Abstract
Background
This study aims to evaluate the impact of multidisciplinary pre-dialysis care (MDPC) on the risks of peritonitis, technique failure and mortality in peritoneal dialysis (PD) patients.
Methods
Incident end-stage kidney disease patients who received peritoneal dialysis (PD) for more than 90 days were recruited in this study from 1 January 1, 2007 to December 31, 2018. Patients were classified into two groups, the MDPC group and the control group, that received the usual care by nephrologists. Risks of the first episode of peritonitis, technique failure and mortality were compared between the two groups.
Results
There were 126 patients under the usual care and 546 patients under the MDPC. Patients in the MDPC group initiated dialysis earlier than those in the non-MDPC group. There was no significant difference between these two groups in time to the first episode of peritonitis. Compared to the non-MDPC group, the MDPC group was at similar risks of technique failure (adjusted HR = 0.85, 95% CI = 0.64–1.15) and mortality (adjusted HR = 0.66, 95% CI = 0.42–1.02). Among patients with diabetes, the risk of mortality was significantly reduced in the MDPC group with an adjusted HR of 0.45 (95% CI = 0.25–0.80).
Conclusions
There was no significant difference in time to develop the first episode of peritonitis, and risks of technique failure and mortality between these two groups. Diabetic PD patients under MDPC had a lower risk of mortality than those under the usual care.
Similar content being viewed by others
Introduction
Chronic kidney disease (CKD), defined by reduced glomerular filtration rate, proteinuria or structural kidney disease, is an important global health issue with the prevalence around 12–16% and high associated mortality [1, 2]. CKD is generally progressive and irreversible, and could progress to end-stage kidney disease (ESKD). Patients with ESKD require renal replacement therapy to maintain their lives. Because of a shortage of organ donation, the majority of ESKD patients in Taiwan undergo maintenance dialysis, including hemodialysis (HD) and peritoneal dialysis (PD) [3]. PD is cost-effective dialysis modality associated with better preservation of residual renal function compared to HD [4, 5]. However, the majority of ESKD patients in Taiwan received HD rather than PD. A higher technique failure rate and a higher risk of peritonitis in patients treated with PD compared to those treated with HD are major challenges for caring PD patients [6, 7].
As patients with CKD usually have multiple coexisting comorbidities, a coordinated multidisciplinary care may be needed to improve the management and outcome of these patients [8,9,10,11,12,13,14]. A nationwide multidisciplinary pre-dialysis care (MDPC) program has been established since November 2006 in Taiwan to improve the quality and outcomes of pre-dialysis care. The team of MDPC consists of nephrologists, dietitians and nurses and provides standardized pre-dialysis education according to the Kidney Disease Outcomes Quality Initiative guidelines. Dietitians provide dietary consultation. Nurses of case management contact patients to ensure regular follow-up and deliver knowledge of nutrition, life style modification, nephrotoxin avoidance, medication, risk factors and complications of kidney disease, pre-dialysis preparation, and dialysis modality every 1–3 months. The benefits, disadvantages and self-care for different renal replacement modalities are explained. However, studies about the impact of MDPC on the outcome of PD patients are limited [13, 15]. The aim of this study is to evaluate the impact of MDPC on the risks of post-dialysis peritonitis, technique failure and mortality in PD patients using recent data at a tertiary medical center in Taiwan.
Materials and methods
Data source
The medical records of patients with ESKD undergoing PD from January 1, 2007 to December 31, 2018 were collected for this study at the China Medical University Hospital, one of the major teaching medical centres in Taiwan. The medical records contained the information of demographic data, medical history, underlying comorbid conditions, laboratory data and treatment at the beginning of the PD therapy. This study was performed in compliance with guidelines of the Declaration of Helsinki. This retrospective observational study was approved by the Research Ethics Committee of China Medical University Hospital [CMUH103-REC2-070 (CR5)]. Because this study involved retrospective review of existing data, the Research Ethics Committee of China Medical University Hospital specifically waived the need for informed consent.
