Abstract
Background
Left ventricular hypertrophy (LVH) is common among patients undergoing dialysis. However, the dynamic structural changes of LV are rarely discussed. The study aimed to investigate the prognostic significance of left ventricular mass index (LVMI)-progression in incident peritoneal dialysis (PD) patients, and explore risks factors for LVMI-progression.
Methods
Incident PD patients between February 2008 and July 2018 were recruited. Echocardiography was performed yearly to collect LVMI and evaluate its changes. Participants were divided into three subgroups: group with LVMI-regression, group with LVMI stable and group with LVMI-progression. The end points include all-cause mortality, cardiovascular mortality and cardiovascular events. Cox regression models were performed to identify the associations between LVMI-progression and these endpoints. Multivariate logistic regression was conducted to identify risk factors for LVMI-progression.
Results
A total of 216 PD patients (130 men,60.2%) with a mean age of 54.3 ± 16.8 years were recruited. LVMI-progression was identified in 72 patients (33.3%) after PD initiation. The cohort was followed for a median duration of 65.9 months. Multivariable Cox regression analysis revealed that LVMI-progression was an independent predictor of all-cause mortality (HR, 1.419; 95% CI, 1.016–1.982; p = 0.040), cardiovascular mortality (HR, 1.836; 95%CI, 1.084–3.108; p = 0.024), and cardiovascular events (HR, 1.494; 95%CI, 1.063–2.099; p = 0.021). Multivariable logistic regression showed that hemoglobin, ferritin, blood pressure and fibrinogen were significantly associated with LVMI-progression.
Conclusion
Early LVMI-progression was independently associated with all-cause mortality and cardiovascular outcomes in PD patients. The dynamic monitoring of LVMI might therefore help identify high-risk patients.
Similar content being viewed by others

Introduction
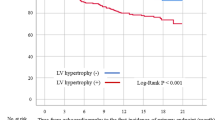
Cardiovascular mortality in dialysis patients is 10 to 20 times higher than in the general population [1]. Given the far greater cardiovascular risks among dialysis patients, it is important to assess the role of traditional and nontraditional risk factors for cardiovascular disease. Alterations in left ventricular (LV) mass and structure are common in patients with chronic kidney disease (CKD) [2]. Among patients treated by hemodialysis or peritoneal dialysis (PD), it is reported that the prevalence of left ventricular hypertrophy (LVH) is approximately 75% [3].
Echocardiogram is one of the most common imaging tests to evaluate the LV geometry. And high left ventricular mass index (LVMI) is widely used as a quantitative tool to assess LVH [4]. Previous reports have shown that LVH in CKD contributes to high cardiovascular mortality [5,6,7], and increased LVMI predicts higher mortality in PD patients [8, 9]. LVMI thus has been widely accepted as an indicator of prognosis in dialysis patients. Although many studies have investigated the results of a single echocardiogram in PD patients, very few studies reviewed serial echocardiograms dynamically. As early as in 1992 [10], Hüting J, et al. have found that LVH could be changeable during the long-term CAPD treatment. However, the structural change of LV over time and its impact on PD patients are rarely discussed.
In the present study, we regularly followed up the LVMI of PD patients through echocardiogram. The study aimed to investigate the prognostic significance of LVMI-progression in patients on PD, and explore risks factors for LVMI-progression.
Materials and methods
Population and study design
It was designed as a prospective observational study. From February 2008 to July 2018, adult incident PD patients from Huashan Hospital, Fudan University, China were recruited. Patients were excluded if they were transferred from hemodialysis or failed renal transplantation. Patients exhibited an ongoing infection, neoplasia, or unstable cardiovascular disease with a life expectancy of less than 6 months were also excluded. Participants were followed from enrollment until death, a switch to hemodialysis (HD), renal transplantation, a transfer, or March 2020. The study was approved by the Ethics Committee of Huashan Hospital, Fudan University and all participants provided written informed consent.
