Abstract
Background
Idiopathic membranous nephropathy (MN) is one of the major glomerulonephritis that cause nephrotic syndrome. The phospholipase A2 receptor (PLA2R) has recently been identified as an endogenous antigen of idiopathic MN. Thrombotic thrombocytopenic purpura (TTP) is a disorder characterized by schistocytes, hemolytic anemia, thrombocytopenia, and organ dysfunction which occurs as a result of thrombi. Patients with acquired TTP have autoantibodies against a disintegrin and metalloprotease with thrombospondin type 1 motif 13 (ADAMTS13). These autoantibodies act as an inhibitor and cause ADAMTS13 deficiency. Idiopathic MN and acquired TTP are usually considered as independent autoimmune diseases. We experienced a patient who developed TTP during the conservative treatment of idiopathic MN, with the coexistence of ADAMTS13 inhibitor and anti-PLA2R antibody.
Case presentation
A 73-year-old man presented with thrombocytopenia, hemolytic anemia, disturbance of consciousness, and acute kidney injury after 4-year course of biopsy-proven idiopathic MN. ADAMTS13 activity was undetectable and the ADAMTS13 inhibitor was identified. Additionally, he was positive for anti-PLA2R antibody. The patient did not have any diseases that could cause secondary thrombotic microangiopathy, and he was diagnosed with acquired TTP. Steroid therapy and plasma exchange were initiated and the acquired TTP resolved. MN achieved remission 3 months after the anti-PLA2R antibody disappeared.
Conclusions
This is the first reported case of acquired TTP developed during conservative treatment of idiopathic MN, with both ADAMTS13 inhibitor and anti-PLA2R antibody positive at the onset of the TTP. The present case suggests that idiopathic MN might be associated with the development of some cases of acquired TTP.
Similar content being viewed by others
Background
Membranous nephropathy (MN) is a kidney disease that often causes nephrotic syndrome. MN is roughly classified into 2 types: idiopathic MN, which develops without any underlying disease, and secondary MN, which develops due to collagen disease, malignant disease, infection, or drug use. The phospholipase A2 receptor (PLA2R) and thrombospondin type-I domain-containing 7A (THSD7A) have been identified as endogenous antigens of idiopathic MN [1, 2]. Additionally, it has been reported that serum anti-PLA2R antibody levels are associated with disease activity, such as the level of proteinuria, and the therapeutic response in idiopathic MN [3]. Therefore, the serum anti-PLA2R antibody level can be used in the diagnosis of idiopathic MN.
Thrombotic thrombocytopenic purpura (TTP) is a disorder characterized by schistocytes, hemolytic anemia, thrombocytopenia, and organ dysfunction caused by thrombi. In terms of renal pathology, the microscopic features of TTP are the same as those of thrombotic microangiopathy, with ectatic glomerular capillary lumina, enlargement of the subendothelial space, mesangiolysis, and reduplication of the glomerular capillary basement membranes. Recent studies have shown that TTP is caused by a deficiency in a disintegrin and metalloprotease with thrombospondin type 1 motif 13 (ADAMTS13), which cleaves von Willebrand factor and prevents excessive platelet aggregation. Patients with acquired TTP have autoantibodies against ADAMTS13, causing a deficiency of ADAMTS13. As a result, platelet thrombi are formed in the microvessels of multiple organs.
Idiopathic MN and acquired TTP are usually regarded as independent diseases. However, herein, we report a case of acquired TTP developed 4 years after the onset of idiopathic MN. In our case, in response to plasma exchange and steroid therapy, both the anti-PLA2R antibody level and ADAMTS13 inhibitor level became undetectable, and these 2 diseases resolved.
Case presentation
A 69-year-old man was referred to our department with proteinuria and edema of the lower limbs in July 2012. His urinary protein excretion was 7.8 g/day and serum creatinine level was 2.10 mg/dL. He was diagnosed with nephrotic syndrome, and a renal biopsy was performed. Light microscopy showed glomerular capillary thickening with periodic acid-Schiff staining (Fig. 1a), and a bubbly appearance and spike formation in the glomerular capillary walls, with periodic acid silver-methenamine staining (Fig. 1b). Immunofluorescence staining showed granular 2 + deposition of immunoglobulin G and complement C3 in the glomerular capillary walls (Fig. 1c). Electron microscopy demonstrated subepithelial deposits. MN was diagnosed as stage II (Ehrenreich-Churg classification) (Fig. 1d). Examinations for collagen disease and malignancy were performed and were negative. Additionally, he did not use any drugs that could cause nephrotic syndrome. Based on these results, he was diagnosed with idiopathic MN.
