Abstract
Background
Patients on hemodialysis often suffer from reduced muscle strength and exercise capacity due to the decreased quantity and quality of muscle. Cumulative studies showed ultrasound echo intensity (EI) had great potential in evaluating muscle quality. The objective of this study was to evaluate the relationship between EI of skeletal muscle and physical function of patients on maintenance hemodialysis.
Methods
Cross-sectional area (CSA) and mean EI of the right rectus femoris were measured by ultrasound to evaluate the quantity and quality of the muscle, respectively. Physical function was measured by handgrip strength (HGS), gait speed, sit-to-stand 60 s (STS-60) test, and instrumental activities of daily living (IADL) scale.
Results
A total of 107 patients on hemodialysis were included, with women accounting for 37.3% (n = 40), and a mean age of 53.53 ± 12.52 years. Among the patients on hemodialysis, EI was moderately and negatively correlated with HGS (r = − 0.467, P < 0.001), gait speed (r = − 0.285, P = 0.003), and STS-60 (r = − 0.313, P = 0.001). Multiple regression analyses adjusted for CSA showed that the enhanced EI of patients on hemodialysis remained associated with worse HGS (β = − 0.207, P = 0.047), lower gait speed (β = − 0.002, P = 0.001), less STS-60 (β = − 0.136, P = 0.049), and a higher likelihood of dependency in IADL (Odds Ratio: 1.070, 95% CI: [1.033–1.111], P = 0.001).
Conclusions
In patients on hemodialysis, enhanced EI in the skeletal muscle measured via ultrasound was correlated with poor physical performance. The combined muscle quality and muscle quantity evaluation provide more information for assessing the level of physical function of the patients.
Similar content being viewed by others
Introduction
Patients on hemodialysis often suffer from reduced muscle strength and exercise capacity, lowering their autonomy and quality of life and shortening survival time [1]. Decreased muscle mass is one of the main causes of reduced muscle strength and exercise capacity [2, 3]. A recent study showed that muscle quality is decreased significantly at the same time as or before the decrease in muscle mass [4]. Studies of the elderly have also reported that the decline of muscle quality is closely related to the decline in physical function and poor prognosis of patients [5, 6]. Therefore, both the quantity and quality of muscle affect muscle function. The joint evaluation may help formulate intervention measures and improve prognosis.
Muscle quality is a relatively new term that refers to the microscopic and macroscopic changes in muscle structure and composition and the level of muscle function from each muscle mass unit [7]. The infiltration of non-contractile tissue such as adipose and fibrotic tissue has been the focus of numerous reports in the last decade [6, 8]. While changes in muscle composition occurred with aging, numerous factors might make chronic kidney disease (CKD) patients disposed to these alterations [9].
Multiple existing imaging methods are used to get estimated muscle composition to evaluate muscle quality. Computed tomography (CT), magnetic resonance imaging (MRI), and muscle radiation attenuation by CT evaluate muscle quality by providing the measurements of adipose tissue and lipid infiltration, loss in tissue elasticity, and relative fluid expansion [10]. Unfortunately, only a few imaging studies focused on patients on hemodialysis, possibly for the reason that CT and MRI are limited by difficulties to access due to the clinical setting, the cost, and radiation exposure. Nevertheless, a study demonstrated that CT measurement of mid-thigh intermuscular adipose tissue predicts usual gait velocity and 6-min walk distance of patients on hemodialysis [2].
