Abstract
Background
There are a growing number of studies on ethnic differences in progression and mortality for pre-dialysis chronic kidney disease (CKD), but this literature has yet to be synthesised, particularly for studies on mortality.
Methods
This scoping review synthesized existing literature on ethnic differences in progression and mortality for adults with pre-dialysis CKD, explored factors contributing to these differences, and identified gaps in the literature. A comprehensive search strategy using search terms for ethnicity and CKD was taken to identify potentially relevant studies. Nine databases were searched from 1992 to June 2017, with an updated search in February 2020.
Results
8059 articles were identified and screened. Fifty-five studies (2 systematic review, 7 non-systematic reviews, and 46 individual studies) were included in this review. Most were US studies and compared African-American/Afro-Caribbean and Caucasian populations, and fewer studies assessed outcomes for Hispanics and Asians. Most studies reported higher risk of CKD progression in Afro-Caribbean/African-Americans, Hispanics, and Asians, lower risk of mortality for Asians, and mixed findings on risk of mortality for Afro-Caribbean/African-Americans and Hispanics, compared to Caucasians. Biological factors such as hypertension, diabetes, and cardiovascular disease contributed to increased risk of progression for ethnic minorities but did not increase risk of mortality in these groups.
Conclusions
Higher rates of renal replacement therapy among ethnic minorities may be partly due to increased risk of progression and reduced mortality in these groups. The review identifies gaps in the literature and highlights a need for a more structured approach by researchers that would allow higher confidence in single studies and better harmonization of data across studies to advance our understanding of CKD progression and mortality.
Similar content being viewed by others
Background
Chronic kidney disease (CKD) is common and is associated with increased morbidity and mortality [1,2,3]. Risk factors for progression include proteinuria, comorbid conditions such as diabetes and cardiovascular disease, as well as non-modifiable characteristics such as ethnicity [4, 5]. In the UK, a higher proportion of people from ethnic minority groups than Caucasians begin renal replacement therapy (RRT) [6]. In the United States (US), the rate of RRT initiation for end-stage kidney disease (ESKD) is also disproportionately higher for ethnic minority groups (such as African-American, Hispanic and Native Americans) compared to Caucasians, despite similar prevalence for early stages of CKD [7]. Higher RRT prevalence in ethnic minority groups has been attributed to faster progression of CKD and better CKD survival [7, 8]. Understanding which ethnic groups have worse outcomes and which factors influence adverse outcomes can help clinicians and policy makers target health care efforts and resources and improve outcomes for individuals, as well as inform policies to reduce health inequities.
To our knowledge, no study has systematically scoped studies exploring the range of risk factors for ethnic differences in CKD progression and mortality. A systematic review published in 2010 [9] that investigated ethnic differences in CKD progression, and had similar inclusion criteria to the current study, identified 5 relevant studies and concluded little evidence for ethnic differences in CKD progression and a lack of appropriately designed studies to assess ethnic differences in CKD progression. However, there have since been further studies in the field. Furthermore, no studies have systematically synthesised evidence for ethnic differences in mortality for people with pre-dialysis CKD, which would further contribute to understanding of ethnic differences in CKD progression – a competing risk. A scoping review rapidly examines the extent, range, and nature of existing knowledge in a diverse body of literature, summarises research findings and identifies research gaps [10]. In contrast to systematic reviews, scoping reviews identify and synthesise the breadth of knowledge in a given area without in depth assessment of study quality. The aim of this scoping review was to identify and present findings from studies addressing ethnic differences in pre-dialysis CKD progression and mortality, the key factors that underpin these ethnic differences, and identify areas where further research is needed. Understanding the patterns and potential mechanisms in ethnic minorities in high-income countries may contribute to CKD prevention and care in countries where they are the main ethnic groups. We conducted a scoping review to synthesize the existing literature comparing CKD progression and mortality for ethnic minority and non-ethnic minority adults with pre-dialysis CKD.
Methods
Our scoping review was conducted in line with the five-stage framework outlined by Arskey and O’Malley (2005) [10]. This framework includes formulating the research question, identifying relevant studies, study selection, charting the data, and collating, summarizing and reporting results. We acknowledge that classification of ethnicity can be complex and challenging. In this study, ethnic minority was defined as belonging to a particular group of people with common social, physical, national, linguistic, cultural, ancestral backgrounds and other such attributes living in a country where they differ from the majority [11]. Ethnic minority groups include African-Americans, Hispanic, Asian (East Asia, Southeast Asia, Indian subcontinent), Native Hawaiian or Pacific Islanders, and American Indian or Alaska Native in US studies, and Afro-Caribbean, South Asian (comprising Indians, Pakistanis, Bangladeshis), East Asians [e.g.: Chinese], and other Asian countries, and First Nation populations in UK and other countries [e.g.: Aboriginal Australians]. Pre-dialysis CKD was defined by glomerular filtration rate (GFR) or a combination of urinary albumin to creatinine ratio and GFR, and not requiring RRT.