LVMI acquirement
In each patient, standard transthoracic echocardiogram was performed at PD initiation and followed up yearly. All measurement data were read and confirmed by professional cardiac sonographer according to the recommendations of the American Society of Echocardiography (ASE) [11]. Left ventricular end-diastolic diameter (LVDd), interventricular septum thickness (IVST), posterior wall thickness (PWT) during diastole, left ventricular ejection fraction (LVEF) and E/A ratio were evaluated and recorded. LVMI was calculated using the following equations [4]:
Definition of LVMI changes and the grouping method
LVMIs in the first 2 years after PD initiation were collected. ∆LVMI was calculated to define LVMI changes (LVMI0 = the baseline LVMI, LVMI2 = LVMI in the second year, ∆LVMI = LVMI2-LVMI0). Participants were divided into 3 subgroups according to ∆LVMI:
-
Group 1: participants with LVMI-regression: ∆LVMI ≤ -5%LVMI0;
-
Group 2: participants with LVMI stable: -5%LVMI0 < ∆LVMI < 5%LVMI0;
-
Group 3: participants with LVMI-progression: ∆LVMI ≥ 5%LVMI0.
Clinical and laboratory data
Demographic characteristics including sex, age, history of diabetes, smoking status and medication status (use of statins, anti-hypertensive drugs and renin–angiotensin–aldosterone system [RAAS] antagonists) were obtained at the time of enrollment. Height, weight, BSA, body mass index (BMI), blood pressure, laboratory parameters and dialysis-related parameters were also recorded at baseline and collected semiannually. The laboratory data include hemoglobin, albumin, iron metabolism parameters, calcium-phosphate metabolism parameters, lipid metabolism parameters, plasma fibrinogen (FIB), N-terminal pro brain natriuretic peptide (NT-proBNP) and high-sensitivity C-reactive protein (hsCRP). The PD-related data include urinary output, total Kt/V, residual Kt/V, total clearance of creatinine (Ccr), residual Ccr, normalized protein catabolic rate (nPCR), glucose concentration in dialysate and volume of dialysate. Dialysis adequacy was evaluated using Baxter PD Adequest 2.0 software (Baxter Healthcare Corporation, Deerfield, IL, USA). The average values of all these indexes in the first two years were calculated.
End points
The primary endpoint of the study was all-cause mortality. The secondary endpoints were cardiovascular events (CVEs) and cardiovascular mortality. CVEs were defined when patients exhibited myocardial infarction, acute coronary syndrome, non-hemorrhagic or hemorrhagic stroke, heart failure, arterial embolization, diabetic foot or non-traumatic amputation. Mortality caused by CVEs was defined as cardiovascular death.
Statistical analysis
Statistical analyses were carried out with SPSS 22.0 (SPSS, Inc., Chicago, IL, USA). Numeric variables were presented as mean values ± standard deviation or as median with quartile ranges. Categorical variables were presented as percentages. One‐way analysis of variance (ANOVA), and two‐way ANOVA analysis were performed in continuous variables to make comparisons while Chi-square test was performed in categorical variables as appropriate. Univariate and multivariate Cox regression models were used to explore risk factors for all-cause mortality, CVEs and cardiovascular mortality and to clarify the associations between LVMI changes and these endpoints. Binary logistic regressions were conducted to identify the risk factors for LVMI-progression.
All statistical tests were 2-sided and a p value < 0.05 was considered statistically significant.
Results
Characteristics of study population
The study population included 216 patients (130 men; mean age of 54.3 ± 16.8 years) with a mean BMI of 22.4 ± 3.3 kg/m2. LVMI-regression was identified in 113 patients (52.3%) while 72 patients (33.3%) were detected to have LVMI-progression. Clinical data changes in the first 2 years after PD initiation were displayed in Table 1. And Table 2 showed the average values of all these clinical indexes among 3 groups. Compared to patients with LVMI-regression, patients with LVMI-progression had higher blood pressure, NT-proBNP, hsCRP and FIB but lower level of hemoglobin, ferritin and iron saturation. Besides, HDL and ApoA1 were lower in the group with LVMI-progression. No difference was observed in medication status and dialysis-related variables among three groups.
The duration of the observational period was 65.9 (31.9–87.4) months for all patients. Overall, 53 patients experienced CVEs, 56 patients died with 22 of them died from CVEs. More all-cause mortality, cardiovascular morbidity and mortality occurred in the group with LVMI-progression. During the follow-up, 2 patients transferred to other centers, 17 switched to HD and 15 received renal transplantation.
