Abstract
Background
The number of chronic kidney disease (CKD) patients continues to increase worldwide. CKD patients need to take phosphate binders to manage serum phosphorus concentrations. Currently, several types of phosphate binder, including lanthanum carbonate, are used. However, they each have disadvantages.
Methods
In this study, we evaluated cerium oxide as a new phosphate binder in vitro and in vivo. First, cerium oxide was mixed with phosphoric acid at pH 2.5 or 7.0, and residual phosphoric acid was measured by absorption photometry using colorimetric reagent. Second, cerium oxide was fed to 5/6 nephrectomy model rats (5/6Nx), a well-known renal damage model. All rats were measured food intake, water intake, feces volume, and urine volume, and collected serum and urine were analyzed for biochemical markers.
Results
Cerium oxide can adsorb phosphate at acidic and neutral pH, while lanthanum carbonate, which is a one of popular phosphate binder, does not dissolve at neutral pH. Cerium oxide-treatment reduced serum phosphate concentrations of 5/6Nx rats without an increase in serum alanine transaminase levels that would indicate hepatotoxicity, and cerium oxide-treatment maintained serum creatinine and blood urea nitrogen levels, while those of normal 5/6Nx rats increased slightly.
Conclusions
These results suggest that cerium oxide can be a potential phosphate binder. Decreased body weight gain and increased water intake and urine volume in 5/6Nx rats were thought to be an effect of nephrectomy because these changes did not occur in sham operation rats. Additional investigations are needed to evaluate the longer-term safety and possible accumulation of cerium oxide in the body.
Similar content being viewed by others
Background
Chronic kidney disease (CKD) is a disease in which renal function declines, and the number of CKD patients continues to increase worldwide. Progression of CKD results in end stage kidney disease (ESKD), and ESKD patients need dialysis or renal transplantation [1]. In Japan, the number of CKD patients in 2005 was estimated at 13.3 million, accounting for 12.9% of the adult population [2]. The number of dialysis patients reached 347,671 in 2020 [3], and the increase of CKD and ESKD patients has become a serious problem.
Mineral excretion is reduced in CKD patients, and decreased phosphorus excretion leads to elevated serum phosphorus levels, resulting in hyperphosphatemia. Hyperphosphatemia in CKD patients is known to be closely associated with the development of cardiovascular disease [4]. In hyperphosphatemia patients, serum phosphorus and calcium are increased, resulting in an increase in calcium-phosphate products associated with vascular calcification [4,5,6]. To prevent cardiovascular events, hyperphosphatemia patients should eat minimal phosphorus-containing protein and take phosphate binders. One popular phosphate binder is lanthanum carbonate (Fosrenol®) [7,8,9,10,11,12,13], and the others are precipitated calcium carbonate [7, 9, 10, 12, 13], sevelamer hydrochloride [12,13,14,15,16,17], bixalomer [13, 16, 17], ferric citrate hydrate [13, 18, 19], and sucroferric oxyhydroxide [13, 20, 21].
Lanthanum carbonate ionizes in gastric acid, and the ions bind to phosphate ions. Lanthanum phosphate has very low solubility and, has been reported to precipitates in the feces. Consequently, phosphate is egested without systemic absorption, and serum phosphorus levels are decreased.
However, lanthanum carbonate has two disadvantages. First, lanthanum carbonate ionizes only at low pH. The influx of stomach contents after a meal makes the stomach pH more neutral. Therefore, in clinical use, lanthanum carbonate cannot fully exert its phosphate adsorption capacity. Second, it has been reported that lanthanum depositions are observed in patients treated with lanthanum carbonate [22,23,24,25]. The effects of these depositions on living organisms have not yet been elucidated, and there are reports that lanthanum depositions are associated with cancer or inflammation [23,24,25]. For these reasons, a new phosphate binder is needed.
In this study, we focused on cerium oxide, which is a lanthanoid compound. Cerium oxide is used as a phosphate adsorbent and has been studied as a treatment to avoid aluminum hydroxide toxicity [26, 27]. Shibamoto et al. found that cerium oxide can be adsorbed as an anion under pH 7 by ion exchange [26]. They evaluated the phosphate adsorption capacity of cerium oxide using hyperphosphatemic dogs and column-filled cerium oxide supported on porous polymers. However, they did not orally administer cerium oxide, but used a column for passing blood. Cerium oxide as an orally administered phosphate binder has been reported by Ogura et al. in 1986 [28]. There are few or no studies of cerium oxide as an orally administered phosphate binder. In this study, we examined the effect of cerium oxide as an orally administered phosphate binder using 5/6 nephrectomy model rats (5/6Nx).
Materials and methods
Animals
All animal experiments were approved by the Animal Protection and Ethics Committee of Shibaura Institute of Technology (approval number #20005, permission on Aug 25th, 2020), which conform to the current Japanese laws. The 5/6 nephrectomy and sham-operated male Wistar rats (six weeks old) were purchased from Japan SLC, Inc. (Shizuoka, Japan). They were housed at a temperature of 22 ± 2 °C with a 12-h light–dark cycle, and ad libitum access to food and water. They were experimented on after a week of habituation and given Labo MR Stock (Nosan Corp., Kanagawa, Japan) during habituation.
Materials
Lanthanum carbonate was purchased from NIKKI CORPORATION (Saitama, Japan). AIN-93G and AIN-93G (substituted with soy protein) were obtained from Funabasi Farm Co. Ltd., (Chiba, Japan). BIOMOL® Green Reagent was purchased from Enzo Life Sciences, Inc. (New York, USA). All other materials were obtained from commercially available sources unless otherwise stated.