Unlike MRI and CT, ultrasound has the advantages of bedside operation, convenience, non-invasiveness, and lack of radiation exposure, and is widely studied as a tool in muscle assessment. Ultrasound can effectively assess muscle mass and muscle quality with good internal and external consistency [11]. Diagnostic ultrasound has been proposed as an alternative imaging modality for the use of sarcopenia screening and staging [12]. Echo intensity (EI) is an important indicator for ultrasound assessment of muscle quality. The foundational premise of EI is that skeletal muscle is comprised not only of contractile proteins, but also non-contractile elements, such as intramuscular adipocytes and fibrous tissue [13]. Enhanced EI indicates increased infiltration of fibrotic tissue and/or adipose tissue in the muscle and represents reduced muscle quality [14]. Numerous studies in the elderly have shown that an increase in EI is related to decline in muscle strength and physical function [15,16,17]. A recent study involving 61 patients with CKD showed that patients with high EI had low physical function [18]. However, there are no relevant studies evaluating ultrasound derived EI in patients on hemodialysis to date. Hence, this study clarified the application value of ultrasound EI of skeletal muscle in patients on hemodialysis by assessing the relationship between ultrasound EI and physical function.
Methods
Study population
We performed an observational and cross-sectional study on outpatients who were on maintenance hemodialysis at Menghai county hospital (Yunnan province, China). Patients were recruited between July 2020 and August 2020, and enrolled if they had undergone more than 3 months of hemodialysis. Patients were excluded if they could not walk or be completely blind were excluded. We screened a total of 110 patients and excluded 3 patients who could not walk. A total of 107 subjects were eventually enrolled in this exploratory study. The research was conducted in accordance with the Helsinki Declaration. The patients’ demographic information, past medical history, and duration of dialysis were collected before hemodialysis. Various body measurements and assessments of physical function were performed before hemodialysis. A complete ultrasound examination of the right rectus femoris was also performed after hemodialysis.
Body measurements and assessments of physical function
Measurement of body mass index (BMI)
BMI measurement of individual research subjects was calculated based on body dry weight and the following formula: BMI (Kg/m2) = body weight/height2.
Measurement of handgrip strength (HGS)
HGS of individual subjects was measured before hemodialysis. Patients with internal arteriovenous fistula were subjected to HGS measurement on the non-fistula hand twice to obtain the maximum value. Patients using tunneled cuffed catheters were subjected to the HGS measurement on the dominant hand twice to obtain the maximum value.
Gait speed
Individual subjects were required to walk independently for a 4-m distance, followed by recording of the completion time and calculation of the gait speed (meter/second) twice to obtain the maximum value.
Sit-to-stand 60 s (STS-60) test
The number of times an individual patient stood up and sat down on a chair within 60 s was calculated. This STS-60 test assessed the strength and endurance of muscles of the lower limbs of the tested subject [19]. The patient was asked to complete the sit down-stand up-sit down movement as many times as possible within 60 s by holding his/her hands in front of the chest and starting from the sitting position. The number of STS-60 movements was recorded at the end of the test.
Dependence in IADL
Physical function was also measured using the instrumental activities of daily living (IADL) scale [20, 21]. The IADL scale included 8 items: ability to shop on the street, do outdoor activities, cook meals, do household activities, wash clothes, use the telephone, take medications, and handle personal finances. The evaluation of the results of each item was divided into 3 levels: completion with no assistance, completion with partial assistance, and completion with full assistance. The IADL scale of individuals with any item which needed assistance (including partial or full assistance) was characterized as impaired IADL, otherwise IADL was characterized as intact.
Ultrasound measurement
A portable ultrasound machine with a 60-mm width curvilinear transducer (2–5 MHz, S II, SonoSite, USA) was used to gain enough ultrasound window to cover the whole width of the rectus femoris muscle. All ultrasounds were standardized by the default machine settings (gain, and focus), and the scanning depth was set at 56 mm. One experienced pain physician who had been practicing musculoskeletal ultrasound for more than 10 years obtained all the images.
B-mode 2D ultrasonography of the right rectus femoris was taken immediately once hemodialysis was finished. The patient would lie in the supine position at a 45° angle, with the rectus femoris muscle relaxed. A line at the midpoint between the anterior superior iliac spine and the superior patellar border was marked on the skin. The ultrasound transducer was placed perpendicular to the longitudinal axis of the thigh, overlying the marked line. Minimal pressure was applied to avoid muscle distortion. In the transverse ultrasound image, the rectus femoris muscle was confirmed and the muscle epimysium boundary was identified as an oval circle wrapping around the muscle fibers. Two images from the same point for each person were saved.