Study population
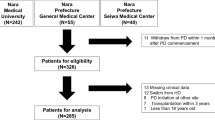
Patients aged 18 years and older receiving PD for more than 90 days were identified and classified into two groups: those who had received the MDPC program as the study group and those who received the usual care by nephrologists as the control group. The pre-dialysis was defined as at least 90 days before the initiation of dialysis. An earlier study from our center revealed that icodextrin use was associated with lower risks of both technique and death [16]. A recent study also from our center demonstrated that APD is associated a lower risk of technique failure than CAPD [17]. Thus, the use of icodextrin or APD was considered as a covariate in data analysis. Patients who had received icodextrin for at least 30 days were defined as the users of icodextrin. Similarly, patients who received APD for at least 30 days were defined as the users of APD. The others were classified as the continuous ambulatory peritoneal dialysis (CAPD) group. The dose of PD was prescribed incrementally to aim for a weekly Kt/V ≥ 1.7 [18, 19]. The adequacy of PD was measured one month after the initiation of PD and every 6 months. All patients were followed up until transfer from PD to HD, renal transplantation, transfer to another hospital, death, or December 31, 2018, whichever came first.
Outcomes and covariates
During the follow-up period, incident rates of the first episode of peritonitis, technique failure, and mortality were estimated. Demographic variables included age, gender, life style variables of smoking and alcohol drinking, comorbidities, laboratory data, and treatment.
Statistical analysis
The baseline characteristics between patients with and without MDPC were compared and tested by Chi-square test and Student’s t test for categorical variables and continuous variables, respectively. The Kaplan–Meier method was used to estimate and plot cumulative incidence of outcomes. The multivariate Cox proportional hazards model was used to estimate the adjusted hazard ratio (HR) and 95% CI after controlling for variables with a p value < 0.25 in the univariate Cox model. The subhazard ratio (SHR) and 95% confidence interval (CI) was also calculated with considering deaths as a competing risk [20]. Technique failure was defined as transfer to HD for at least 30 days or death on PD [21, 22]. Death is one of the major causes of drop-out in PD patients. In addition, complications of PD such as ultrafiltration failure, peritonitis, mineral bone disease, cardiovascular disease, and encapsulating peritoneal sclerosis may lead to death. Thus, death was included as a cause of technique failure. Renal transplantation, transfer to another hospital for care, and alive at the end of the study period were censored for technique survival analysis. If patients died within 90 days after switching to HD, the death was attributed to PD and counted as a death event. Otherwise, transfer to HD, renal transplantation, transfer to other hospital for care, and alive at the end of the study period (December 31, 2018) were censored for patient survival analysis. Stratification analysis by diabetes status was also performed to estimate its impact on outcomes. The statistical software SAS (version 9.4; SAS Institute, Inc., Cary, NC, USA) and R (version 2.1) was utilized to perform the analysis.
Results
There were 126 patients under the usual care and 546 patients under the MDPC program. 34 patients were transferred to other hospitals and 43 patients received transplantation in the MDPC group. 6 patients were transferred to other hospitals and 8 patients underwent transplantation in the control group. The mean follow-up time for patients in MDPC and usual care groups were 5.20 ± 3.18 years and 5.41 ± 3.48 years (p = 0.53), respectively. The MDPC group consisted of more women and elderly patients than the control group (Table 1). Less than 20% of patients were smokers or had alcohol drinking. Patients in the MDPC group were more likely to use automated peritoneal dialysis (APD) and less likely to have gout. 3 patients and 5 patients in the MDPC and control groups had anuria respectively (data not shown). The MDPC group had a higher mean renal Kt/V than that in control patients (0.66 ± 0.43 versus (vs.) 0.47 ± 0.36, p < 0.001), but a lower mean peritoneal Kt/V (1.31 ± 0.36 vs 1.46 ± 0.37, p < 0.001). However, there was no difference in renal Kt/V between the two group for diabetes patients (1.90 ± 0.38 versus (vs.) 1.89 ± 0.41, p = 0.90). The proportion of patients under MDPC increased in the most recent year. The top three causes of ESKD were diabetes, chronic glomerulonephritis and hypertension.
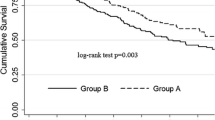
Figure 1 shows that the cumulative incident rates of the first episode of peritonitis, and technique failure and survival probability of patients were not different between the MDPC and control groups.
The incident rates of the first episode of peritonitis were similar between the MDPC group and controls (Table 2). The incidence of technique failure was lower in the MDPC group than in controls, but the estimated HRs were all not significant. The MDPC group had a lower mortality rate than the control group (0.47 versus 0.56 per 10 person-years), with an adjusted HR of 0.63 (95% CI = 0.41–0.97) after controlling for gender, age, smoking, diabetes, hypertension, cardiovascular disease, liver cirrhosis, gout, hepatitis C, icodextrin use, APD use, peritoneal permeability, Kt/V, albumin and hemoglobin. Using another model replacing total Kt/V by renal Kt/V, the adjusted HR for mortality became 0.66 (95% CI = 0.42–1.02).