Phosphate adsorption test
Cerium oxide dispersion liquid (average particle size: 4 nm, 0.1 g) was added to 1.5 mL tubes, and Milli-Q water was added to make a final weight of 1 g. This was used as a 10-fold diluted dispersion liquid. The following were added to new tubes: Milli-Q water (800, 760, 740, 720, 700 μL), 500 mM glycine–HCl buffer pH 2.5 (100 μL), 10-fold diluted dispersion liquid (0, 40, 60, 80, 100 μL), and approximately 30 mM phosphoric acid solution (100 μL), resulting a final volume of 1 mL. The mixed solutions were incubated (38 °C, 1 h) and centrifuged (10,000 rpm, 5 min, room temperature (R/T)). The supernatant fraction (80 μL) was added to Milli-Q (920 μL) and mixed completely, and a part of the solution (80 μL) was added to Milli-Q (880 μL) and mixed completely. To each well of a 96-well plate, the diluted solution (50 μL) and BIOMOL® Green Reagent (100 μL) were added and incubated (R/T, 25 min). The absorbance at 620 nm was measured using a UV/Vis microplate and cuvette spectrophotometer (Multiskan GO, Thermo Fisher Scientific, Inc.; Waltham, USA). Finally, we measured the concentration of phosphoric acid solution and calculated the phosphate adsorption capacity.
This experiment was also performed using 500 mM Bis–Tris HCl pH 7.0 to evaluate the phosphate adsorption capacity at neutral pH.
Animal experiments
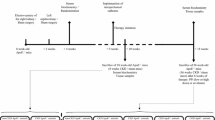
After an acclimatization period of one week, 5/6Nx or sham rats were divided by body weight into six (5/6Nx-s–n, 5/6Nx-s-La, 5/6Nx-s-Ce, 5/6Nx-c-n, 5/6Nx-c-La, or 5/6Nx-c-Ce) or two (Sham-s or Sham-c) groups (n = 6), respectively. During the experimental period, the rats were provided AIN-93G or AIN-93G (substituted with soy protein) as shown in Table 1. In Sham-s, Sham-c, 5/6Nx-s-n, and 5/6Nx-c-n, food was provided without the food additive. In 5/6Nx-s-La and 5/6Nx-c-La, food was provided with 0.5% lanthanum from lanthanum carbonate according to a previous report [29]. Additionally, we used 1.31% cerium oxide added to food in animal experiments due to its commensurate capacity compared to 0.5% lanthanum. In 5/6Nx-s-Ce and 5/6Nx-c-Ce, food was provided with 1.31% cerium oxide from the cerium oxide dispersion liquid. The group conditions and experimental design are indicated in Table 2 and Fig. 1, respectively. During the experimental period, the rats were placed in metabolic cages to measure food intake, water intake, feces volume, and urine volume, and urine was sampled weekly. Additionally, the rats were weighed and blood was collected from tail veins weekly. After four weeks, the rats were fasted for approximately 19 h, sacrificed, and blood was collected. The blood was stored at R/T for 1 h and centrifuged at 1400 × g for 20 min to separate serum. The serum and urine were stored at − 80 °C until biochemical analysis.
Biochemical analysis
The serum and urine were analyzed for biochemical markers including inorganic phosphate (IP), calcium (Ca), creatinine (CRE), urea nitrogen (BUN), aspartate transaminase (AST), alanine transaminase (ALT), total cholesterol (T-CHO), high density lipoprotein cholesterol (HDL-C), total protein (TP), albumin (ALB), albumin-globulin ratio (A/G), ureic acid (UA), sodium (Na), potassium (K), chlorine (Cl), amylase (AMY), triglyceride (TG), total bilirubin (T-BIL), total bile acid (TBA) and glucose (GLU) by an animal inspection service (Oriental Yeast Co., Ltd., Tokyo, Japan).
Statistical analysis
Data were expressed as means ± SEM and analyzed with GraphPad Prism Version 9.2.0 for Windows 64-bit (GraphPad Software Inc., San Diego, USA). Comparisons between the eight groups were performed by the Tukey–Kramer method.
Results
Cerium oxide absorbs phosphate in a concentration-dependent manner
The phosphate adsorption capacity of cerium oxide and lanthanum carbonate was evaluated using a colorimetric reagent (Figs. 2 and 3). Cerium oxide adsorbed phosphate in a concentration-dependent manner at pH 2.5 and 7.0. The phosphate adsorption capacity of cerium oxide at pH 2.5 was stronger than that at pH 7.0. The amount of cerium oxide necessary to adsorb phosphate at pH 7.0 was 1.4-fold that at pH 2.5. However, the decrease in phosphate adsorption capacity caused by neutral pH was not large. Lanthanum carbonate adsorbed phosphate in a concentration-dependent manner at pH 2.5, and could not be evaluated at pH 7.0 due to its poor solubility.
Cerium oxide did not reduce the significant increase in water intake and urine volume in 5/6Nx rats
The body weight, water intake, and urine volume of each rat group are shown in Fig. 4. The body weight of the 5/6Nx rats was nominally (but not significantly) lower than that of the sham rats. The body weights of lanthanum carbonate- and cerium oxide-treated 5/6Nx rats were nominally (but not significantly) lower than those of the sham rats in the presence or absence of casein in the food. There was no difference in the amount of food intake by each group. The water intake and urine volumes were significantly increased in the 5/6Nx rats compared to those of the sham rats. The both volumes of cerium oxide-treated 5/6Nx rats (5/6Nx-s-Ce and 5/6Nx-c-Ce) were nominally (but not significantly) increased compared to those of the other 5/6Nx rats. However, a significant increase in the urine volume in the 5/6Nx-s-Ce group compared to the 5/6Nx-s–n or 5/6Nx-s-La groups was only seen at one and two weeks.