Association of LVMI changes with all-cause mortality and cardiovascular outcomes
In the group with LVMI-regression, a total of 23 (20.4%) patients experienced CVEs; 20 (17.7%) patients died and 8 (7.1%) died from CVEs. Compared to the LVMI-regression group, more all-cause mortality (28,38.9%; p = 0.020) and cardiovascular events (22, 30.6%; p = 0.037) occurred in the group with LVMI-progression.
In univariable Cox regression models, LVMI-progression was a risk factor for both all-cause mortality and cardiovascular events while not for cardiovascular mortality (Table 3). In multivariable Cox regression analysis after adjustment, LVMI-progression turned out to be an independent predictor for all-cause mortality (HR, 1.419; 95% CI, 1.016–1.982; p = 0.040), cardiovascular events (HR, 1.494; 95%CI, 1.063–2.099; p = 0.021) and cardiovascular mortality (HR, 1.836; 95%CI, 1.084–3.108; p = 0.024) (Table 4). In addition, a negative correlation was observed between LVMI and LVEF (Supplementary Fig. 1). The correlation coefficient was -0.287 with p < 0.001, verifying the adverse role of LVH progression in cardiac function.
Risk factors for LVMI-progression
To explore risk factors for LVMI-progression, participants with LVMI-regression and LVMI-stable were merged as a new group without LVMI-progression. Age, sex, BMI,use of anti-hypertensive drugs, glucose concentration in dialysate, MAP, hemoglobin, ferritin, iron saturation, ApoA1, NT-proBNP and FIB were added to binary logistic regressions. The multivariate analysis showed that MAP, hemoglobin, ferritin and FIB were independent risk factors for LVMI-progression (Table 5).
Discussion
In this study, we followed up the patients’ LVMI changes after PD initiation and identified early LVMI-progression was an independent predictor for all-cause mortality, cardiovascular events, and cardiovascular mortality. We also explored the risk factors related to LVMI-progression. It revealed that MAP, hemoglobin, ferritin and fibrinogen were significantly associated with the process. The findings indicated that dynamic follow-up of LVMI after PD initiation might be useful for the early stratification of patients with a high risk of cardiovascular disease and mortality.
Before our research, several observational studies have confirmed the association between LVMI and high cardiovascular mortality in dialysis patients [5, 12, 13]. However, most of them were cross-sectional and performed in prevalent dialysis patients. Only Zoccali C. et al. have monitored LVMI among hemodialysis (HD) patients and verified that changes in LVMI had an independent prognostic value for cardiovascular events in HD patients [14]. Recently, Tamara's group have found that concentric LVH at PD initiation was independently associated with survival in PD patients [15]. Similarly, they advocated early evaluation of LV geometry at PD initiation. Although the group didn’t evaluate changes in LV geometry over time, they suggested that monitoring of LV geometry over time might provide more accurate predictions regarding patients’ prognosis. Our study to some extent could compensate the limitation of their exploration. To our knowledge, it’s the first study that focuses on the relationship between early-stage LVH changes after PD initiation and the prognosis of patients.
According to the suggestion made by the 2007 ESH/ESC guidelines [16], LVH was defined as LVMI more than 125 g/m2 in men and more than 110 g/m2 in women. Previous reports showed that at the initiation of maintenance dialysis, 70–80% of patients had evidence of LVH [17,18,19]. Consistent with the data, 73.6% patients in our study was diagnosed with LVH at baseline. It is noteworthy that more than half of the participants (52.3%) in our study got LVMI reduced after 2-year PD treatment. Like our data, Rebic D et al. have observed a significant reduction in LVMI and a significant improvement of LV function after 1-year PD treatment [20]. It is explainable. LVMI is a modifiable factor. For patients with end-stage renal disease (ESRD), PD offers the advantage of continuous dialysis, which is useful in preventing disequilibrium syndrome such as hypertension, poor extracellular volume control, and anemia. As displayed in Table 1, the median LVMI of all participants was 128 g/m2 at baseline and it decreased to 115.9 g/m2 one year later. Concomitantly, parameters such as hemoglobin, blood pressure, NT-proBNP and CRP were all improved one year after PD initiation.
