Abstract
Background
Syphilis is a multisystemic infection that causes a wide variety of symptoms and thus has been dubbed one of the great medical mimickers. Due to recent global re-emergence of syphilis, it has become important to recognize its various presentations. Relative to the kidney, syphilitic infections generally present themselves with nephrotic range proteinuria, and are most often associated with pathological features of a membranous glomerulonephritis with subepithelial immune complex deposition. However, other rare renal presentations have been reported. One of these includes a rapidly progressive glomerulonephritis picture. All described cases have been successfully resolved with the treatment of the underlying syphilis infection.
Case presentation
The patient was an elderly woman of Caribbean descent who presented with lower extremity weakness, anasarca and proteinuria, hematuria with progressive renal failure. On kidney biopsy, she was found to have a pauci-immune crescentic glomerulonephritis pattern and a concomitant acute tubulointerstitial nephritis. She had a positive Treponema pallidum particle agglutination test and a negative syphilis rapid plasma reagin test with clinical evidence of polyneuropathy suggestive chronic syphilis infection.
Conclusion and discussion
It is important in the context of pauci-immune crescentic glomerulonephritis to explore all differential diagnoses. Given the positive syphilis serologies, clinical context and presence of tubulointerstitial nephritis, she was determined to have syphilitic glomerulonephritis that resolved with a course of both penicillin and steroids.
Similar content being viewed by others
Background
Rapidly progressive glomerulonephritis (RPGN) is diagnosed when a patient presents with hematuria, proteinuria and increasing serum creatinine over a short period of time. The histopathologic correlate of RPGN is crescentic glomerulonephritis and is commonly classified based on three main patterns: type I is defined as anti-glomerular basement membrane (GBM) positive disease, type II is immune-complex mediated disease and type III is pauci-immune disease. Several infections lead to immunologic dysregulations that result in type II RPGN. The most common infections being post-streptococcal glomerulonephritis caused by streptococcal infection and membranoproliferative glomerulonephritis caused by viral infections such as hepatitis. However, a much rarer cause is syphilis, with only three reported cases [1,2,3]. Generally, syphilitic infection has been associated with nephrotic-range proteinuria caused by membranous glomerulonephritis [4,5,6]. As there is a global rise in syphilis cases, it is important to recognize atypical syphilitic presentations, especially if there is a pattern of RPGN. We present a case of a patient with proteinuria, hematuria and progressive renal failure compatible with a pattern of RPGN.
Case presentation
A retired 77-year-old Caribbean woman presented to the emergency department with general fatigue and lower extremity weakness. She is known for type II diabetes mellitus, hypertension, and a recent episode of small bowel obstruction that had resolved without surgery.
On examination, she had a bilateral severe axonal sensory and motor polyneuropathy (confirmed by nerve conduction studies and electromyography) which was manifested by severe bilateral lower extremity weakness. She also had diffuse anasarca. She did not have any skin findings nor any ophthalmologic symptoms.
She had a previously normal creatinine of 70 μmol/L (0.79 mg/dL), but on admission was found to have an acute kidney injury with a serum creatinine of 122 μmol/L (1.38 mg/dL). The creatinine peaked over 3 weeks to 231 μmol/L (2.61 mg/dL) (Table 1). This was associated with a decreasing albumin and increasing urine protein (Table 1). Urinalysis showed significant proteinuria and hematuria and urine microscopy showed active sediments including dysmorphic erythrocytes. Urine eosinophils were positive twice. A serological panel, which included cytoplasmic and perinuclear anti-neutrophilic antibodies anti-cytoplasmic (p-ANCA and c-ANCA), complement (C3 and C4), anti-nuclear antibodies (ANA), hepatitis B, hepatitis C and human immunodeficiency virus (HIV) serologies were all negative (Table 1). Rheumatoid factor (RF), C-reactive protein (CRP) and the immunoglobulin profile (IMM) were consistent with inflammation. Both the urine protein electrophoresis and the serum protein electrophoresis were normal. Given the neurological symptoms and impressive proteinuria, a Venereal Disease Research Laboratory (VDRL) and Treponema pallidum particle agglutination test (TP-PA) were requested and both were positive. A peripheral electromyography (EMG) with nerve conduction studies, was also consistent with syphilis-induced neuropathy. The patient declined a lumbar puncture to confirm neurosyphilis and confirmed she had never been diagnosed with nor specifically been treated for syphilis before.