Differences in body weight, water intake, and urine volume between sham model rats and 5/6 nephrectomy model rats (5/6Nx) at each week. Sham rats and three different treated (normal, lanthanum carbonate, and cerium oxide) 5/6Nx rats fed soy protein or casein were used (Sham-s, n = 6; 5/6Nx-s–n, n = 6; 5/6Nx-s-La, n = 6; 5/6Nx-s-Ce, n = 6; Sham-c, c = 6; 5/6Nx-c-n, n = 6; 5/6Nx-c-La, n = 6; 5/6Nx-c-Ce, n = 6). The data are shown as means ± SD and were analyzed using the Tukey–Kramer method. *p < 0.05 and **p < 0.01 compared to food-matched sham rats (Sham-s or Sham-c). †p < 0.05 compared to food-matched normal 5/6Nx rats (5/6Nx-s–n or 5/6Nx-c-n). ‡p < 0.05 compared to food-matched lanthanum carbonate-treated 5/6Nx rats (5/6Nx-s-La or 5/6Nx-c-La)
Cerium oxide significantly reduced serum IP
Figure 5 shows the serum IP and Ca levels before and after each diet was applied for up to three weeks. The IP levels of lanthanum carbonate- and cerium oxide-treated 5/6Nx rats (5/6Nx-s-La, 5/6Nx-s-Ce, 5/6Nx-c-La, and 5/6Nx-c-Ce) were significantly lower than those of each normal diet group (Sham-s, 5/6Nx-s–n, Sham-c, and 5/6Nx-c-n). Serum Ca levels of lanthanum carbonate- or cerium oxide-treated 5/6Nx rats (5/6Nx-s-La, 5/6Nx-s-Ce, 5/6Nx-c-La, and 5/6Nx-c-Ce) were significantly higher than those of each normal diet group (Sham-s, 5/6Nx-s–n, Sham-c, and 5/6Nx-c-n). The increase of serum Ca levels occurred in parallel with the serum IP decrease.
Differences in serum IP and Ca levels at each week. Sham rats and three different treated (normal, lanthanum carbonate, and cerium oxide) 5/6Nx rats fed soy protein or casein were used (Sham-s, n = 6; 5/6Nx-s–n, n = 6; 5/6Nx-s-La, n = 6; 5/6Nx-s-Ce, n = 6; Sham-c, c = 6; 5/6Nx-c-n, n = 6; 5/6Nx-c-La, n = 6; 5/6Nx-c-Ce, n = 6). The data are shown as means ± SD and were analyzed using the Tukey–Kramer method. *p < 0.05 and **p < 0.01 compared to food-matched sham rats (Sham-s or Sham-c). †p < 0.05 and ††p < 0.01 compared to food-matched normal 5/6Nx rats (5/6Nx-s–n or 5/6Nx-c-n)
Figure 6 shows the urinary IP (U-IP) and Ca (U-Ca) levels before and after each diet was applied up to four weeks. The U-IP levels of lanthanum carbonate- or cerium oxide-treated 5/6Nx rats (5/6Nx-s-La, 5/6Nx-s-Ce, 5/6Nx-c-La, and 5/6Nx-c-Ce) were approximately 0 mg/day from one to four weeks. The U-Ca levels of the same four groups were significantly increased compared to those of each normal diet group (Sham-s, 5/6Nx-s–n, Sham-c, and 5/6Nx-c-n). These alterations in U-IP and U-Ca occurred in parallel with alterations of IP and Ca, respectively.
Differences in urine IP and Ca levels at each week. Sham rats and three different treated (normal, lanthanum carbonate, and cerium oxide) 5/6Nx rats fed soy protein or casein were used (Sham-s, n = 6; 5/6Nx-s–n, n = 6; 5/6Nx-s-La, n = 6; 5/6Nx-s-Ce, n = 6; Sham-c, c = 6; 5/6Nx-c-n, n = 6; 5/6Nx-c-La, n = 6; 5/6Nx-c-Ce, n = 6). The data are shown as means ± SD and were analyzed using the Tukey–Kramer method. *p < 0.05 and **p < 0.01 compared to food-matched sham rats (Sham-s or Sham-c). †p < 0.05 and ††p < 0.01 compared to food-matched normal 5/6Nx rats (5/6Nx-s–n or 5/6Nx-c-n). ‡p < 0.05 and ‡‡p < 0.01 compared to food-matched lanthanum carbonate-treated 5/6Nx rats (5/6Nx-s-La or 5/6Nx-c-La)
Figure 7 shows the serum CRE and BUN levels before and after each diet was applied up to three weeks. The CRE and BUN levels of the 5/6Nx rats were significantly higher than those of the sham rats. The CRE and BUN levels of these two groups (5/6Nx-s–n and 5/6Nx-s-La) were slightly increased. However, the CRE level of 5/6Nx-s-Ce did not change before the start of feeding the cerium oxide diet. The CRE and BUN levels of casein-fed cerium oxide-treated rats (5/6Nx-c-Ce) at the period from zero to two weeks were nominally (but not significantly) high compared to all other groups in the presence of casein in the food. However, their CRE and BUN levels were lower than those of casein-fed normal or lanthanum carbonate-treated rats (5/6Nx-c-n and 5/6Nx-c-La) at three weeks.
Differences in serum CRE and BUN levels at each week. Sham rats and three different treated (normal, lanthanum carbonate, and cerium oxide) 5/6Nx rats fed soy protein or casein were used (Sham-s, n = 6; 5/6Nx-s–n, n = 6; 5/6Nx-s-La, n = 6; 5/6Nx-s-Ce, n = 6; Sham-c, c = 6; 5/6Nx-c-n, n = 6; 5/6Nx-c-La, n = 6; 5/6Nx-c-Ce, n = 6). The data are shown as means ± SD and were analyzed using the Tukey–Kramer method. *p < 0.05 and **p < 0.01 compared to food-matched sham rats (Sham-s or Sham-c). †p < 0.05 compared to food-matched normal 5/6Nx rats (5/6Nx-s–n or 5/6Nx-c-n)
After the feeding period, all rats were dissected to isolate sera, and IP, Ca, CRE, and BUN levels were measured (Fig. 8). The serum IP levels of casein-fed normal or soy protein-fed lanthanum carbonate-treated 5/6Nx rats (5/6Nx-s–n and 5/6Nx-c-La) were significantly higher than those of the Sham-s and Sham-c groups, respectively. The serum Ca levels were not significantly different among any group. The CRE level of 5/6Nx rats was significantly higher than that of the sham rats. The CRE level of soy protein-fed cerium oxide-treated 5/6Nx rats (5/6Nx-s-Ce) was significantly lower than that of the 5/6Nx-s–n rats. The BUN levels of soy protein-fed normal or lanthanum carbonate-treated 5/6Nx rats (5/6Nx-s–n and 5/6Nx-s-La) were significantly higher than that of the Sham-s rats. The BUN levels of casein-fed 5/6Nx rats (5/6Nx-c-n, 5/6Nx-c-La, and 5/6Nx-c-Ce) were significantly higher than those of the Sham-c rats, and the BUN level of the 5/6Nx-c-Ce group was significantly lower than that of 5/6Nx-c-La.