Histopathological findings in the kidney biopsy from the patient. (a): A glomerulus with thickened basement membranes and normal cellularity is shown (periodic acid-Schiff stain, × 200) (b): A glomerulus with a bubbly appearance and spike formation of the glomerular capillary walls is shown (periodic acid silver-methenamine stain, × 400). (c): Intense immunofluorescence staining for immunoglobulin G on the glomerular capillary walls is shown (× 200) (d): Subepithelial electron-dense deposits and effacement of the podocyte foot processes are observed on electron microcopy (× 8000)
During hospitalization, paroxysmal atrial fibrillation occurred, and oral warfarin was started. Steroid therapy was initiated for MN, and complete remission was achieved. However, the nephrotic syndrome relapsed while the patient was on prednisolone (PSL) at a dose of 5 mg per day. As hypoalbuminemia and edema were manageable by conservative treatment, the dosage of the steroid was maintained, and 2.5 years after the onset of MN, steroid therapy was discontinued.
Four years after his MN diagnosis, he lost consciousness at home, and he was subsequently admitted to our hospital. His consciousness level on the Glasgow Coma Scale was E4V2M5. The patient did not have a fever. His blood pressure was 60/30 mmHg. His pulse rate was 90 beats/min and irregular. Physical examination showed lower extremity edema and petechiae on his front chest. Laboratory blood data on admission were as follows; white blood cell count, 8200 /μL; hemoglobin, 4.8 g/dL; platelet count, 1.7 × 104 /μL; direct bilirubin, 1.4 mg/dL; indirect bilirubin, 4.5 mg/dL; lactate dehydrogenase, 1477 IU/L; C-reactive protein, 2.85 mg/dL; fibrinogen, 275 mg/dL; and fibrin degradation products, 15.9 μg/mL. A direct Coombs test was negative, and schistocytes (4.4%) were detected. As a result, he was diagnosed with thrombotic microangiopathy (TMA).
The patient’s serum creatinine level at the latest visit was 1.83 mg/dL, and it had worsened to 3.76 mg/dL on admission. On the day after hospitalization (day 2), fever was observed, thus his medical condition was consistent with TTP. Disseminated intravascular coagulation (DIC), hemolytic uremic syndrome (HUS), and secondary TMA were considered as differential diagnoses. However, his blood, urine, and sputum cultures were negative. In addition, whole-body contrast-enhanced computed tomography revealed no cancer or a focus of infection. Although diarrhea was observed before admission, his stool culture and serum anti-lipopolysaccharide antibodies were negative, and no bloody stool was observed. Therefore, the possibility of HUS was excluded. Furthermore, he had no history with medications that could cause secondary TMA, and there were no laboratory results suggestive of collagen disease. From these results, acquired TTP was strongly suspected, and plasma exchange was started on the day of admission (day 1). On day 13, his ADAMTS13 activity was found to be undetectable (< 0.5%) and his ADAMTS13 inhibitor level was 1.2 Bethesda U/mL on the admission blood sample. Thus, he was diagnosed with acquired TTP. On the same day, 1 mg/kg (55 mg) per day of PSL was started. On day 23, we withdrew the plasma exchange because the ADAMTS13 inhibitor level became undetectable and the ADAMTS13 activity was 30.2%. On day 68, he was discharged from our hospital.
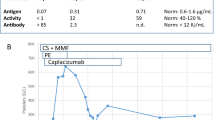
The urinary protein creatinine ratio (g/gCr) at the onset of TTP was 1.8 and thereafter increased to a maximum level of 26.6, then decreased to 7.9 at discharge. The anti-PLA2R antibody level was 89.3 RU/mL at the onset of TTP, and 6.6 RU/mL at the end of the plasma exchange. On day 175, the anti-PLA2R antibody level became undetectable. The ADAMTS13 activity was 23.0% and the ADAMTS13 inhibitor level kept undetectable. On day 259, complete remission of MN was achieved with 10 mg /day of PSL, and his serum creatinine level recovered to 0.96 mg/dL. Since then, there has been no recurrence of either disease (Fig. 2). Anti-THSD7A IgG level was undetectable.