Table 3 presents the impact of MDPC in patients with and without diabetes. Diabetes patients receiving MDPC had significantly reduced risk of mortality compared to controls with diabetes (adjusted HR = 0.45, 95% CI = 0.25–0.80).
The most common causes of technique failure were death and peritonitis (Table 4), while the most common causes of mortality were cardiovascular disease and infection (Table 5).
Discussion
Our study demonstrated that the overall risks of developing the first episode of peritonitis, technique failure, and mortality between the MDPC group and the non-MDPC group were not significant. However, diabetic PD patients receiving MDPC had a lower risk of mortality compared to those receiving the usual care.
MDPC for pre-dialysis CKD patients has been shown to be associated with a lower risk of all-cause mortality, a slower estimated glomerular filtration decline, and a decreased risk of progression to ESKD, a lower risk of hospitalization, more planned dialysis starts and a higher proportion of patients initiating dialysis with PD [12, 14, 23, 24]. A retrospective cohort study in the US evaluating 6978 elderly patients with CKD stage 3–5 not yet on dialysis demonstrated that MDPC was associated with a 50% reduction in the risk of death [23]. An open-label, controlled cohort study from Taiwan also revealed that MDPC may decrease the risk of all-cause mortality and reduce the hazard of progression to ESKD for stage 3–5 pre-dialysis CKD patients [14]. Similarly, a recent meta-analysis based on 21 studies also revealed that MDPC reduced the risk of all-cause mortality for patients with stage 4–5 pre-dialysis CKD [12].
The beneficial effects of MDPC might extend to the post-dialysis periods. A small prospective study in Canada including both HD and PD patients revealed that MDPC was associated with a lower risk of deaths after the initiation of dialysis independent of residual renal function, medication use, and laboratory data [9]. A prospective study evaluated the effectiveness of MDPC for patients initiating dialysis at two tertiary care institutions in Vancouver of Canada and in Cremona of Italy [8]. Patients in the MDPC group initiated dialysis at a higher estimated glomerular filtration rate, and had higher hemoglobin, albumin, and calcium compared to those in the non-MDPC group. The non-MDPC group were at an elevated risk of death with a HR of 2.17, compared to the MDPC group. A prospective study from Taiwan found that MDPC was significantly associated with a lower risk of getting the first episode of peritonitis in PD patients [15]. A prospective study in Brazil compared the outcomes between early pre-dialysis care (90 days of follow-up by a nephrology team) and late pre-dialysis care (absent or less than 90 days) in a national cohort of 4107 incident PD patients [13]. The results showed that early pre-dialysis care was associated with better patient survival, but the time to the first episode of peritonitis and technique survival were similar [13]. However, this study failed to adjust residual renal function [13]. In our study, patients in the MDPC group had a higher residual renal function than patients in the non-MDPC group. Thus, patients in the MDPC group were more likely to initiate dialysis earlier than those in the non-MDPC group. In our study, there was no significant difference in laboratory data of albumin, phosphate, hemoglobin, and glycated hemoglobin, distribution of comorbidities, and duration of break-in period between the two groups. In a model without adjustment for residual renal function, MDPC was associated with a lower risk of mortality. However, there was no significant difference between the two groups in risks of mortality after adjustment for residual renal function. The adjustment of residual renal function could reduce the lead-time bias.
Diabetes is a major risk factor for peritonitis, technique failure, and mortality in PD patients [7, 25]. In other words, PD patients with diabetic have a worse prognosis than those without diabetes. There was no difference in the care of MDPC program between diabetic and non-diabetic patients. In our study, the subgroup analysis demonstrated that PD patients with diabetes under the care of MDPC program had a much lower risk of mortality than those in the non-MDPC group.
Although care of PD patients after dialysis initiation are also multidisciplinary approach with involvement of nephrologists, dietitians, and nurses, there might be a legacy effect of MDPC. The positive effects of MDPC include selecting healthier PD candidate, adaptation of positive attitude toward illness, enablement of self-care technique, improvement in patient compliance with treatment, maintenance of a healthier lifestyle, timely initiation of renal replacement therapy, and greater understanding of PD complications. The possible reasons why the diabetic PD patients might get more benefits through this program included better residual renal function preservation, better glycemic control, better blood pressure control, etc. [26]
The strength of this study is the use of a well-organized database of medical records collected in a recent decade with the sample size large enough to evaluate outcomes after a long follow-up period. There are limitations in this study. This study was observational and retrospective in design. Medications such as angiotensin-converting enzyme inhibitors and angiotensin receptor blockers were not included in the analysis. The majority of the study population were less than 70 years. Thus, we did not further analyze the female younger subgroup. In addition, the assignment of MDPC was up to the preference of physicians and patients. There might be a selection bias. However, multivariate analyses were preformed to reduce the bias. The observational study contains valuable information that could be conveyed to the nephrology community.