Differences in serum IP, Ca, CRE and BUN levels upon dissection. Sham rats and three different treated (normal, lanthanum carbonate, and cerium oxide) 5/6Nx rats fed soy protein or casein were used (Sham-s, n = 6; 5/6Nx-s–n, n = 6; 5/6Nx-s-La, n = 6; 5/6Nx-s-Ce, n = 6; Sham-c, c = 6; 5/6Nx-c-n, n = 6; 5/6Nx-c-La, n = 6; 5/6Nx-c-Ce, n = 5). The data are shown as means ± SD and were analyzed using the Tukey–Kramer method. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001
Cerium oxide did not increase the serum ALT level.
The results of the serum parameters are shown in Fig. 9. There was no significant difference in AST level among any group. The ALT level of soy protein-fed lanthanum carbonate-treated 5/6Nx rats (5/6Nx-s-La) was significantly higher than that of the other soy protein-fed rats (Sham-s, 5/6Nx-s–n, and 5/6Nx-s-Ce). The ALT level of casein-fed lanthanum carbonate-treated 5/6Nx rats (5/6Nx-c-La) was significantly higher than that of the casein fed cerium oxide-treated 5/6Nx rats (5/6Nx-c-Ce). The T-CHO levels of the 5/6Nx rat groups were significantly higher than those of each sham group, except for soy protein-fed cerium oxide-treated 5/6Nx rats (5/6Nx-s-Ce). Additionally, the T-CHO of soy protein-fed cerium oxide-treated 5/6Nx rats (5/6Nx-s-Ce) was significantly lower than that of the soy protein-fed normal 5/6Nx rats (5/6Nx-s–n). The HDL-C levels of the 5/6Nx rat groups were higher than those of the sham rat groups, except for the soy protein-fed cerium oxide-treated 5/6Nx rats (5/6Nx-s-Ce), which showed no significant difference compared to the Sham-s group. The soy protein-fed cerium oxide-treated 5/6Nx rats (5/6Nx-s-Ce) had similarly lower values of HDL-C than the soy protein-fed normal 5/6Nx rats (5/6Nx-s–n).
Differences in AST, ALT, T-CHO, and HDL-C levels upon dissection. Sham rats and three different treated (normal, lanthanum carbonate, and cerium oxide) 5/6Nx rats fed soy protein or casein were used (Sham-s, n = 6; 5/6Nx-s–n, n = 6; 5/6Nx-s-La, n = 6; 5/6Nx-s-Ce, n = 6; Sham-c, c = 6; 5/6Nx-c-n, n = 6; 5/6Nx-c-La, n = 6; 5/6Nx-c-Ce, n = 5). The data are shown as means ± SD and were analyzed using the Tukey–Kramer method. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001
Other serum indexes are shown in additional file 1 (Fig. 10). The TP levels of cerium oxide-treated 5/6Nx rats (5/6Nx-s-Ce and 5/6Nx-c-Ce) were significantly lower than those of the Sham-s and Sham-c rats, respectively. The casein-fed normal 5/6Nx rats (5/6Nx-c-n) showed similarly low TP levels compared to the Sham-c rats. The ALB levels of the 5/6Nx rat groups were significantly lower than those of the sham rat groups, except for the soy protein-fed lanthanum carbonate-treated 5/6Nx rats (5/6Nx-s-La). However, treatment with lanthanum carbonate did not improve serum ALB levels. The A/G level of soy protein-fed normal 5/6Nx rats (5/6Nx-s–n) was significantly lower than those of the other soy protein fed rats (Sham-s, 5/6Nx-s-La, and 5/6Nx-s-Ce). The casein-fed normal 5/6Nx rats (5/6Nx-c-n) showed significantly lower A/G level compared to the Sham-c rats. The Cl levels of cerium oxide-treated 5/6Nx rats (5/6Nx-s-Ce and 5/6Nx-c-Ce) were significantly different compared to each normal diet group (Sham-s, 5/6Nx-s–n, Sham-c, and 5/6Nx-c-n). The other serum indexes including UA, Na, K, AMY, TG, T-BIL, TBA, and GLU were not significantly different among any groups.
Discussion
First, phosphate adsorption capacity of cerium oxide was evaluated in vitro. Cerium oxide showed phosphate adsorption at not only acidic pH but also neutral pH (Fig. 2). This is advantageous for a phosphate binder due to the increase in gastric pH after eating. The phosphate adsorption capacity of lanthanum carbonate was evaluated by a similar method (Fig. 3). The trivalent cation, Lanthanum ion binds to the trivalent anion, phosphate ion, at a ratio of 1 to 1 in an ideal environment. Our experiments indicated almost a theoretical phosphate adsorption capacity of lanthanum carbonate at pH 2.5. However, lanthanum carbonate could not be evaluated at pH 7.0 due to its poor solubility. These results indicate that cerium oxide may be a better phosphate binder than lanthanum carbonate.