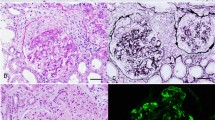
While waiting for the serological testing, a kidney biopsy was performed due to the increasing creatinine with an active urine sediment. The biopsy showed crescentic and necrotizing glomerulonephritis in 9/23 glomeruli (Fig. 1a). Some of the crescentic glomeruli even showed Bowman’s capsule rupture. Numerous plasma cells surrounded the crescentic glomeruli and some of the small blood vessels. There was also concomitant acute tubulo-interstitial nephritis with abundant plasma cells and occasional eosinophils (Figs. 1 and 2). The plasma cells showed an increased proportion (50%) of IgG-4 versus IgG (Fig. 2). There was moderate interstitial fibrosis and tubular atrophy as well as moderate atherosclerosis. No granulomatous inflammation was identified. Glomerular basement spikes or vacuolization were not found. No Treponema pallidum were found by immunohistochemistry and there was no evidence for monoclonality by kappa/lambda in situ hybridization. Immunofluorescence showed only minimal focal non-specific C3 mesangial staining (1+) and fibrinogen positive crescents (3+). IgA, IgG, IgM, C1q, kappa and lambda immunofluorescence were negative. Electron microscopy did not show any significant electron dense deposits in any of the glomerular compartments (Fig. 1c).
a Light microscopy of kidney biopsy showing crescent formation in the glomerulus. Periodic Acid-Schiff stain (400X). b Light microscopy showing tubulointerstitial nephritis with large infiltration of plasma cells and eosinophils. Haematoxylin and Eosin stain (200X) (c) Electron microscopy did not show any significant immune complex deposits (3000X)
Overall, the case was diagnosed as a pauci-immune crescentic glomerulonephritis with a concomitant acute tubulointerstitial nephritis. This was combined with atypical perivascular and periglomerular nonclonal, but significantly positive IgG-4 plasma cells infiltrates. The histological differential diagnosis would include a pauci-immune vasculitis, an atypical post-infectious glomerulonephritis, a secondary drug reaction or inflammatory reaction. Background vascular findings were attributed to the patient’s diabetes type 2 and hypertension. Our biopsy findings being similar to Nandikanti et al. [1] and given the clinical context, this presentation was most likely due to syphilitic infection. Absence of hypocomplementemia and of other organ involvement (thoraco-abdominal and pelvic computerized tomography showed no abnormalities) made IgG4-related disease and tubulointerstitial nephritis unlikely based on the proposed diagnostic criteria for IgG4-related disease [7, 8]. Furthermore, IgG4-disease with a lymphoplasmacytic pattern could not be diagnosed in the presence of an infection such as syphilis [9]. The immunoglobulin profile and RF were likely inflammatory given the C-reactive protein (CRP) of 174 mg/L. An elevated CRP is unlikely to be present IgG4-related disease [8]. The patient had a negative ANA and no symptoms of Sjogren’s syndrome and thus this diagnosis was excluded.
Given that we did not have the confirmatory syphilis test nor the ANCA serology test results initially and given this crescentic pauci-immune picture suggestive of ANCA vasculitis, we gave this elderly patient both intravenous (IV) penicillin G for 2 weeks and half-dose pulse methylprednisolone (500 mg IV daily X 3 days) concurrently. Once the ANCA results were negative, we continued with only the IV penicillin and a fast Prednisone taper. With this treatment, the patient’s serum creatinine decreased from a peak of 231 μmol/L (2.61 mg/dL) to 134 μmol/L (1.51 mg/dL) within 1 week. The serum creatinine remained at a new baseline of 110 μmol/L 1 month afterwards and 97 μmol/L a year later.
Discussion and conclusions
We present a case of syphilitic RPGN in this patient with concomitant neurological symptoms due to neurosyphilis. As mentioned previously, RPGN typically presents in three broad immunohistopathological categories. It is important to consider the clinical context and send for the appropriate serological tests. In the case of syphilis, given the symptoms may be non-specific and mild, it is important to send a syphilis screen in the context of nephrotic range proteinuria and, as this case presentation and others have shown [1, 3], in the context of active urine sediment with hematuria and proteinuria. Timing is crucial, especially in crescentic-necrotizing renal disease - the earlier the diagnosis, the greater the chance of regression of crescent formation and thereby the greater the chance of renal recovery. In our case, the patient’s creatinine decreased in 2 days with treatment.
Similar to syphilitic membranous glomerulonephritis, management of syphilitic RPGN includes treating the underlying syphilitic infection [5, 6, 10,11,12]. As for immunosuppression with steroids, given the paucity of cases in literature of syphilitic RPGN, it is unknown whether steroids are beneficial for induction in the acute treatment phase. Two case reports have successfully treated with penicillin alone [1, 2]. Walker et al. treated a patient with RPGN who required dialysis with both penicillin and methylprednisolone [3]. We opted to give steroids given the cellular crescents and the pauci-immune appearance of the biopsy which was also suspicious for ANCA-associated RPGN. Intravenous penicillin was given for 14 days due to the suspicion of neurosyphilis. In brief, we believe it is prudent to consider steroids if the diagnosis is not clear, especially in the context of crescentic-necrotizing disease. However, the rapid and sustained response to penicillin and a short course of high dose steroids with rapid taper is not typical of an ANCA vasculitis and supports our diagnosis of syphilitic glomerulonephritis.
In conclusion, as the prevalence of syphilis increases, physicians should be mindful of the different renal manifestations of syphilitic infection. As latent syphilis may present vague symptoms or no symptoms, it is important to always consider latent syphilitic infection in any patient with isolated increasing proteinuria and any patient presenting with a RPGN pattern.
Availability of data and materials
Not applicable.