In conclusion, patients in the MDPC group were more likely to initiate dialysis earlier than those in the non-MDPC group. There were no significant differences in time to the first episode peritonitis, and risks of technique failure and mortality between the MDPC group and the non-MDPC group. The MDPC program could reduce the risk of death for patients with diabetes, compared to those under the usual care.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Murphy D, McCulloch CE, Lin F, Banerjee T, Bragg-Gresham JL, Eberhardt MS, Morgenstern H, Pavkov ME, Saran R, Powe NR, et al. Trends in prevalence of chronic kidney disease in the United States. Ann Intern Med. 2016;165(7):473–81.
Wen CP, Cheng TY, Tsai MK, Chang YC, Chan HT, Tsai SP, Chiang PH, Hsu CC, Sung PK, Hsu YH, et al. All-cause mortality attributable to chronic kidney disease: a prospective cohort study based on 462 293 adults in Taiwan. Lancet. 2008;371(9631):2173–82.
Wang IK, Lin CL, Yen TH, Lin SY, Sung FC. Comparison of survival between hemodialysis and peritoneal dialysis patients with end-stage renal disease in the era of icodextrin treatment. Eur J Intern Med. 2018;50:69–74.
Mohnen SM, van Oosten MJM, Los J, Leegte MJH, Jager KJ, Hemmelder MH, Logtenberg SJJ, Stel VS, Hakkaart-van Roijen L, de Wit GA. Healthcare costs of patients on different renal replacement modalities - Analysis of Dutch health insurance claims data. PLoS ONE. 2019;14(8):e0220800.
Moist LM, Port FK, Orzol SM, Young EW, Ostbye T, Wolfe RA, Hulbert-Shearon T, Jones CA, Bloembergen WE. Predictors of loss of residual renal function among new dialysis patients. J Am Soc Nephrol. 2000;11(3):556–64.
Li PK, Szeto CC, Piraino B, de Arteaga J, Fan S, Figueiredo AE, Fish DN, Goffin E, Kim YL, Salzer W, et al. ISPD peritonitis recommendations: 2016 update on prevention and treatment. Perit Dial Int. 2016;36(5):481–508.
Wang IK, Lu CY, Muo CH, Chang CT, Yen TH, Huang CC, Li TC, Sung FC. Analysis of technique and patient survival over time in patients undergoing peritoneal dialysis. Int Urol Nephrol. 2016;48(7):1177–85.
Curtis BM, Ravani P, Malberti F, Kennett F, Taylor PA, Djurdjev O, Levin A. The short- and long-term impact of multi-disciplinary clinics in addition to standard nephrology care on patient outcomes. Nephrol Dial Transplant. 2005;20(1):147–54.
Goldstein M, Yassa T, Dacouris N, McFarlane P. Multidisciplinary predialysis care and morbidity and mortality of patients on dialysis. Am J Kidney Dis. 2004;44(4):706–14.
Imamura Y, Takahashi Y, Uchida S, Iwamoto M, Nakamura R, Yamauchi M, Ogawara Y, Goto M, Takeba K, Yaguchi N, et al. Effect of multidisciplinary care of dialysis initiation for outpatients with chronic kidney disease. Int Urol Nephrol. 2021;53(7):1435–44.
Ino J, Kasama E, Kodama M, Sato K, Eizumi H, Kawashima Y, Sekiguchi M, Fujiwara T, Yamazaki A, Suzuki C, et al. Multidisciplinary team care delays the initiation of renal replacement therapy in diabetes: a five-year prospective. Single-center Study Intern Med. 2021;60(13):2017–26.
Shi Y, Xiong J, Chen Y, Deng J, Peng H, Zhao J, He J. The effectiveness of multidisciplinary care models for patients with chronic kidney disease: a systematic review and meta-analysis. Int Urol Nephrol. 2018;50(2):301–12.
Spigolon DN, de Moraes TP, Figueiredo AE, Modesto AP, Barretti P, Bastos MG, Barreto DV, Pecoits-Filho R, Investigators B. Impact of pre-dialysis care on clinical outcomes in peritoneal dialysis patients. Am J Nephrol. 2016;43(2):104–11.