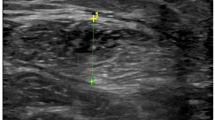
Ultrasound images were transferred to the computer for measurements using Image J software (National Institute of Health, USA). The whole rectus femoris muscle within its epimysium was circled as the region of interest for cross sectional area (CSA) and mean EI measurement (Fig. 1). The software returned measures of CSA and the mean EI, which was expressed as a value between 0 (black) and 255 (white) (Fig. 1). Two images in the same region for the same patient were obtained to assess test–retest reliabilities for EI values, and the average CSA and mean EI values of the two images were analyzed.
Representative ultrasound image of a relaxed rectus femoris. Rectus Femoris cross-sectional area is outlined by the dotted line. The histogram analysis for computerized quantitative grayscale analysis is illustrated at the lower right corner of the image. RF, rectus femoris; VL, vastus lateralis; VI, vastus intermedius
Statistical analysis
Continuous data are presented as mean ± standard deviation, and categorical data are presented as a percentage. For continuous data, one-way ANOVA was used to compare two groups when normal distribution was fulfilled; otherwise, the non-parametric Wilcoxon rank sum test was performed. For categorical data, the chi-square test was performed. Test–retest reliability for EI values was assessed by intra-class correlation coefficient.
The association of EI with physical function was examined using Pearson’s correlation analysis. Multiple linear regression (continuous outcomes) and logistics regression (dichotomous outcomes) were performed to assess the independent association of EI and CSA with physical function. The two model methods were as follows: Model 1—only adjusted for EI and CSA; Model 2—further adjusted for gender, Model 3—further adjusted for age, gender, and dialysis duration. All statistical analyses were performed using MedCalc Software (Version 18.2.1 MedCalc Software Ltd, Belgium). P < 0.05 was considered statistically significant.
Results
Patient characteristics
As shown in Table 1, a total of 107 patients on maintenance hemodialysis were enrolled in this study. Patients were 37.3% female (n = 40), mean age was 53.53 ± 12.52 years, average dialysis duration was 2.97 ± 2.32 years, and average BMI was 21.43 ± 3.77 kg/m2. In addition, 15.9% of the patients had diabetes (n = 17), 93.5% of the patients had hypertension (n = 100), and 26.2% (n = 28) of the patients had dependency in IADL. The ultrasound measurements for EI and CSA are 62.34 ± 16.18 and 6.37 ± 1.58 respectively. The EI value of the male group was significantly lower than in the female group (58.21 ± 16.59 versus 69.26 ± 12.93, P < 0.001), and the CSA of the male group was significantly higher than the female group (6.91 ± 1.55 versus 5.46 ± 1.20, P < 0.001).
The intra-class correlation coefficient showed an excellent test–retest reliability for EI values (ICC = 0.948, 95% [0.925,0.965], P < 0.001). In addition, older age was moderated correlated with enhanced EI (r = 0.323, P = 0.001), while dialysis vintage was not correlated with EI (r = 0.088, P = 0.368).
Relationship between muscle quality and physical function
As shown in Fig. 2, the EI of the overall patient population on hemodialysis was negatively correlated with HGS (r = − 0.467, P < 0.001), gait speed (r = − 0.285, P = 0.003), and STS-60 (r = − 0.313, P = 0.001).