In the univariate Cox regression analysis, we found that both baseline LVMI and LVMI-progression were risk factors for all-cause mortality and cardiovascular events (Table 3). However, when they were put into the multivariate models together, the predictive role of LVMI was diluted while LVMI-progression was still strongly linked to all these endpoints. Therefore, LVMI-progression was related to mortality and cardiovascular risks independently of LVMI and of a large series of traditional risk factors. Compared to a single cross-sectional LVMI, LVMI-changes might be a more powerful indicator for PD patients’ prognosis.
In PD patients, the most prominent hemodynamic parameters include hypertension, volume overload and anemia [21]. Anemia, together with hypertension, has been reported to be one of the main etiological factors for the development of LVH in dialysis patients [22, 23]. Similarly, our results showed that MAP, hemoglobin and ferritin were independently associated with LVMI-progression in PD patients. Fibrinogen is considered as an established predictor of cardiovascular events [24]. In our data, its adverse role in LVMI-progression was further verified. Volume overload is also thought to be a major factor in the development of LVH [25, 26]. In the present study, the association of NT-proBNP with LVMI-progression could not be demonstrated in the logistic regression models. Firstly, NT-proBNP isn’t the gold standard for volume overload. Secondly, this may be due to the short-term of PD duration. Earlier studies have suggested that patients on short-term CAPD were less volume overload than those on long-term CAPD [27]. We only followed up the first 2-year clinical data after PD initiation. Therefore, the role of volume overload might have been attenuated. In addition, antihypertensive treatment didn’t affect LVH progression in our results. It was reasonable. In our study, over 90% of participants used anti-hypertensive drugs. Interindividual variations of anti-hypertensive therapy might be too small to distinguish statistical differences. Nevertheless, antihypertensive treatment can never be neglected in clinic. Our results showed MAP was an independent risk factor for LVMI-progression, illustrating the importance of blood pressure control.
Several limitations remained in this study. First, the study was single-center based with a relatively small sample size. Further multicenter research with more participants is required to confirm our conclusions. Second, number of events especially the cardiovascular death in our study was small and it may affect the statistical power. Some potential risk factors could be missed. Third, the observational nature of this study didn’t not allow us to examine whether therapeutic intervention on anemia etc. could slow LVMI-progression, improving the survival of PD patients. Further studies were needed to explore the causal relationship between them. Finally, we only collected the clinical data in the first 2 years after PD initiation and didn’t further evaluate changes of LVMI over time. It is reported that long-term CAPD is disadvantageous for volume clearance, so it is associated with higher LVMI compared to short-term CAPD [28, 29]. Although a long-time monitoring of left ventricular structure might provide more accurate predictions for patients’ prognosis, our results showed that the long-term outcomes of PD patients could be predicted by LVMI-progression at an early point of PD initiation.
Conclusion
In conclusion, early LVMI-progression after PD initiation was a predictor for all-cause mortality, cardiovascular events and cardiovascular in PD patients. The dynamic monitoring of LVMI after PD initiation might therefore help identify patients with a high risk of cardiovascular disease and mortality. In addition, the study found that hypertension, anemia, iron deficiency and high level of fibrinogen were independent risk factors for LVMI-progression. These factors could be the possible point for interventions. Further investigations are needed to clarify whether early treatment for factors such as anemia could improve outcomes in PD patients.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Abbreviations
- ASE:
-
Society of Echocardiography
- ANOVA:
-
Analysis of variance
- BMI:
-
Body mass index
- CAPD:
-
Continuous ambulatory peritoneal dialysis
- CKD:
-
Chronic kidney disease
- CVEs:
-
Cardiovascular events
- Ccr:
-
Creatinine clearance
- CHO:
-
Cholesterol
- DBP:
-
Diastolic blood pressure
- ESRD:
-
End-stage renal disease
- FGF-23:
-
Fibroblast growth factor-23
- FIB:
-
Fibrinogen
- HDL:
-
High-density lipoprotein
- HD:
-
Hemodialysis
- hsCRP:
-
High-sensitivity C-reactive protein
- IVST:
-
Interventricular septum thickness
- Kt/V:
-
Urea clearance
- LV:
-
Left ventricular
- LVH:
-
Left ventricular hypertrophy
- LVDd:
-
Left ventricular end-diastolic diameter
- LVEF:
-
Left ventricular ejection fraction
- LVMI:
-
Left ventricular mass index
- LDL:
-
Low-density lipoprotein
- MAP:
-
Mean arterial pressure
- NT-proBNP:
-
N-terminal pro brain natriuretic peptide
- nPCR:
-
Normalized protein catabolic rate
- PD:
-
Peritoneal dialysis
- PWT:
-
Posterior wall thickness
- RAAS:
-
Renin-angiotensin-aldosterone system
- SBP:
-
Systolic blood pressure
- TG:
-
Triglyceride
References
Coresh J, Longenecker JC, Miller ER, Young HJ, Klag MJ. Epidemiology of cardiovascular risk factors in chronic renal disease. J Am Soc Nephrol. 1998;9(12 Suppl):S24-30.