Wu IW, Wang SY, Hsu KH, Lee CC, Sun CY, Tsai CJ, Wu MS. Multidisciplinary predialysis education decreases the incidence of dialysis and reduces mortality–a controlled cohort study based on the NKF/DOQI guidelines. Nephrol Dial Transplant. 2009;24(11):3426–33.
Hsu CK, Lee CC, Chen YT, Ting MK, Sun CY, Chen CY, Hsu HJ, Chen YC, Wu IW. Multidisciplinary predialysis education reduces incidence of peritonitis and subsequent death in peritoneal dialysis patients: 5-year cohort study. PLoS One. 2018;13(8):e0202781.
Wang IK, Li YF, Chen JH, Liang CC, Liu YL, Lin HH, Chang CT, Tsai WC, Yen TH, Huang CC. Icodextrin decreases technique failure and improves patient survival in peritoneal dialysis patients. Nephrology (Carlton). 2015;20(3):161–7.
Wang IK, Yu TM, Yen TH, Lin SY, Chang CL, Lai PC, Li CY, Sung FC. Comparison of patient survival and technique survival between continuous ambulatory peritoneal dialysis and automated peritoneal dialysis. Perit Dial Int. 2020;40(6):563–72.
Blake PG, Dong J, Davies SJ. Incremental peritoneal dialysis. Perit Dial Int. 2020;40(3):320–6.
Brown EA, Blake PG, Boudville N, Davies S, de Arteaga J, Dong J, Finkelstein F, Foo M, Hurst H, Johnson DW, et al. International Society for Peritoneal Dialysis practice recommendations: Prescribing high-quality goal-directed peritoneal dialysis. Perit Dial Int. 2020;40(3):244–53.
Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509.
Lan PG, Clayton PA, Johnson DW, McDonald SP, Borlace M, Badve SV, Sud K, Boudville N. Duration of hemodialysis following peritoneal dialysis cessation in Australia and New Zealand: proposal for a standardized definition of technique failure. Perit Dial Int. 2016;36(6):623–30.
See EJ, Johnson DW, Hawley CM, Pascoe EM, Badve SV, Boudville N, Clayton PA, Sud K, Polkinghorne KR, Borlace M, et al. Risk Predictors and causes of technique failure within the first year of peritoneal dialysis: an Australia and New Zealand Dialysis and Transplant Registry (ANZDATA) Study. Am J Kidney Dis. 2018;72(2):188–97.
Hemmelgarn BR, Manns BJ, Zhang J, Tonelli M, Klarenbach S, Walsh M, Culleton BF. Association between multidisciplinary care and survival for elderly patients with chronic kidney disease. J Am Soc Nephrol. 2007;18(3):993–9.
Marron B, Ortiz A, de Sequera P, Martin-Reyes G, de Arriba G, Lamas JM, Martinez Ocana JC, Arrieta J, Martinez F. Spanish Group for CKD: Impact of end-stage renal disease care in planned dialysis start and type of renal replacement therapy–a Spanish multicentre experience. Nephrol Dial Transplant. 2006;21(Suppl2):ii51-55.
Chen HL, Tarng DC, Huang LH. Risk factors associated with outcomes of peritoneal dialysis in Taiwan: an analysis using a competing risk model. Medicine (Baltimore). 2019;98(6):e14385.
Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358(6):580–91.
Acknowledgements
We are grateful to Health Data Science Center, China Medical University Hospital for providing administrative, technical and funding support.
Funding
This study is supported in part by the Ministry of Health and Welfare, Taiwan (MOHW110-TDU-B-212–124004), and China Medical University Hospital (DMR-110–200, DMR-110–037, DMR-107–053, and DMR-109–175).
Author information
Authors and Affiliations
Contributions
I.K.W., T.M.Y., T.H.Y., H.T.Y., P.C.L., and C.Y. L. designed the study and drafted the manuscript. H.T. Y. conducted the statistical analysis. K.T.S., and F.C.S. revised the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed in compliance with guidelines of the Declaration of Helsinki. The Research Ethics Committee of China Medical University Hospital, Taichung, Taiwan, approved the study [CMUH103-REC2-070 (CR5)]. Informed consent was waived by the Research Ethics Committee of China Medical University Hospital, Taichung, Taiwan because this study involved retrospective review of existing data.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, IK., Yu, TM., Yen, TH. et al. The impact of multidisciplinary pre-dialysis care on the outcomes of incident peritoneal dialysis patients. BMC Nephrol 23, 173 (2022). https://doi.org/10.1186/s12882-022-02800-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-022-02800-z