.
Song MK, Ward SE, Happ MB, Piraino B, Donovan HS, Shields AM, Connolly MC. Randomized controlled trial of SPIRIT: an effective approach to preparing African-American dialysis patients and families for end of life. Res Nurs Health. 2009;32(3):260–73.
Al Saraireh FA, Aloush SM, Al Azzam M, Al Bashtawy M. The effectiveness of cognitive behavioral therapy versus psychoeducation in the management of depression among patients undergoing haemodialysis. Issues Ment Health Nurs. 2018;39(6):514–8.
Babamohamadi H, Sotodehasl N, Koenig HG, Al Zaben F, Jahani C, Ghorbani R. The effect of Holy Qur’an recitation on depressive symptoms in hemodialysis patients: a randomized clinical trial. J Relig Health. 2017;56(1):345–54.
Chen HC, Zhu L, Chan WS, Cheng KFK, Vathsala A, He HG. The effectiveness of a psychoeducational intervention on health outcomes of patients undergoing haemodialysis: a randomized controlled trial. Int J Nurs Pract. 2023;29(3):e13123.
Látos M, Lázár G, Ondrik Z, Szederkényi E, Hódi Z, Horváth Z, Csabai M. Positive psychology intervention to improve recovery after renal transplantation: a randomized controlled trial. J Contemp Psychother. 2022;52(1):35–44.
Mehrotra R, Cukor D, Unruh M, Rue T, Heagerty P, Cohen SD, Dember LM, Diaz-Linhart Y, Dubovsky A, Greene T, et al. Comparative efficacy of therapies for treatment of depression for patients undergoing maintenance hemodialysis: a randomized clinical trial. Ann Intern Med. 2019;170(6):369–79.
Tsai SH, Wang MY, Miao NF, Chian PC, Chen TH, Tsai PS. The efficacy of a nurse-led breathing training program in reducing depressive symptoms in patients on hemodialysis: a randomized controlled trial. Am J Nurs. 2015;115(4):24–32. quiz 33, 42.
Tsay SL, Hung LO. Empowerment of patients with end-stage renal disease–a randomized controlled trial. Int J Nurs Stud. 2004;41(1):59–65.
Tsay SL, Lee YC, Lee YC. Effects of an adaptation training programme for patients with end-stage renal disease. J Adv Nurs. 2005;50(1):39–46.
Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–70.
Julian LJ. Measures of anxiety: state-trait anxiety inventory (STAI), Beck anxiety inventory (BAI), and hospital anxiety and depression scale-anxiety (HADS-A). Arthritis Care Res (Hoboken). 2011;63(Suppl 11):467-S472.
Hays RD, Kallich JD, Mapes DL, Coons SJ, Carter WB. Development of the kidney disease quality of life (KDQOL) instrument. Qual Life Res. 1994;3(5):329–38.
Fu V, Weatherall M, McNaughton H. Estimating the minimal clinically important difference for the physical component summary of the short form 36 for patients with Stroke. J Int Med Res. 2021;49(12):3000605211067902.
Tang Q, Yang B, Fan F, Li P, Yang L, Guo Y. The impact of individualized exercise plans on the physical function, psychological dimensions, and health-related quality of life of patients with chronic kidney disease: A randomized controlled trial in China. Int J Nurs Pract. 2017;23(2). https://doi.org/10.1111/ijn.12519.
Valsaraj BP, Bhat SM, Latha KS. Cognitive behavioral therapy for anxiety and depression in hemodialysis patients: A randomized controlled trial. J Clin Diagn Res. 2016;10(8):VC06–10.
Palmer SC, Vecchio M, Craig JC, Tonelli M, Johnson DW, Nicolucci A, Pellegrini F, Saglimbene V, Logroscino G, Hedayati SS, et al. Association between depression and death in people with CKD: a meta-analysis of cohort studies. Am J Kidney Dis. 2013;62(3):493–505.
Kimmel PL, Weihs K, Peterson RA. Survival in hemodialysis patients: the role of depression. J Am Soc Nephrol. 1993;4(1):12–27.
Durose CL, Holdsworth M, Watson V, Przygrodzka F. Knowledge of dietary restrictions and the medical consequences of noncompliance by patients on hemodialysis are not predictive of dietary compliance. J Am Diet Assoc. 2004;104(1):35–41.
Kugler C, Maeding I, Russell CL. Non-adherence in patients on chronic hemodialysis: an international comparison study. J Nephrol. 2011;24(3):366–75.
Mellon L, Regan D, Curtis R. Factors influencing adherence among Irish haemodialysis patients. Patient Educ Couns. 2013;92(1):88–93.
Pang SK, Ip WY, Chang AM. Psychosocial correlates of fluid compliance among Chinese haemodialysis patients. J Adv Nurs. 2001;35(5):691–8.
Farrokhi F, Abedi N, Beyene J, Kurdyak P, Jassal SV. Association between depression and mortality in patients receiving long-term dialysis: a systematic review and meta-analysis. Am J Kidney Dis. 2014;63(4):623–35.
Shorey S, Ng ED, Wong CHJ. Global prevalence of depression and elevated depressive symptoms among adolescents: a systematic review and meta-analysis. Br J Clin Psychol. 2022;61(2):287–305.
Pascoe MC, Thompson DR, Castle DJ, McEvedy SM, Ski CF. Psychosocial interventions for depressive and anxiety symptoms in individuals with chronic kidney disease: systematic review and meta-analysis. Front Psychol. 2017;8:992.
Cuijpers P, Karyotaki E, Eckshtain D, Ng MY, Corteselli KA, Noma H, Quero S, Weisz JR. Psychotherapy for depression across different age groups: a systematic review and meta-analysis. JAMA Psychiat. 2020;77(7):694–702.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
HY: designed and made search strategy, reviewed included studies titles and abstracts, selected Studies, and analyzed the data.LQ: reviewed included studies titles and abstracts, and selected Studies. DMP: made a decision independently when an article disagreement was need on the inclusion of a study.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1:
Table S1. Search strategy of PubMed, Embase, Cochrane Library and Web of science.
Additional file 2:
Table S2. GRADE level of evidence.
Additional file 3:
Table S3. Publication bias of summarized outcomes.
Additional file 4: Figure S1.
Risk of bias assessment of included studies using the revised Cochrane risk of bias tool. Figure S2. Forest plot of subgroup analyses on changes of depression. Figure S3. Forest plot of changes of subgroup analyses on quality of life. Figure S4. Funnel plot for pooled results of included studies on changes of BDI. Figure S5. Funnel plot for pooled results of included studies on changes of HADS. Figure S6. Funnel plot for pooled results of included studies on changes of KDQOL-SF. Figure S7. Funnel plot for pooled results of included studies on changes of SF-36. Figure S8. Funnel plot for pooled results of included studies on changes of SPRT. Figure S9. Results of sensitivity analysis for pooled results of included studies on changes of BDI. Figure S10. Results of sensitivity analysis for pooled results of included studies on changes of HADS. Figure S11. Results of sensitivity analysis for pooled results of included studies on changes of KDQOL-SF. Figure S12. Results of sensitivity analysis for pooled results of included studies on changes of SF-36. Figure S13. Results of sensitivity analysis for pooled results of included studies on changes of SPRT.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, H., Qi, L. & Pei, D. Effect of psychosocial interventions for depression in adults with chronic kidney disease: a systematic review and meta-analysis. BMC Nephrol 25, 17 (2024). https://doi.org/10.1186/s12882-023-03447-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-023-03447-0