Discussion and conclusion
In the present case, the acquired TTP developed during the conservative treatment of idiopathic MN. Secondary MN and secondary TMA can be caused by collagen diseases such as systemic lupus erythematosus and Sjogren's syndrome, malignancies, and certain drugs, and can sometimes coexist [4]. In contrast, idiopathic MN and acquired TTP have different etiologies, and their coexistence is unusual. In this case, it is possible that these 2 diseases merged by chance. However, as far as our literature review, 3 cases of idiopathic MN and acquired TTP co-existing have been reported [5,6,7], which suggested to us a possible association between these 2 diseases. Several mechanisms which could explain this association might be proposed. First, the presence of a common epitope in PLA2R and ADAMTS13 may have induced the production of antibodies to ADAMTS13. The structures of PLA2R and ADAMTS13 have a cysteine-rich domain in common. However, the cysteine-rich domains of PLA2R and ADAMTS13 have low homology on Protein BLAST (National Center for Biotechnology information, U.S. National Library of Medicine, Bethesda, MD), suggesting that a single antibody is unlikely to recognize both PLA2R and ADAMTS13. Second, epitope spreading could be considered. It has been reported that epitope spreading can induce an autoimmune disease during chronic inflammation [8]. A cysteine-rich domain has been reported to be the major antigenic epitope in idiopathic MN [9]. C-type lectin-like domain (CTLD)1 and CTLD7 have been newly recognized as antigens by intramolecular epitope spreading [10]. In addition, ADAMTS13 may also be newly recognized as an antigen through intermolecular epitope spreading, producing the ADAMTS13 inhibitor, resulting in the development of acquired TTP. There are some reports of anti-glomerular basement membrane glomerulonephritis and vasculitic glomerulonephritis superimposed on MN [11,12,13], suggesting that intermolecular epitope spreading could occur in MN by the same mechanism. Lastly, ADAMTS13 expressed on podocytes might become endogenous antigens, and cause MN. ADAMTS13 is mainly expressed in the liver, but is also known to be expressed on the podocytes of glomerular epithelial cells [14], and it may also be a new endogenous antigen for MN.
The anti-PLA2R antibody was detected in the serum of our patient. The anti-PLA2R antibody test has high sensitivity (82%) and specificity (89%) for idiopathic MN [15]. In a cohort of 131 Japanese patients with MN, there were no false positives for anti-PLA2R antibodies [16]. In this case, none of the tests suggested the presence of a collagen disease, viral infection, or malignancy, and the anti-PLA2R antibody test was positive and high (89.3 RU/mL). Thus, we considered that idiopathic MN was present at the onset of TTP. Furthermore, ADAMTS13 activity was undetectable and the ADAMTS13 inhibitor was detected. A severe decrease in ADAMTS13 activity (< 0.5%) is more likely in acquired TTP than in secondary TMA [17]. Acquired TTP is fatal if untreated, but can be dramatically improved by plasma exchange therapy [18,19,20]. Our patient recovered rapidly with plasma exchange therapy, and the remission of TTP has been maintained for several years. Therefore, this case is consistent with acquired TTP.
As mentioned above, 3 cases of the coexistence of idiopathic MN and an acquired TTP have been reported. In the first case, nephrotic syndrome was diagnosed at the onset of the TTP, and a renal biopsy showed characteristic findings of both MN and TMA [6]. However, the anti-PLA2R antibody and ADAMTS13 inhibitor were not measured. In the second case, a renal biopsy also showed characteristic findings of MN and TMA, although the case had a history of hepatitis C, with no history of proteinuria [7]. Anti-PLA2R antibody and ADAMTS13 inhibitor were detected in the serum, and immunofluorescence staining for renal tissue showed positive for the PLA2R and IgG1 subclass, but not for IgG4, whereas in idiopathic MN, IgG4 is usually predominant with immunofluorescence detection of the IgG subclass [21]. It has been reported that PLA2R staining in renal biopsies may also be positive for secondary MN which is associated with hepatitis C [22]. In the third case, a patient with frequently relapsing acquired TTP developed nephrotic syndrome 7 years after remission of the TTP, and a renal biopsy showed stage II MN, not TMA. Our case differs from these 3 cases in the following manner. Firstly, primary MN had been proven by renal biopsy prior to the acquired TTP. Secondly, anti-PLA2R antibody and ADAMTS13 inhibitor were positive at the onset of the TTP. Lastly, we confirmed that the levels of these 2 antibodies disappeared, and urinary protein levels became undetectable in response to treatment.