Second, we evaluated cerium oxide as a potential of phosphate binder when orally administered in vivo. That the body weights of 5/6Nx rats were lower than those of sham rats (Fig. 4). It has been reported elsewhere that 5/6Nx rats have low body weights compared to sham rats [30,31,32], which is thought to be an effect of nephrectomy. Additionally, lanthanum carbonate- or cerium oxide-treated groups had suppressed body weight gain (Fig. 4). Yoshida et al. reported that lanthanum carbonate-treated rats had slightly suppressed body weight gain [29]. They found that the cause of suppressed body weight gain was the indirect effect of low IP conditions as opposed to the direct action of lanthanum. In this study, inhibited body weight gain not only occurred in lanthanum carbonate-treated 5/6Nx rats (5/6Nx-s-La and 5/6Nx-c-La), but also in cerium oxide-treated 5/6Nx rats (5/6Nx-s-Ce and 5/6Nx-c-Ce). Therefore, the suppression of body weight gain is an effect of low IP conditions.
Water intake and urine volumes of 5/6Nx rats were increased compared to the sham rats (Fig. 4). Barata et al. and Liu et al. reported similar results in which water intake and urine volumes in 5/6Nx rats were larger than in sham rats [31, 32], which is thought to be an effect of nephrectomy. Water intake and urine volumes of the cerium oxide-treated 5/6Nx rats (5/6Nx-s-Ce and 5/6Nx-c-Ce) were slightly larger than those of the other 5/6Nx rat groups, which is apparently an effect of eating a diet that includes the cerium oxide dispersion liquid. Urine volume was changed in concert with water intake volume in each group. These results suggests that water balance in the body was maintained during cerium oxide treatment.
The four groups of lanthanum carbonate- or cerium oxide-treated 5/6Nx rats (5/6Nx-s-La, 5/6Nx-s-Ce, 5/6Nx-c-La, and 5/6Nx-c-Ce) had low IP and U-IP levels compared to each normal diet group (Sham-s, 5/6Nx-s–n, Sham-c, and 5/6Nx-c-n) (Figs. 5 and 6). This suggests that 1.31% cerium oxide had a phosphate adsorption capacity comparable to 0.5% lanthanum from lanthanum carbonate. Cerium oxide probably adsorbed phosphate in the stomach and small intestine, resulting in inhibition of phosphate intake. As a result, low IP and U-IP levels in cerium oxide-treated 5/6Nx rats (5/6Nx-s-Ce and 5/6Nx-c-Ce) were observed. In this study, 5/6Nx rats did not have hyperphosphatemia, because the IP levels were equal between normal 5/6Nx rats (5/6Nx-s–n and 5/6Nx-c-n) and sham rats (Sham-s and Sham-c). Lanthanum carbonate- and cerium oxide-treated 5/6Nx rats (5/6Nx-s-La, 5/6Nx-s-Ce, 5/6Nx-c-La, and 5/6Nx-c-Ce) had low IP levels compared to each normal diet group (Sham-s, 5/6Nx-s–n, Sham-c, and 5/6Nx-c-n), which implies normal IP levels. The lanthanum carbonate- or cerium oxide-treated 5/6Nx rats (5/6 N-s-La, 5/6Nx-s-Ce, 5/6Nx-c-La, and 5/6Nx-c-Ce) had high Ca and U-Ca levels compared to each normal diet group (Sham-s, 5/6Nx-s–n, Sham-c, and 5/6Nx-c-n; Figs. 5 and 6). These variations appeared negatively correlated with variations in IP and U-IP. Yoshida et al. reported that Ca is increased by lanthanum carbonate treatment [29]. They discussed that this phenomenon is caused by low IP levels, resulting in deossification or diminishing calcium intake inhibition by phosphoric acid. Additionally, Ben-Dov et al. reported similar results in which the Ca levels of lanthanum carbonate-treated rats were increased inversely with the IP level [33].
The IP and Ca levels at dissection are shown in Fig. 8. The IP levels of lanthanum carbonate- or cerium oxide-treated 5/6Nx rats (5/6Nx-s-La, 5/6Nx-s-Ce, 5/6Nx-c-La, and 5/6Nx-c-Ce) were not as low but were comparable to normal 5/6Nx rats (5/6Nx-s–n and 5/6Nx-c-n). The Ca levels of lanthanum carbonate- or cerium oxide-treated 5/6Nx rats (5/6Nx-s-La, 5/6Nx-s-Ce, 5/6Nx-c-La, and 5/6Nx-c-Ce) were not high compared to each normal diet group (Sham-s, 5/6Nx-s–n, Sham-c, and 5/6Nx-c-n). These data indicated a different trend with that found at 1–3 weeks, which may be an effect of fasting before sacrifice. The variations in IP and Ca levels in lanthanum carbonate- or cerium oxide-treated 5/6Nx rats (5/6Nx-s-La, 5/6Nx-s-Ce, 5/6Nx-c-La, and 5/6Nx-c-Ce) were due to the effects of each treatment. Therefore, the IP and Ca levels after fasting were comparable in all 5/6Nx rat groups.
The CRE and BUN levels of 5/6Nx rats were significantly higher than those of the sham rats (Figs. 7 and 8). These results are consistent with previous reports [30,31,32] and indicated a decline in renal function. The CRE and BUN levels of normal or lanthanum carbonate-treated 5/6Nx rats (5/6Nx-s–n, 5/6Nx-s-La, 5/6Nx-c-n, and 5/6Nx-c-La) slowly increased. The CRE and BUN levels of cerium oxide-treated 5/6Nx rats (5/6Nx-s-Ce and 5/6Nx-c-Ce) were maintained or decreased. In 5/6Nx-s-Ce, the CRE and BUN levels were slightly lower at the start. Therefore, it is less certain that cerium oxide affects the CRE and BUN levels. However, cerium oxide conceivably had a protective effect on renal function. Nemoto et al. reported that 5/6Nx rats treated with sucroferric oxyhydroxide, which binds with phosphate, attenuated glomerulosclerosis and tubulointerstitial injury [34]. Additionally, it was reported that phosphate loading induced a decline in renal function [35]. In our study, we used two phosphate binders, namely lanthanum carbonate and cerium oxide, and each treated group showed commensurate IP levels. However, lanthanum carbonate-treated groups (5/6Nx-s-La and 5/6Nx-c-La) did not show either maintenance or decrease of the CRE and BUN levels. We believe that the mechanism of the renal protective effect of cerium oxide is unrelated to the decline in phosphate loading.