Information sources and search strategies
Search terms for ethnic groups included ‘ethnic groups, race, minority, Asian, Caucasian, Hispanic, Continental population groups, and African’ [11]. Search terms for CKD included ‘kidney diseases, renal insufficiency, glomerular filtration rate’. Searches were expanded using truncation symbols and search terms were combined using Boolean operators. The electronic databases Medline OVID, Embase, CINAHL, PsycINFO, Web of Science, Scopus, Social Care Online, Applied Social Sciences Index and Abstracts (ASSIA), and Cochrane Database of Promoting Health Effectiveness Reviews were searched from 1992 to July 2017. An updated search was conducted in February 2020 to identify more recent eligible studies. A time frame of 1992 onwards was set to capture evidence from the last 28 years and the searches were limited to the English language. Searches were conducted without a study design filter. Bibliography searches of key papers were performed. The search strategies for each database can be found in Appendix 1.
Article selection
A comprehensive and iterative approach to the literature searches for evidence was taken to ensure that a broad range of perspectives was captured. Articles were included in the review if the following criteria were met: (1) used an adult study population and (2) compared risk of progression [e.g., (decline in) (estimated) glomerular filtration rate (GFR)] and/or mortality for ≥2 ethnic groups with pre-dialysis CKD. Articles that did not compare outcomes between ethnic minority and non-ethnic minority groups or focused on dialysis/transplant patients were excluded. Two reviewers (HH and RH) reviewed articles to determine eligibility for inclusion. Any discrepancies were resolved by discussion with a third reviewer (SF).
Data extraction and synthesis
Data were extracted into a series of evidence tables, developed a priori, by one reviewer (HH). One evidence table was produced for each outcome group. Each table included details on the authors, date of publication, country in which the study was conducted, study aims, ethnic groups included in the study, study design, outcomes of interest, and key findings on factors associated with any ethnic differences in CKD outcomes for each study. Evidence tables can be found in Appendix 2. A narrative synthesis approach was taken to summarise the evidence.
Results
Search strategy, study selection and data extraction
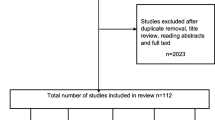
The results of the search strategy and selection process are shown in Fig. 1. 8059 citations were identified from the search. After removing duplicates and title and abstract screening 227 studies met the criteria for full text review, from which 50 were selected for inclusion in the review. Our updated searches identified 5 relevant studies published between July 2017 and February 2020, resulting in a total of 55 studies included in the review. These 55 studies included 1 systematic review [9] and 8 literature reviews on CKD progression [12,13,14,15,16,17,18,19]. Together these reviews included 19 of the individual studies identified in our searches. The reviews were published between 2004 and 2018, addressed slightly different research questions, and included different studies (Table 1). Only two of the reviews [9, 19] used a systematic search strategy and reported clear inclusion/exclusion criteria. Forty-two studies (n = 15,204,453 individuals; median: 3785, IQR: 1208-25,774) examined differences in CKD progression and thirty studies (n = 4,480,316 individuals, median: 3939, IQR: 1798-22,634) assessed ethnic differences in survival.
Ethnic differences in CKD progression
African-Americans and Afro-Caribbean ethnicity
Thirty-six studies explored CKD progression for African-Americans or Afro-Caribbeans and Caucasians (Table 2). Studies were conducted in the US, Canada, UK, and Norway and most were prospective or retrospective cohort studies. Overall, most (n = 24) studies reported higher risk of CKD progression in African-American/Afro-Caribbean (adjusted (for varying covariates) HRs ranging in 15 studies from 1.16 (95% CI: 1.09–2.62) to 4.00 (95% CI: 2.99–5.35)) [13,14,15,16, 19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38]. However, some (n = 12) studies found no significant ethnic differences in risk of CKD progression, which may be partly due to smaller study sample size, duration of follow up, and/or adjustment for confounders and mediators [9, 38,39,40,41,42,43,44,45,46,47,48]. Studies adjusted for demographics factors (such as age and sex, with fewer studies adjusting for socioeconomic status), biological factors (such as baseline estimated glomerular filtration (eGFR) levels, proteinuria, baseline creatinine, cholesterol, haemoglobin, blood pressure), comorbidities (hypertension, diabetes, cardiovascular disease). Some studies also adjusted for body mass index, smoking, and prescribed medication.