A pathological feature of TTP is TMA, and proteinuria is usually detected in TMA. Because TTP is often associated with poor general condition and causes marked decrease in platelets, it is difficult to perform a renal biopsy during the acute phase of TTP. Therefore, even if some cases of TTP are associated with MN, the MN may be overlooked. Therapies for TTP include plasma exchange and steroid therapy, which also reduce anti-PLA2R antibody levels as observed in our case [23]. Therefore, the treatment of TTP will ultimately improve the MN because the therapy can reduce the level of ADAMTS13 inhibitor if ADAMTS13 is an antigen for MN. In this case, 3 months after both antibodies’ levels became undetectable, the MN completely resolved, which is consistent with research that states that the disappearance of circulating anti-PLA2R antibodies precedes clinical remission by several months in idiopathic MN [24].
In summary, we experienced a patient with idiopathic MN who developed acquired TTP a few years later. Two autoantibodies, the anti-PLA2R antibody and ADAMTS13 inhibitor, were detected at the onset of the TTP. We found these antibodies were related to the activity of the TTP and MN in this case. Additionally, there is a possibility that idiopathic MN might be associated with the etiology of some cases of acquired TTP.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- MN:
-
Membranous nephropathy
- PLA2R:
-
Phospholipase A2 receptor
- THSD7A:
-
Thrombospondin type-I domain-containing 7A
- TTP:
-
Thrombotic thrombocytopenic purpura
- ADAMTS13:
-
A disintegrin and metalloprotease with thrombospondin type 1 motif 13
- PSL:
-
Prednisolone
- TMA:
-
Thrombotic microangiopathy
- DIC:
-
Disseminated intravascular coagulation
- HUS:
-
Hemolytic uremic syndrome; CTLD: C-type lectin-like domain
References
Beck LH Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21.
Tomas NM, Beck LH Jr, Meyer-Schwesinger C, Seitz-Polski B, Ma H, Zahner G, et al. Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med. 2014;371:2277–87.
Hoxha E, Thiele I, Zahner G, Panzer U, Harendza S, Stahl RA. Phospholipase A2 receptor autoantibodies and clinical outcome in patients with primary membranous nephropathy. J Am Soc Nephrol. 2014;25:1357–66.
Abe H, Tsuboi N, Yukawa S, Tsuji S, Hayashi H, Yukawa N, et al. Thrombotic thrombocytopenic purpura complicating Sjogren’s syndrome with crescentic glomerulonephritis and membranous nephritis. Mod Rheumatol. 2004;14:174–8.
Friedlander MA, Jacobs GH. Recurrent thrombotic thrombocytopenic purpura associated with membranous glomerulopathy. Am J Kidney Dis. 1991;17:83–5.
Kuppachi S, Chander P, Yoo J. Membranous nephropathy and thrombotic thrombocytopenic purpura treated with rituximab. J Nephrol. 2009;22:561–4.
Al-Rabadi L, Quillen K, Shashar M, Al Marji C, Jaberi A, Chitalia V, et al. Concurrent Presentation of thrombotic thrombocytopenic purpura and membranous nephropathy. Kidney Int Rep. 2018;3:476–81.
Vanderlugt CL, Miller SD. Epitope spreading in immune-mediated diseases: implications for immunotherapy. Nat Rev Immunol. 2002;2:85–95.
Fresquet M, Jowitt TA, Gummadova J, Collins R, O’Cualain R, McKenzie EA, et al. Identification of a major epitope recognized by PLA2R autoantibodies in primary membranous nephropathy. J Am Soc Nephrol. 2015;26:302–13.
Seitz-Polski B, Dolla G, Payre C, Girard CA, Polidori J, Zorzi K, et al. Epitope Spreading of autoantibody response to PLA2R associates with poor prognosis in membranous nephropathy. J Am Soc Nephrol. 2016;27:1517–33.
Tse WY, Howie AJ, Adu D, Savage CO, Richards NT, Wheeler DC, et al. Association of vasculitic glomerulonephritis with membranous nephropathy: a report of 10 cases. Nephrol Dial Transplant. 1997;12:1017–27.
Sano T, Kamata K, Shigematsu H, Kobayashi Y. A case of anti-glomerular basement membrane glomerulonephritis superimposed on membranous nephropathy. Nephrol Dial Transplant. 2000;15:1238–41.