Comparison of physical function with muscle quality (EI) and muscle quantity (CSA)
Further multiple linear regression analyses and logistic regression analyses were performed to assess the relationship between EI, CSA, and physical function. As shown in Table 2, Model 1 adjusted for the EI and CSA, Model 2 further adjusted for gender, and Model 3 further adjusted for age, gender, and dialysis duration. Enhanced EI was associated with worse HGS (β = − 0.207, P = 0.047), lower gait speed (β = − 0.002, P = 0.001), less STS-60 (β = − 0.136, P = 0.049), and a higher likelihood of dependency in IADL in Model 1 (OR: 1.070; 95% CI: [1.030–1.111], P = 0.001). These associations remained statistically significant after further adjusting for sex in Model 2, but enhanced EI was only associated with dependency in IALD (P = 0.001) in Model 3. Except for STS-60, increased CSA was a significant predictor of better HGS, higher gait speed, and lower IALD disability among all Model 1, Model 2, and Model 3 in patients on hemodialysis.
Discussion
This study was the first to report the relationship between ultrasound-derived EI of the skeletal muscle and physical function in patients on hemodialysis. Our results showed that high EI was closely related to low physical function including HGS, gait speed, STS-60, and IADL disability in patients on hemodialysis.
The pathophysiological mechanisms of decreased muscle quality include muscle fiber reduction, mitochondrial dysfunction of myocytes, intramuscular fat infiltration, and increased fibrosis [22]. The existing assessment methods of muscle quality mainly include CT, MRI, and ultrasound. Although CT and MRI directly evaluate the degree of intramuscular fat infiltration, they are expensive and require patients to go to a specific place or have a certain degree of radiation, which affects patient compliance with the examination. Ultrasound is being increasingly implemented by investigators in the fields of muscle measurements, taking ultrasound EI as a vital indicator of muscle quality. Compared with percent intramuscular fat measurements derived from high-resolution T1-weighted MRI imaging, ultrasound EI proved to be a practical and reproducible method that can be used as an imaging technique for examination of percent intramuscular fat [23]. Age-related increases in EI, which represents decreases in muscle quality with increasing intramuscular adipose and fibrous tissue, have been reported in numerous studies. A comparison study demonstrated that the young group had significantly lower EI than that of the older group, and there are significant and moderate correlations between EI and CT values (r = -0.502) and between EI and percentage of low-density muscle area (r = 0.441), suggesting that higher EI at least partly reflects intramuscular lipid infiltration [24].
Numerous studies in the elderly have confirmed the close relationship between ultrasound-derived EI isometric muscle strength and physical function. In elderly men, the ultrasound EI showed a significant negative correlation with muscle strength (r = − 0.333), and multivariate regression analysis revealed that the EI of the knee extensor muscle was independently associated with maximum isometric knee extension strength [16]. A study on volunteer elder men resulted that quadriceps femoris EI was correlated to functional capacity and power countermovement jump, and the maximal number of repetitions completed in the 30-s sit-to-stand test presented weak to moderate significant correlation with EI of four quadriceps components [17]. In a study, participants were categorized into the low, mid, and high EI groups according to the EI of the thigh. The researchers found that the high EI group exhibited a significantly slower 5-m walking time and shorter 6-min walking distance than the low EI group and had a shorter moderate-intensity activity time than the mid EI group [25]. In the field of kidney diseases, only one small-scale study of stages 3–5 CKD pre-dialysis patients has reported that EI is moderately and negatively correlated with the STS-60 test, the incremental shuttle walk test (ISWT) (n = 61), and peak oxygen consumption (VO2 peak) results (n = 32), but not with the gait speed and HGS (n = 29); EI was not correlated with any index of physical function when the CSA was corrected [18]. In this study, EI was moderately and negatively correlated with HGS, gait speed, and STS-60 in patients on hemodialysis. Moreover, after adjusting for muscle area, high EI in patients on hemodialysis remained associated with worse HGS, low gait speed, reduced STS-60, and IADL disability. Thus, this is the first report showing the value of ultrasound-derived EI in muscle evaluation of patients on hemodialysis.