Figure 9 shows the serum AST and ALT levels, which are biomarkers of liver function. The ALT levels of lanthanum carbonate-treated 5/6Nx rats (5/6Nx-s-La and 5/6Nx-c-La) were high compared to the other groups, although the AST levels were comparable. There are many reports that lanthanum carbonate does not increase ALT [33, 36, 37]. However, the drug package insert of Fosrenol®, which includes lanthanum carbonate, describes ALT increase as a side effect that occurs in less than 1% of patients. Lanthanum carbonate possibly has weak hepatic toxicity, though cerium oxide did not increase ALT and AST. This suggests that cerium oxide is a better potential drug than lanthanum carbonate.
The serum T-CHO and HDL-C levels are shown in Fig. 9. The T-CHO and HDL-C levels in normal 5/6Nx rats (5/6Nx-s–n and 5/6Nx-c-n) were high compared to sham rats (Sham-s and Sham-c). In general, it is well known that hyperlipidemia causes CKD. The 5/6Nx rats were had a decline renal function by nephrectomy, and as a result, the T-CHO and HDL-C levels in 5/6Nx rats were increased. Kasiske et al. reported that there was an increase of cholesterol in 5/6Nx rats [38]. It is striking that the T-CHO and HDL-C levels of soy protein-fed cerium oxide-treated 5/6Nx rats (5/6Nx-s-Ce) were significantly lower than in soy protein-fed normal 5/6Nx rats (5/6Nx-s–n). These results are conceivably related to the CRE and BUN levels in cerium oxide-treated 5/6Nx rats (5/6Nx-s-Ce) that suggested a protective effect on renal function.
Additional file 1 shows the serum TP, ALB, A/G, UA, Na, K, Cl, AMY, TG, T-BIL, TBA, and GLU levels. The TP and ALB levels of cerium oxide-treated 5/6Nx rats (5/6Nx-s-Ce and 5/6Nx-c-Ce) were significantly lower than those of the sham rats (Sham-s and Sham-c). The differences were very slight, and the A/G level was not significantly different. For this reason, we do not believe these differences to be important. The Cl levels of cerium oxide-treated groups (5/6Nx-s-Ce and 5/6Nx-c-Ce) were significantly higher than those of each normal diet group (Sham-s, 5/6Nx-s–n, Sham-c, and 5/6Nx-c-n). The chloride ions are believed to be derived from the cerium oxide dispersion liquid that was used in this study; the cerium oxide-treated 5/6Nx rats (5/6Nx-s-Ce and 5/6Nx-c-Ce) consumed chloride ions in their diet, resulting in an increase in Cl levels. The other serum indexes did not show any differences.
In this study, we used the food of casein presence or absence. Casein has cation adsorption capacity [39]. However, all results were not clearly influenced by protein source (i.e., casein or soy protein).
We not researched biological distribution of cerium oxide. Kumari et al. reported that cerium oxide was absorbed in body at orally administration [40]. They orally administered cerium oxide nanoparticles (30, 300, 600 mg/kg bw/day) to rats for 28 days. As a result, cerium detected in liver, kidney, brain, spleen, heart and blood. High dose (600 mg/kg bw/day) group was 4–10 μg/g in these organs. These results suggest that orally administered cerium oxide is retained in some organs. Thus, the bioaccumulation and safety of the long-term use of cerium oxide have to be evaluated in future research.
Conclusion
In this study, we found that cerium oxide is a potential orally administered phosphate binder. We evaluated cerium oxide as a phosphate adsorption reagent in vitro and in vivo. Our results indicated that cerium oxide has phosphate adsorption capacity at acidic and neutral pH in vitro. The animal experiment using 5/6Nx rats suggested that cerium oxide can decrease serum IP levels similarly to lanthanum carbonate without hepatotoxicity. In addition, cerium oxide-treated 5/6Nx rats showed slightly lowered levels of CRE and BUN, which suggested a potential protective effect on renal function. Therefore, cerium oxide is a capability of phosphate binder. However, it is necessary to evaluate its protective effect on renal function with further research.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Abbreviations
- 5/6Nx rats:
-
5/6 Nephrectomy model rats
- CKD:
-
Chronic kidney disease
- ESKD:
-
End stage kidney disease
- IP:
-
Serum inorganic phosphate
- Ca:
-
Serum calcium
- CRE:
-
Serum creatinine
- BUN:
-
Serum urea nitrogen
- AST:
-
Aspartate transaminase
- ALT:
-
Alanine transaminase
- T-CHO:
-
Total cholesterol
- HDL-C:
-
High density lipoprotein cholesterol
- TP:
-
Serum total protein
- ALB:
-
Serum albumin
- A/G:
-
Albumin-globulin ratio
- UA:
-
Serum ureic acid
- Na:
-
Serum sodium
- K:
-
Serum potassium
- Cl:
-
Serum chlorine
- AMY:
-
Serum amylase
- TG:
-
Triglyceride
- T-BIL:
-
Serum total bilirubin
- TBA:
-
Total bile acid
- GLU:
-
Serum glucose
- U-IP:
-
Urinary inorganic phosphate
- U-Ca:
-
Urinary calcium
References
Lysaght MJ. Maintenance Dialysis Population Dynamics: Current Trends and Long-Term Implications. J Am Soc Nephrol. 2002;13(suppl 1):S37-40.
Imai E, Horio M, Watanabe T, Iseki K, Yamagata K, Hara S, et al. Prevalence of chronic kidney disease in the Japanese general population. Clin Exp Nephrol. 2009;13:621–30.