Abbreviations
- RPGN:
-
Rapidly progressive glomerulonephritis
- GBM:
-
Anti-glomerular basement membrane
- p-ANCA:
-
Perinuclear anti-neutrophilic anti-cytoplasmic antibodies
- c-ANCA:
-
Cytoplasmic anti-neutrophilic anti-cytoplasmic antibodies
- ANA:
-
Anti-nuclear antibodies
- IMM:
-
Immunoglobulin profile
- CRP:
-
C-reactive protein
- C3 and C4:
-
Complement 3 and complement 4
- RF:
-
Rheumatoid factor
- HIV:
-
Human immunodeficiency virus
- RPR:
-
Rapid plasma reagin test
- VDRL:
-
Venereal Disease Research Laboratory
- TP-PA:
-
Treponema pallidum particle agglutination test
- EMG:
-
Electromyography
- IV:
-
Intravenous
- H & E:
-
Haematoxylin and eosin
References
Nandikanti DK, George LK, Walker PD, Kumar A, Wall BM. Resolution of syphilis-related rapidly progressive glomerulonephritis with penicillin therapy: case report. Clin Nephrol. 2020;93(2):106–10. https://doi.org/10.5414/CN109847.
Tognetti L, Cinotti E, Tripodi S, Garosi G, Rubegni P. Unusual presentation of secondary syphilis: membranoproliferative glomerulonephritis andmuco-cutaneous lesions. Int J STD AIDS. 2018;29(4):410–3. https://doi.org/10.1177/0956462417733351.
Walker PD, Deeves EC, Sahba G, Wallin JD, O'Neill WM Jr. Rapidly progressive glomerulonephritis in a patient with syphilis. Identification of antitreponemal antibody and treponemal antigen in renal tissue. Am J Med. 1984;76(6):1106–12. https://doi.org/10.1016/0002-9343(84)90866-0.
Tourville DR, Byrd LH, Kim DU, Zajd D, Lee I, Reichman LB, et al. Treponemal antigen in immunopathogenesis of syphilitic glomerulonephritis. Am J Pathol. 1976;82(3):479–92.
Handoko ML, Duijvestein M, Scheepstra CG, de Fijter CW. Syphilis: a reversible cause of nephrotic syndrome. BMJ Case Rep. 2013;2013.
Hunte W. al-Ghraoui F, Cohen RJ. Secondary syphilis and the nephrotic syndrome. J Am Soc Nephrol. 1993;3(7):1351–5. https://doi.org/10.1681/ASN.V371351.
Raissian Y, Nasr SH, Larsen CP, Colvin RB, Smyrk TC, Takahashi N, et al. Diagnosis of IgG4-related tubulointerstitial nephritis. J Am Soc Nephrol. 2011;22(7):1343–52. https://doi.org/10.1681/ASN.2011010062.
Saeki T, Kawano M. IgG4-related kidney disease. Kidney Int. 2014;85(2):251–7. https://doi.org/10.1038/ki.2013.393.
Stone JR, Bruneval P, Angelini A, Bartoloni G, Basso C, Batoroeva L, et al. Consensus statement on surgical pathology of the aorta from the Society for Cardiovascular Pathology and the Association for European Cardiovascular Pathology: I. Inflammatory diseases. Cardiovasc Pathol. 2015;24(5):267–78. https://doi.org/10.1016/j.carpath.2015.05.001.
Mora Mora MT, Gallego Dominguez MS, Castellano Cervino MI, Novillo Santana R, Gomez-Martino Arroyo JR. Membranous glomerulonephritis in a patient with syphilis. Nefrologia. 2011;31(3):372–3. https://doi.org/10.3265/Nefrologia.pre2011.Mar.10819.
Ishiwatari A, Hasegawa J, Hoshino Y, Kaga T, Abe Y, Wakai S. Simultaneous nephrotic syndrome and hepatitis in secondary syphilis: case report and review of the literature. CEN Case Rep. 2015;4(2):223–7. https://doi.org/10.1007/s13730-015-0173-2.
Roh M, Sohn JH, Kim TY, Kim SJ, Kim JS, Chung SJ, et al. Gastric syphilis and membranous glomerulonephritis. Clin Endosc. 2015;48(3):256–9. https://doi.org/10.5946/ce.2015.48.3.256.
Acknowledgements
Not applicable.
Funding
There were no funders nor institutional support nor industry involvement in this case report.
Author information
Authors and Affiliations
Contributions
AQ and LP analyzed and interpreted the patient data regarding the renal disease. PF performed the histological examination of kidney biopsy and was a major contributor in writing and editing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
No ethics approval was required for this case report.
Consent for publication
Participation consent form signed by patient and available upon request.
Competing interests
AQ and LP have no competing interests to declare. POF has no competing interests related to this work to declare. POF has received honoraria from Amgen, EMD Serono, Bristol Myers Squibb, AstraZeneca, Merck Canada, Pfizer Canada, and Roche Canada, outside the submitted work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Qi, A., Fiset, P.O. & Pilozzi-Edmonds, L. Syphilis-related rapidly progressive glomerulonephritis: a case presentation. BMC Nephrol 22, 196 (2021). https://doi.org/10.1186/s12882-021-02404-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-021-02404-z