As expected, analysis of the ultrasound parameters of the rectus femoris showed that the CSA of the male group was significantly higher than in the female group, and the EI value of the male group was markedly lower than in the female group. Male muscles are stronger, with larger CSA of the rectus femoris muscle. These phenomena are consistent with other studies. A CT imaging study investigating skeletal muscle quantity and quality in end-stage renal disease showed that muscle CSA was significantly greater in men than in women [2]. The proportion of body fat in the females was generally higher than that of males, and then, the EI value of the female skeletal muscle was higher. A number of studies have reported that EI values are greater in women than in men in both young and old adult populations [13]. In addition, our study lighted that after adjusting for sex in Model 2 of multiple linear regression analyses and logistic regression, enhanced EI remained associated with worse HGS, lower gait speed, less STS-60, and a higher likelihood of dependency in IADL. Therefore, the relationship between physical function and EI (and CSA) is likely to be independent to sex. This result was just consistent with previous studies [10, 26].
In addition to muscle quality, muscle quantity is also an essential indicator for muscle evaluation of patients. A wealth of evidence from multiple studies has shown that muscle size is closely related to the physical function of patients on hemodialysis [2, 3, 27]. When we further corrected for factors such as age, gender, and the duration of dialysis, only CSA was related to the level of physical function of the patients. Thus, in the clinical setting, the combined evaluation of muscle quantity and muscle quality may provide more information for accurately assessing muscle strength and physical function in patients [10].
Loss of muscle quantity and quality in patients on hemodialysis may be related to increased oxidative stress, accumulation of uremic toxins, reduced exercise activities, and malnutrition [28, 29]. Exercise training is an effective way to improve muscle function. In patients with CKD, ultrasound measurement of CSA has been used to effectively assesses muscle mass gain after exercise intervention [30]. Impendence movement reduced the accumulation of fat in the muscles of the elderly [31]. However, there are quite a few researches on the improvement of muscle quality through exercise in patients on hemodialysis. A recent study revealed that seven months of intradialytic aerobic exercise training improved functional capacity and prevented thigh muscle mass loss in hemodialysis patients, and indicated that muscle ultrasonography could play a pivotal role in assessing muscle quality changes in hemodialysis patients [32]. However, the researchers used the vastus lateralis fascicle angle as the ultrasound measurement of the muscle quality in that study. Indeed, further research is needed to verify whether EI can be used as an evaluation index for the improvement of muscle quality.
One of the concerns about the validity of muscle echogenicity as a surrogate measure of muscle quality in dialysis patients is that ultrasound technology is relatively less established and highly tester-and analyzer-dependent by nature. Unfortunately, so far no consistent protocol has arrived for image acquisition and analysis in the field of muscle measurements. EI values may differ between different studies and are not comparable. In our study, we applied some methods to make the results reliable. First, we used a portable ultrasound device in our study, and all the parameters (gain, focus, dynamic range and frame rate) were kept in the default settings of the machine, with the scanning depth at 56 mm. We didn’t change these parameters during the whole process of measurement for the worry that any change of the parameters would complicate the comparison of the data obtained from different patients. Second, the CSA and mean EI values of the two images obtained in the same region for the same patient were averaged to analyze to minimize the instability of ultrasound probe held by the operator. Last, considering the effects of fluid status on ultrasound muscle measurement in patients on hemodialysis, we took ultrasonography of the right rectus femoris after hemodialysis process. Ultrasound-based evaluation of muscle quantity and quality of patients on hemodialysis provided more information for assessing the level of physical function in patients. It has significant clinical value and broad application prospects in the muscle evaluation of patients on hemodialysis. Nevertheless, there is an urgent need to develop a consensus on scanning technique and image analysis for ultrasound muscle measurement.