Hanafusa N, Abe M, Joki N, Hoshino J, Kikuchi K, Goto S, et al. 2020 Annual Dialysis Data Report, JSDT Renal Data Registry. Nihon Toseki Igakkai Zasshi. 2021;54:611–57.
Hruska KA, Mathew S, Lund R, Qiu P, Pratt R. Hyperphosphatemia of chronic kidney disease. Kidney Int. 2008;74:148–57.
Marchais SJ, Metivier F, Guerin AP, London GM. Association of hyperphosphataemia with haemodynamic disturbances in end-stage renal disease. Nephrol Dial Transplant. 1999;14:2178–83.
Block GA, Raggi P, Bellasi A, Kooienga L, Spiegel DM. Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int. 2007;71:438–41.
Hutchison AJ, Maes B, Vanwalleghem J, Asmus G, Mohamed E, Schmieder R, et al. Efficacy, tolerability, and safety of lanthanum carbonate in hyperphosphatemia: a 6-month, randomized, comparative trial versus calcium carbonate. Nephron - Clin Pract. 2005;100:8–19.
Komaba H, Kakuta T, Suzuki H, Hida M, Suga T, Fukagawa M. Survival advantage of lanthanum carbonate for hemodialysis patients with uncontrolled hyperphosphatemia. Nephrol Dial Transplant. 2015;30:107–14.
Brennan A, Akehurst R, Davis S, Sakai H, Abbott V. The Cost-Effectiveness of Lanthanum Carbonate in the Treatment of Hyperphosphatemia in Patients with End-Stage Renal Disease. Value Heal. 2007;10:32–41.
Shigematsu T, Mune, Negi, Mima, Sakaguchi, Orita, et al. The management of hyperphosphatemia by lanthanum carbonate in chronic kidney disease patients. Int J Nephrol Renovasc Dis. 2012;5:81.
Xu J, Zhang YX, Yu XQ, Liu ZH, Wang LN, Chen JH, et al. Lanthanum carbonate for the treatment of hyperphosphatemia in CKD 5D: Multicenter, double blind, randomized, controlled trial in mainland China. BMC Nephrol. 2013;14:1–11.
Zhang C, Wen J, Li Z, Fan J. Efficacy and safety of lanthanum carbonate on chronic kidney disease-mineral and bone disorder in dialysis patients: a systematic review. BMC Nephrol. 2013;14:226.
Ruospo M, Palmer SC, Natale P, Craig JC, Vecchio M, Elder GJ, Strippoli GFM. Phosphate binders for preventing and treating chronic kidney disease-mineral and bone disorder (CKD-MBD). Cochrane Database Syst Rev. 2018;Issue 8. Art. No.: CD006023. https://doi.org/10.1002/14651858.CD006023.pub3.
Goldsmith DR, Scott LJ, Cvetković RS, Plosker GL. Sevelamer hydrochloride: a review of its use for hyperphosphataemia in patients with end-stage renal disease on haemodialysis. Drugs. 2008;68:85–104.
Chertow GM, Burke SK, Dillon MA, Slatopolsky E. Long-term effects of sevelamer hydrochloride on the calcium × phosphate product and lipid profile of haemodialysis patients*. Nephrol Dial Transplant. 1999;14:2907–14.
Akizawa T, Origasa H, Kameoka C, Kaneko Y, Kawasaki S. Randomized Controlled Trial of Bixalomer Versus Sevelamer Hydrochloride in Hemodialysis Patients With Hyperphosphatemia. Ther Apher Dial. 2014;18:122–31.
Hatakeyama S, Murasawa H, Narita T, Oikawa M, Fujita N, Iwamura H, et al. Switching hemodialysis patients from sevelamer hydrochloride to bixalomer: a single-center, non-randomized analysis of efficacy and effects on gastrointestinal symptoms and metabolic acidosis. BMC Nephrol. 2013;14:222.
Yokoyama K, Hirakata H, Akiba T, Fukagawa M, Nakayama M, Sawada K, et al. Ferric Citrate Hydrate for the Treatment of Hyperphosphatemia in Nondialysis-Dependent CKD. Clin J Am Soc Nephrol. 2014;9:543–52.
Yokoyama K, Fukagawa M, Akiba T, Nakayama M, Ito K, Hanaki K, et al. Randomised clinical trial of ferric citrate hydrate on anaemia management in haemodialysis patients with hyperphosphataemia: ASTRIO study. Sci Rep. 2019;9:8877.
Floege J, Covic AC, Ketteler M, Mann JFE, Rastogi A, Spinowitz B, et al. Long-term effects of the iron-based phosphate binder, sucroferric oxyhydroxide, in dialysis patients. Nephrol Dial Transplant. 2015;30:1037–46.
Kendrick J, Parameswaran V, Ficociello LH, Ofsthun NJ, Davis S, Mullon C, et al. One-Year Historical Cohort Study of the Phosphate Binder Sucroferric Oxyhydroxide in Patients on Maintenance Hemodialysis. J Ren Nutr. 2019;29:428–37.
Davis RL, Abraham JL. Lanthanum deposition in a dialysis patient. Nephrol Dial Transplant. 2009;24:3247–50.
Shitomi Y, Nishida H, Kusaba T, Daa T, Yano S, Arakane M, et al. Gastric lanthanosis (lanthanum deposition) in dialysis patients treated with lanthanum carbonate. Pathol Int. 2017;67:389–97.
Tabuchi M, Takeshima F, Akazawa Y, Nakao K. Early Gastric Cancer with Diffuse Lanthanum Deposition. Intern Med. 2019;58:447–8.
Takatsuna M, Takeuchi M, Usuda H, Terai S. Case of early-stage gastric cancer identified in the gastric mucosa with lanthanum phosphate deposition. Endosc Int Open. 2019;07:E893–5.