Surindran S, Ayalon R, Hasan N, Beck LH Jr, Salant DJ, Barisoni L, et al. Coexistence of ANCA-associated glomerulonephritis and anti-phospholipase A2 receptor antibody-positive membranous nephropathy. Clin Kidney J. 2012;5:162–5.
Manea M, Kristoffersson A, Schneppenheim R, Saleem MA, Mathieson PW, Morgelin M, et al. Podocytes express ADAMTS13 in normal renal cortex and in patients with thrombotic thrombocytopenic purpura. Br J Haematol. 2007;138:651–62.
Qin W, Beck LH Jr, Zeng C, Chen Z, Li S, Zuo K, et al. Anti-phospholipase A2 receptor antibody in membranous nephropathy. J Am Soc Nephrol. 2011;22:1137–43.
Akiyama S, Akiyama M, Imai E, Ozaki T, Matsuo S, Maruyama S. Prevalence of anti-phospholipase A2 receptor antibodies in Japanese patients with membranous nephropathy. Clin Exp Nephrol. 2015;19:653–60.
Zheng XL, Kaufman RM, Goodnough LT, Sadler JE. Effect of plasma exchange on plasma ADAMTS13 metalloprotease activity, inhibitor level, and clinical outcome in patients with idiopathic and nonidiopathic thrombotic thrombocytopenic purpura. Blood. 2004;103:4043–9.
Lara PN Jr, Coe TL, Zhou H, Fernando L, Holland PV, Wun T. Improved survival with plasma exchange in patients with thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Am J Med. 1999;107:573–9.
Rock GA, Shumak KH, Buskard NA, Blanchette VS, Kelton JG, Nair RC, et al. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. Canadian Apheresis Study Group. N Engl J Med. 1991;325:393–7.
Vesely SK, George JN, Lammle B, Studt JD, Alberio L, El-Harake MA, et al. ADAMTS13 activity in thrombotic thrombocytopenic purpura-hemolytic uremic syndrome: relation to presenting features and clinical outcomes in a prospective cohort of 142 patients. Blood. 2003;102:60–8.
Qu Z, Liu G, Li J, Wu LH, Tan Y, Zheng X, et al. Absence of glomerular IgG4 deposition in patients with membranous nephropathy may indicate malignancy. Nephrol Dial Transplant. 2012;27:1931–7.
Larsen CP, Messias NC, Silva FG, Messias E, Walker PD. Determination of primary versus secondary membranous glomerulopathy utilizing phospholipase A2 receptor staining in renal biopsies. Mod Pathol. 2013;26:709–15.
Fervenza FC, Appel GB, Barbour SJ, Rovin BH, Lafayette RA, Aslam N, et al. Rituximab or Cyclosporine in the Treatment of Membranous Nephropathy. N Engl J Med. 2019;381:36–46.
Beck LH Jr, Fervenza FC, Beck DM, Bonegio RG, Malik FA, Erickson SB, et al. Rituximab-induced depletion of anti-PLA2R autoantibodies predicts response in membranous nephropathy. J Am Soc Nephrol. 2011;22:1543–50.
Acknowledgments
We thank Toshihumi Shimoda, Toshihiro Nakamura, Kazuki Konishi, and Ryota Nakamura for their daily support in plasma exchange therapy.
Funding
No funding was received.
Author information
Authors and Affiliations
Contributions
RI managed the patient, made decisions regarding the patient’s treatment, and wrote the whole manuscript. SA measured the anti-PLA2R antibody level and provided scientific advice. KM, YI, HO, KS, NO, KT, and ST are nephrologists at our hospital and analyzed and interpreted the patient data regarding kidney disease. HF performed the histological examination of the kidney. MM measured the levels of ADAMTS13 activity and ADAMTS13 inhibitor, and provided scientific advice. TH is a director at our department and supervised the patient’s treatment and revised the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent for publication of this case report and any accompanying images was obtained from the patient and his relative.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Iio, R., Akiyama, S., Mitsumoto, K. et al. Thrombotic thrombocytopenic purpura developed during the conservative treatment of anti-phospholipase A2 receptor antibody-positive idiopathic membranous nephropathy: a case report. BMC Nephrol 21, 431 (2020). https://doi.org/10.1186/s12882-020-02086-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-020-02086-z