This study had some limitations: First, as no standardized measurement method for EI is currently available, we used the raw data of EI. Subcutaneous adipose thickness can attenuate ultrasound waves and influence muscle EI reliability; an additional method in ultrasound muscle quality measurement is to adjust EI [13]. However, besides depth, the transmission of ultrasound beams through the tissue can be attenuated due to complicated factors, such as reflection, dispersion, or absorption of the sound waves. Second, we measured regional leg muscles to estimate systemic muscle quality in patients on hemodialysis, the relation of EI of leg muscle with other limb functionality may be reserved, and further studies need to clarify the relationship. Third, this was a cross-sectional study, so it is impossible to clarify the etiological inference of the decline in muscle quality represented by EI and the decreased physical function of patients on hemodialysis. In addition, this study was carried out in a single dialysis center in China, and the generalization of our conclusions requires international, multi-center studies.
Conclusion
The present study revealed that enhanced ultrasound-derived EI was correlated with poor physical performance in patients on hemodialysis. The combined muscle quality and muscle quantity evaluation provides more information for assessing the level of physical function of the patients.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Change history
25 May 2022
We have switched the affiliation numbers for author Dongsheng Cheng.
Abbreviations
- EI:
-
Echo intensity
- CSA:
-
Cross-sectional area
- HGS:
-
Handgrip strength
- STS-60:
-
Gait speed, sit-to-stand 60 s
- IADL:
-
Instrumental activities of daily living
- CT:
-
Computed tomography; MRI: magnetic resonance imaging
- CKD:
-
Chronic kidney disease
- BMI:
-
Body mass index
- ISWT:
-
Incremental shuttle walk test
- SD:
-
Standard deviation
- SE:
-
Standard error
References
Jassal SV, Karaboyas A, Comment LA, Bieber BA, Morgenstern H, Sen A, et al. Functional Dependence and Mortality in the International Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2016;67(2):283–92. https://doi.org/10.1053/j.ajkd.2015.09.024.
Cheema B, Abas H, Smith B, O’Sullivan AJ, Chan M, Patwardhan A, et al. Investigation of skeletal muscle quantity and quality in end-stage renal disease. Nephrology (Carlton). 2010;15(4):454–63. https://doi.org/10.1111/j.1440-1797.2009.01261.x.
McIntyre CW, Selby NM, Sigrist M, Pearce LE, Mercer TH, Naish PF. Patients receiving maintenance dialysis have more severe functionally significant skeletal muscle wasting than patients with dialysis-independent chronic kidney disease. Nephrol Dial Transplant. 2006;21(8):2210–6. https://doi.org/10.1093/ndt/gfl064.
Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez-Mieyer P, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90(6):1579–85. https://doi.org/10.3945/ajcn.2009.28047.
Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60(3):324–33. https://doi.org/10.1093/gerona/60.3.324.
Correa-de-Araujo R, Harris-Love MO, Miljkovic I, Fragala MS, Anthony BW, Manini TM. The need for standardized assessment of muscle quality in skeletal muscle function deficit and other aging-related muscle dysfunctions: a symposium report. Front Physiol. 2017;8:87. https://doi.org/10.3389/fphys.2017.00087.
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. https://doi.org/10.1093/ageing/afy169.
Wilkinson TJ, Gould DW, Nixon DGD, Watson EL, Smith AC. Quality over quantity? Association of skeletal muscle myosteatosis and myofibrosis on physical function in chronic kidney disease. Nephrol Dial Transplant. 2019;34(8):1344–53. https://doi.org/10.1093/ndt/gfy139.
Dong J, Dong Y, Chen Z, Mitch WE, Zhang L. The pathway to muscle fibrosis depends on myostatin stimulating the differentiation of fibro/adipogenic progenitor cells in chronic kidney disease. Kidney Int. 2017;91(1):119–28. https://doi.org/10.1016/j.kint.2016.07.029.
Bourgeois B, Fan B, Johannsen N, Gonzalez MC, Ng BK, Sommer MJ, et al. Improved strength prediction combining clinically available measures of skeletal muscle mass and quality. J Cachexia Sarcopenia Muscle. 2019;10(1):84–94. https://doi.org/10.1002/jcsm.12353.