Lower baseline eGFR levels [23, 25, 34], proteinuria [34, 35], albuminuria [26], and higher (treated) blood pressure [31, 41, 48], and glycaemic control [45, 47] predicted increased risk of progression in African-Americans compared to Caucasians. Existing diabetes, cardiovascular diseases and congestive heart failure either fully or partially attenuated the association of increased risk of progression observed for African-Americans [26, 31, 43] compared to Caucasians. Apolipoprotein E and variants in the gene encoding apolipoprotein L1 (APOL1) explained some differences in progression for African-Americans compared to Caucasians [28, 33]. For example, there was a mean adjusted difference in eGFR slope of − 1.05 ml per minute per 1.73m2 per year (p < 0.001) for African-Americans in the high risk APOL1 group compared to Caucasians but no significant difference between rate of eGFR decline for African-Americans in the low risk APOL1 group and Caucasians [33].
South Asians
Twelve studies explored CKD progression in South Asians [18, 25, 26, 31, 35, 36, 46,47,48,49,50,51]. These studies were conducted in the US, Canada, and UK. Most were prospective or retrospective studies. Six studies reported similar risk of progression for South Asians compared to Caucasians and 6 reported higher risk for South Asians (for example 2 studies reported odds ratios (95%CI) of 1.44 (1.00–1.85) and 1.41 (1.32–1.51). Studies adjusted for demographic, biological factors, comorbidities, smoking, and prescribed medication and indicated eGFR levels [25], proteinuria [34, 35], higher (treated) blood pressure [48], and renal comorbidities [49] may explain these ethnic differences.
East Asians
Four studies explored CKD progression in East Asians, of which 3 were conducted in the US and 1 in Canada. Three were cohort studies and 1 was a randomised controlled trial (RCT). Three studies found higher risk for East Asians (one study reporting adjusted OR (95% CI): 1.41 (1.32–1.51)) and 1 study found similar risk of progression for East Asians compared to Caucasians [25, 26, 31, 49]. Studies adjusted for demographic, biological and comorbid factors. There was some evidence that renal comorbidities [31, 49] may contribute to South Asians having higher risk of CKD progression that Caucasians.
Pacific islanders
Four US studies included Pacific Islanders [25, 26, 31, 40]. One was a prospective cohort study, 2 retrospective cohort studies, and 1 RCT. Two studies reported higher risk of CKD progression for Pacific Islanders - 1 study reporting adjusted OR (95% CI): 1.41 (1.32–1.51) and another study reporting adjusted HR (95% CI): 3.84 (2.73–5.40), and 1 study found no significant differences in CKD progression for Pacific Islanders compared to Caucasians (adjusted OR (95% CI): 1.02 (0.91–1.15)). Factors contributing to ethnic differences in CKD progression for Pacific Islanders compared to Caucasians were unclear/unexplored.
Hispanics
Seven studies compared CKD progression for Hispanics and Caucasians. Six were conducted in the UK and 1 in Canada. Four were prospective or retrospective cohort studies, 2 were reviews and 1 was an RCT. Six studies reported higher risk of CKD progression for Hispanics compared to Caucasians (effect estimates ranging from adjusted HR (95% CI): 1.93 (1.72–2.17) to HR (95% CI): 2.20 (1.46–3.30), and adjusted OR (95% CI): 1.49 (1.42–1.56)) [15, 25, 26, 31, 38, 52]. One study reported similar risk [9]. Studies suggested eGFR levels [25, 31], albuminuria [26], body mass index [31], blood pressure [31], diabetes [38], and prior cardiovascular disease [31] may explain the higher risk of CKD progression observed for Hispanics.
Native Americans
Two US studies explored CKD progression for Native Americans: 1 retrospective cohort study and 1 RCT. Both studies reported similar risk for CKD progression for Native Americans compared to Caucasians (for example, Go et al. (2018) reported adjusted OR (95% CI):1.57 (0.81–3.04)) [31, 40].
Indigenous populations
Two studies, 1 conducted in Australia and 1 in Canada, focused on CKD progression in Indigenous populations [17, 53]. One was a discussion paper and the other a prospective cohort study. Both studies reported higher risk of CKD progression for Indigenous populations compared to Caucasians. Samuel et al. (2014) reported adjusted HR (95% CI): 18.67 (10.77–32.36) vs 6.33 (5.41–7.40) for First Nation vs non First Nation individuals, respectively. The discussion paper explored pathways through which socioeconomic factors explained these differences.