Park M, Hsu CY, Li Y, Mishra RK, Keane M, Rosas SE, et al. Associations between kidney function and subclinical cardiac abnormalities in CKD. J Am Soc Nephrol. 2012;23(10):1725–34.
Foley RN, Parfrey PS, Sarnak MJ. Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol. 1998;9(12 Suppl):S16-23.
Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57(6):450–8.
Wang AY, Wang M, Woo J, Lam CW, Lui SF, Li PK, et al. Inflammation, residual kidney function, and cardiac hypertrophy are interrelated and combine adversely to enhance mortality and cardiovascular death risk of peritoneal dialysis patients. J Am Soc Nephrol. 2004;15(8):2186–94.
Tripepi G, D’Arrigo G, Mallamaci F, London G, Tangri N, Hsu JY, et al. Prognostic values of left ventricular mass index in chronic kidney disease patients. Nephrol Dial Transplant. 2021;36(4):665–72.
Paoletti E, De Nicola L, Gabbai FB, Chiodini P, Ravera M, Pieracci L, et al. Associations of Left Ventricular Hypertrophy and Geometry with Adverse Outcomes in Patients with CKD and Hypertension. Clin J Am Soc Nephrol. 2016;11(2):271–9.
Silaruks S, Sirivongs D, Chunlertrith D. Left ventricular hypertrophy and clinical outcome in CAPD patients. Perit Dial Int. 2000;20(4):461–6.
Tomura M, Hamasaki Y, Komaru Y, Miyamoto Y, Matsuura R, Matsumoto A, et al. Prognostic significance of concentric left ventricular hypertrophy at peritoneal dialysis initiation. BMC Nephrol. 2021;22(1):135.
Hüting J, Alpert MA. Progression of left ventricular hypertrophy in end-stage renal disease treated by continuous ambulatory peritoneal dialysis depends on hypertension and hypercirculation. Clin Cardiol. 1992;15(3):190–6.
Marwick TH, Gillebert TC, Aurigemma G, Chirinos J, Derumeaux G, Galderisi M, et al. Recommendations on the Use of Echocardiography in Adult Hypertension: A Report from the European Association of Cardiovascular Imaging (EACVI) and the American Society of Echocardiography (ASE). J Am Soc Echocardiogr. 2015;28(7):727–54.
Ateş K, Nergizoğlu G, Keven K, Sen A, Kutlay S, Ertürk S, et al. Effect of fluid and sodium removal on mortality in peritoneal dialysis patients. Kidney Int. 2001;60(2):767–76.
Braunisch MC, Gundel P, Werfel S, Mayer CC, Bauer A, Haller B, et al. Electrocardiographic parameters of left ventricular hypertrophy and prediction of mortality in hemodialysis patients. J Nephrol. 2021.
Zoccali C, Benedetto FA, Mallamaci F, Tripepi G, Giacone G, Stancanelli B, et al. Left ventricular mass monitoring in the follow-up of dialysis patients: Prognostic value of left ventricular hypertrophy progression. Kidney Int. 2004;65(4):1492–8.
Tomura M, Hamasaki Y, Komaru Y, Miyamoto Y, Matsuura R, Matsumoto A, et al. Prognostic significance of concentric left ventricular hypertrophy at peritoneal dialysis initiation. BMC Nephrol. 2021;22(1).
Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. 2007 ESH-ESC Practice Guidelines for the Management of Arterial Hypertension: ESH-ESC Task Force on the Management of Arterial Hypertension. J Hypertens. 2007;25(9):1751–62.
Mitsnefes MM, Daniels SR, Schwartz SM, Khoury P, Strife CF. Changes in left ventricular mass in children and adolescents during chronic dialysis. Pediatr Nephrol. 2001;16(4):318–23.
Sozeri B, Mir S, Kara OD, Levent E. When Does the Cardiovascular Disease Appear in Patients With Chronic Kidney Disease? Pediatr Cardiol. 2010;31(6):821–8.
Yilmaz M, Unsal A, Oztekin E, Kesmezacar O, Harmankaya Kaptanogullari O, Eren N. The Prevalence of Hypertension, Valve Calcification and Left Ventricular Hypertrophy and Geometry in Peritoneal Dialysis Patients. Kidney Blood Press Res. 2012;35(6):431–7.
Rebić D, Rašić S, Valjevac A, Unčanin S, Hamzić-Mehmedbašić A. Biomarkers of cardiovascular remodeling in patients on peritoneal dialysis. Am J Nephrol. 2014;39(2):92–9.
London GM. Left ventricular alterations and end-stage renal disease. Nephrol Dial Transplant. 2002;17(Suppl 1):29–36.
Ataş N, Erten Y, Okyay GU, İnal S, Topal S, Öneç K, et al. Left Ventricular Hypertrophy and Blood Pressure Control in Automated and Continuous Ambulatory Peritoneal Dialysis Patients. Ther Apher Dial. 2014;18(3):297–304.
Nardi E, Palermo A, Mulè G, Cusimano P, Cottone S, Cerasola G. Left ventricular hypertrophy and geometry in hypertensive patients with chronic kidney disease. J Hypertens. 2009;27(3):633–41.
Zoccali C, Benedetto FA, Mallamaci F, Tripepi G, Cutrupi S, Parlongo S, et al. Fibrinogen, inflammation and concentric left ventricular hypertrophy in chronic renal failure. Eur J Clin Invest. 2003;33(7):561–6.
Tangwonglert T, Davenport A. Changes in extracellular water and left ventricular mass in peritoneal dialysis patients. Kidney Res Clin Pract. 2021;40(1):135–42.
Cader RA, Ibrahim OA, Paul S, Gafor HA, Mohd R. Left ventricular hypertrophy and chronic fluid overload in peritoneal dialysis patients. Int Urol Neohrol. 2014;46(6):1209–15.
Faller B, Lameire N. Evolution of clinical parameters and peritoneal function in a cohort of CAPD patients followed over 7 years. Nephrol Dial Transplant. 1994;9(3):280–6.
Lai S, Molfino A, Russo GE, Testorio M, Galani A, Innico G, et al. Cardiac, Inflammatory and Metabolic Parameters: Hemodialysis versus Peritoneal Dialysis. Cardiorenal Med. 2015;5(1):20–30.
Tonbul Z, Altintepe L, Sözlü C, Yeksan M, Yildiz A, Türk S. Ambulatory blood pressure monitoring in haemodialysis and continuous ambulatory peritoneal dialysis (CAPD) patients. J Hum Hypertens. 2002;16(8):585–9.
Acknowledgements
Not applicable.
Funding
This study was supported by National Natural Science Foundation of China (grant number 81670692,81930120 and 81520108006).
Author information
Authors and Affiliations
Contributions
Tongying Zhu designed the study. Yun Chen and Shuqi Dai are major contributors in writing the manuscript and Chuanming Hao reviewed it. Xiaolin Ge and Da Shang interpreted the patient data and Qionghong Xie made analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Huashan Hospital, Fudan University and all participants provided written informed consent. And all methods were performed in accordance with Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Figure 1.
Relationship between LVEF and LVMI.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, Y., Dai, S., Ge, X. et al. Prognostic values of left ventricular mass index progression in incident peritoneal dialysis patients : a prospective cohort study. BMC Nephrol 23, 200 (2022). https://doi.org/10.1186/s12882-022-02831-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-022-02831-6