Nijholt W, Scafoglieri A, Jager-Wittenaar H, Hobbelen JSM, van der Schans CP. The reliability and validity of ultrasound to quantify muscles in older adults: a systematic review. J Cachexia Sarcopenia Muscle. 2017;8(5):702–12. https://doi.org/10.1002/jcsm.12210.
Harris-Love MO, Monfaredi R, Ismail C, Blackman MR, Cleary K. Quantitative ultrasound: measurement considerations for the assessment of muscular dystrophy and sarcopenia. Front Aging Neurosci. 2014;6:172. https://doi.org/10.3389/fnagi.2014.00172.
Stock MS, Thompson BJ. Echo intensity as an indicator of skeletal muscle quality: applications, methodology, and future directions. Eur J Appl Physiol. 2021;121(2):369–80. https://doi.org/10.1007/s00421-020-04556-6.
Grimm A, Teschner U, Porzelius C, Ludewig K, Zielske J, Witte OW, et al. Muscle ultrasound for early assessment of critical illness neuromyopathy in severe sepsis. Crit Care. 2013;17(5):R227. https://doi.org/10.1186/cc13050.
Fukumoto Y, Ikezoe T, Yamada Y, Tsukagoshi R, Nakamura M, Mori N, et al. Skeletal muscle quality assessed from echo intensity is associated with muscle strength of middle-aged and elderly persons. Eur J Appl Physiol. 2012;112(4):1519–25. https://doi.org/10.1007/s00421-011-2099-5.
Watanabe Y, Yamada Y, Fukumoto Y, Ishihara T, Yokoyama K, Yoshida T, et al. Echo intensity obtained from ultrasonography images reflecting muscle strength in elderly men. Clin Interv Aging. 2013;8:993–8. https://doi.org/10.2147/cia.S47263.
Wilhelm EN, Rech A, Minozzo F, Radaelli R, Botton CE, Pinto RS. Relationship between quadriceps femoris echo intensity, muscle power, and functional capacity of older men. Age (Dordr). 2014;36(3):9625. https://doi.org/10.1007/s11357-014-9625-4.
Wilkinson TJ, Gould DW, Nixon DGD, Watson EL, Smith AC. Quality over quantity ? Association of skeletal muscle myosteatosis and myofibrosis on physical function in chronic kidney disease. Nephrol Dial Transplant. 2019;34(8):1344–53. https://doi.org/10.1093/ndt/gfy139.
Rikli RE, Jones CJ. Development and validation of criterion-referenced clinically relevant fitness standards for maintaining physical independence in later years. Gerontologist. 2013;53(2):255–67. https://doi.org/10.1093/geront/gns071.
Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179–86.
Cheng XS, Myers J, Han J, Stedman MR, Watford DJ, Lee J, et al. Physical performance testing in kidney transplant candidates at the top of the waitlist. Am J Kidney Dis. 2020;S0272–6386(20):30728–9. https://doi.org/10.1053/j.ajkd.2020.04.009.
Russ DW, Gregg-Cornell K, Conaway MJ, Clark BC. Evolving concepts on the age-related changes in “muscle quality.” J Cachexia Sarcopenia Muscle. 2012;3(2):95–109. https://doi.org/10.1007/s13539-011-0054-2.
Young HJ, Jenkins NT, Zhao Q, McCully KK. Measurement of intramuscular fat by muscle echo intensity. Muscle Nerve. 2015;52(6):963–71. https://doi.org/10.1002/mus.24656.
Watanabe Y, Ikenaga M, Yoshimura E, Yamada Y, Kimura M. Association between echo intensity and attenuation of skeletal muscle in young and older adults: a comparison between ultrasonography and computed tomography. Clin Interv Aging. 2018;13:1871–8. https://doi.org/10.2147/CIA.S173372.