Nakagawa S, Ogura M, Akiba T, Iwamoto H, Shibamoto T. DEVELOPMENT OF NONALUMINUM PHOSPHATE BINDER HYDROUS CERIUM OXIDE (Ceα(OH)4–2α·nH2O). Am Soc Artif Intern Organs. 1985;31:155–9.
Malaki M. Acute encephalopathy following the use of aluminum hydroxide in a boy affected with chronic kidney disease. J Pediatr Neurosci. 2013;8:81.
Ogura M, Iwamoto H, Akiba T, Nakagawa S. Development of non-aluminum phosphate binder -Hydrous cerium oxide. J Japanese Assoc Dial Physicians. 1986;19:775–8.
Yoshida M, Nishizaki I, Komura E, Hosomi R, Fukunaga K. Effect of difference in dietary protein on serum phoshorus and tissue lanthanum concentration in rats administered lanthanum carbonate. Trace Nutr Res. 2019;36:29–34.
Ji L, Masuda S, Saito H, Inui KI. Down-regulation of rat organic cation transporter rOCT2 by 5/6 nephrectomy. Kidney Int. 2002;62:514–24.
Kyoko B, Midori Y, Ryoji H, Akihiko M. Sequential Alterations in Clinical Biochemical Indicators of Renal Function in 5/6 Nephrectomized Rats: Basic Study for Renal Toxicity Using 5/6 Nephrectomized Rats. J Toxicol Sci. 1998;23:433–42.
Liu F, Li Y, Wang F, Jiang Y-F, Jiang Y-S. Shenfushu Granule and Atropine Attenuate Microvasculature Loss in Rat Models with 5/6 Nephrectomy. Ren Fail. 2012;34:600–9.
Ben-Dov IZ, Pappo O, Sklair-Levy M, Galitzer H, Ilan Y, Naveh-Many T, et al. Lanthanum carbonate decreases PTH gene expression with no hepatotoxicity in uraemic rats. Nephrol Dial Transplant. 2006;22:362–8.
Nemoto Y, Kumagai T, Ishizawa K, Miura Y, Shiraishi T, Morimoto C, et al. Phosphate binding by sucroferric oxyhydroxide ameliorates renal injury in the remnant kidney model. Sci Rep. 2019;9:1732.
Santamaría R, Díaz-Tocados JM, Pendón-Ruiz de Mier MV, Robles A, Salmerón-Rodríguez MD, Ruiz E, et al. Increased phosphaturia accelerates the decline in renal function: a search for mechanisms. Sci Rep. 2018;8:13701.
Hutchison AJ, Barnett ME, Krause R, Kwan JTC, Siami GA. Long-Term Efficacy and Safety Profile of Lanthanum Carbonate: Results for up to 6 Years of Treatment. Nephron Clin Pract. 2008;110:c15-23.
Rombolà G, Londrino F, Corbani V, Falqui V, Ardini M, Zattera T. Lanthanum carbonate: a postmarketing observational study of efficacy and safety. J Nephrol. 2012;25:490–6.
Kasiske BL, O’Donnell MP, Garvis WJ, Keane WF. Pharmacologic treatment of hyperlipidemia reduces glomerular injury in rat 5/6 nephrectomy model of chronic renal failure. Circ Res. 1988;62:367–74.
Suárez-Luque S, Mato I, Huidobro JF, Simal-Lozano J. Determination of major metal cations in milk by capillary zone electrophoresis. Int Dairy J. 2007;17:896–901.
Kumari M, Kumari SI, Grover P. Genotoxicity analysis of cerium oxide micro and nanoparticles in Wistar rats after 28 days of repeated oral administration. Mutagenesis. 2014;29:467–79.
Acknowledgements
Not applicable.
Funding
This work was funded by applausePharma Co., Ltd..
Author information
Authors and Affiliations
Contributions
AH designed the in vivo study, performed the in vitro and in vivo study, analyzed and interpreted the data, and wrote the manuscript. JG performed the in vivo study, analyzed and interpreted the data regarding the in vivo study. YK performed the in vivo study, analyzed and interpreted the data regarding the in vivo study. YO designed and performed the in vitro study, analyzed the data regarding the in vitro study, and interpreted the data. MN analyzed and interpreted the data. MK conceptualized the study and interpreted the data. KF conceptualized the study, designed the in vivo study, interpreted the data regarding the in vivo study, and revised the manuscript. All authors and approved the final manuscript.
Authors’ information
Not applicable.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All animal experiments were approved by the Animal Protection and Ethics Committee of Shibaura Institute of Technology (approval number #20005, permission on Aug 25th, 2020), which conform to the current Japanese laws. All methods were carried out in accordance with relevant guidelines and regulations, and the study was carried out in compliance with the ARRIVE guidelines.
Consent for publication
Not applicable.
Competing interests
MK report that financial support was provided by applause Co., Ltd., which is an affiliated company of applausePharma Co., Ltd.. AH, YO and MN are employed by applause Co., Ltd., which is an affiliated company of applausePharma Co., Ltd.. All other authors declare that they have no conflicts of interest with the contents of this article.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Fig. 10.
Concentrations of biomarkers upon dissection. Sham rats and three different treated (normal, lanthanum carbonate, and cerium oxide) 5/6Nx rats fed soy protein or casein were used (Sham-s, n=6; 5/6Nx-s-n, n=6; 5/6Nx-s-La, n=6; 5/6Nx-s-Ce, n=6; Sham-c, c=6; 5/6Nx-c-n, n=6; 5/6Nx-c-La, n=6; 5/6Nx-c-Ce, n=5). The data are shown as means ± SD and were analyzed using the Tukey-Kramer method. *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hashimoto, A., Gao, J., Kanome, Y. et al. Evaluation of cerium oxide as a phosphate binder using 5/6 nephrectomy model rat. BMC Nephrol 23, 277 (2022). https://doi.org/10.1186/s12882-022-02904-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-022-02904-6