Yoshiko A, Natsume Y, Makino T, Hayashi T, Umegaki H, Yoshida Y, et al. Higher and lower muscle echo intensity in elderly individuals is distinguished by muscle size, physical performance and daily physical activity. Ultrasound Med Biol. 2019;45(9):2372–80. https://doi.org/10.1016/j.ultrasmedbio.2019.05.029.
Sai A, Tanaka K, Ohashi Y, Kushiyama A, Tanaka Y, Motonishi S, et al. Quantitative sonographic assessment of quadriceps muscle thickness for fall injury prediction in patients undergoing maintenance hemodialysis: an observational cohort study. BMC Nephrol. 2021;22(1):191. https://doi.org/10.1186/s12882-021-02347-5.
Giglio J, Kamimura MA, Lamarca F, Rodrigues J, Santin F, Avesani CM. Association of sarcopenia with nutritional parameters, quality of life, hospitalization, and mortality rates of elderly patients on hemodialysis. J Ren Nutr. 2018;28(3):197–207. https://doi.org/10.1053/j.jrn.2017.12.003.
Moorthi RN, Avin KG. Clinical relevance of sarcopenia in chronic kidney disease. Curr Opin Nephrol Hypertens. 2017;26(3):219–28. https://doi.org/10.1097/mnh.0000000000000318.
Song YR, Kim JK, Lee HS, Kim SG, Choi EK. Serum levels of protein carbonyl, a marker of oxidative stress, are associated with overhydration, sarcopenia and mortality in hemodialysis patients. BMC Nephrol. 2020;21(1):281. https://doi.org/10.1186/s12882-020-01937-z.
Gould DW, Watson EL, Wilkinson TJ, Wormleighton J, Xenophontos S, Viana JL, et al. Ultrasound assessment of muscle mass in response to exercise training in chronic kidney disease: a comparison with MRI. J Cachexia Sarcopenia Muscle. 2019;10(4):748–55. https://doi.org/10.1002/jcsm.12429.
Marcus RL, Addison O, Kidde JP, Dibble LE, Lastayo PC. Skeletal muscle fat infiltration: impact of age, inactivity, and exercise. J Nutr Health Aging. 2010;14(5):362–6. https://doi.org/10.1007/s12603-010-0081-2.
Krase AA, Terzis G, Giannaki CD, Stasinaki AN, Wilkinson TJ, Smith AC, et al. Seven months of aerobic intradialytic exercise training can prevent muscle loss in haemodialysis patients: an ultrasonography study. Int Urol Nephrol. 2022;54(2):447–56. https://doi.org/10.1007/s11255-021-02931-6.
Acknowledgements
We wish to thank Dr. Jing Chen and Dr Zhenyi Jia for their assistance and thoughtful academic discussions with the current study.
Funding
This work was supported by grants from the Shanghai Municipal Health Commission [grant number 20194y0347], the Clinical Project of Shanghai Sixth People's Hospital [grant number YNLC201806], and the National Natural Science Foundation of China [grant numbers 81670657,81870504, 81870468].
Author information
Authors and Affiliations
Contributions
JZW and HQL contributed to the conception of the study, performed statistical analysis and wrote the initial manuscript draft. JZW, SRR and LXS participated in study coordination, performed the recruitment, took the measures and collected the data. DSC and NSW contributed to the conception of the study, supervised the study, helped to interpret the results and corrected the draft. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study protocol complied with the Helsinki Declaration standards and was approved by the Ethics Committee of the People's Hospital of Menghai County (approval number 2020-SNK-001) and Shanghai Jiao Tong University Affiliated Sixth People’s Hospital (approval number 2020–175). All subjects signed the written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wu, J., Luo, H., Ren, S. et al. Enhanced echo intensity of skeletal muscle is associated with poor physical function in hemodialysis patients: a cross-sectional study. BMC Nephrol 23, 186 (2022). https://doi.org/10.1186/s12882-022-02816-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-022-02816-5