Ethnic disparities in all-cause mortality
African-Americans and afro-Caribbeans
Twenty-seven studies examined mortality differences for African-American or Afro-Caribbeans compared to Caucasians (Table 3). Most were conducted in the US and were either prospective or retrospective cohort studies. Eighteen studies reported no significant ethnic differences in survival [20,21,22, 26, 33, 34, 36, 38, 39, 42, 44, 46, 54,55,56,57,58,59]. Five studies found higher risk of mortality for African-Americans [13, 23, 60,61,62] (adjusted HRs (95% CI) ranging in studies from 1.30 (1.02–1.65) to 1.83 (1.33–2.52)), and 4 reported lower risk of mortality for African-Americans [25, 43, 63, 64] (adjusted HRs (95% CI) ranging in studies from 0.67 (0.63–0.72) to 0.79 (0.61–0.97)). Studies suggested age [25, 63], biological factors (e.g., higher blood pressure, serum albumin), comorbidities (cardiovascular disease, diabetes) and medication use (e.g.; more frequent use of calcitriol and less use of statins in African-Americans compared to Caucasians) [43, 63] may partly explain ethnic differences in survival for African-Americans/Afro-Caribbeans and Caucasians with CKD. There were mixed findings on the role of socioeconomic status in explaining these differences [60, 61].
South Asians
Eight studies explored ethnic differences in mortality for South Asians compared to Caucasians. Studies were conducted in the US (n = 4), Canada (n = 2), and UK (n = 2). All were either retrospective or prospective cohort studies. Six reported lower risk of mortality for South Asians compared to Caucasians (adjusted HR (95% CI) ranging from 0.33 (0.14–0.64) to 0.73 (0.59–0.88)), and 2 reported no significant differences (e.g., OR (95% CI): − 0.51 (− 3.25 to − 2.23)) [33, 34, 36, 46, 49, 56, 59, 65]. Studies that reported lower risk of mortality for South Asians suggested age and sex, proteinuria, blood pressure, diabetes, cardiovascular disease and medications (antihypertensive drugs and statins), and C-reactive protein partly explained ethnic differences in mortality [49, 59].
East Asians
Five cohort studies – 3 conducted in the US and 2 in Canada- explored mortality differences for East Asians and Caucasians. All 5 studies reported lower risk of mortality for East Asians compared to Caucasians (adjusted HR (95% CI) ranging from 0.58 (0.52–0.65) to 0.69 (0.55–0.88)) [25, 26, 49, 56, 65]. Age and sex, proteinuria, blood pressure, diabetes, cardiovascular disease and medications (antihypertensive drugs and statins), and C-reactive protein [49, 59] partly explained the lower risk of mortality observed for East Asians.
Pacific islanders
Three US studies, 1 prospective and 2 retrospective cohort studies, explored mortality differences for Asians/Pacific Islanders and Caucasians [25, 26, 56]. Two reported lower risk of mortality for Pacific Islanders (adjusted HR (95% CI) ranging from 0.58 (0.52–0.65) to 0.76 (0.61–0.95) and 1 reported no significant differences. Factors contributing to ethnic differences in mortality for Pacific Islanders compared to Caucasians were not fully explored.
Hispanics
Six US studies examined ethnic differences in mortality for Hispanics and Caucasians [25, 26, 38, 52, 56, 61]. Studies were either prospective or retrospective cohort studies. Three studies reported lower risk of mortality for Hispanics compared to Caucasians (adjusted HR (95% CI) ranging from 0.66 (0.50–0.94) to 0.72 (0.66–0.79)) and 3 reported similar risk (adjusted HR (95% CI) ranging from 0.79 (0.59–1.35) to 0.94 (0.74–1.20)). Lower risk of mortality observed for Hispanics was partly explained by differences in urine protein levels [38] (with Hispanics having significantly lower risk of mortality than Caucasians at higher levels of urine protein but no significant ethnic differences at lower levels of urine protein) and not explained by differences in hypertension, diabetes, and use of medication including insulin [52].
Native Americans
One US retrospective cohort study compared mortality differences for Native Americans and Caucasians and reported higher risk of mortality for Native Americans (adjusted HR (95% CI):1.41 (1.08–1.84)) [56]. Factors contributing to mortality differences for Native Americans compared to Caucasians were not clear.
Discussion
We used an established and systematic methodology and searched a range of databases to capture the full range of existing studies on ethnic differences in pre-dialysis CKD progression and mortality. This scoping review identified evidence for higher risk of CKD progression in Afro-Caribbean/African-Americans, Hispanics, Asians compared to Caucasians which was at least partly explained by biological factors (e.g.: blood pressure) and comorbidities (such as diabetes, and cardiovascular disease), and lower risk of mortality for South and East Asians and Pacific Islanders compared to Caucasians. Our scoping review also identified mixed findings on risk of mortality for African-Americans and Hispanics compared to Caucasians. Future studies need to explore this, as studies reporting significant findings did not differ in the range of adjusted confounders compared to studies that found significant differences. The role of medication in the association between ethnicity and progression and mortality is complex, as differences in medication may represent unmet need in certain ethnic groups or may be an indicator of disease severity in individuals.
Gaps in the literature
A key gap in this literature is understanding why Asians (South, East, and Pacific Islanders) and Hispanics live longer, despite having higher prevalence of comorbidities such as diabetes, cardiovascular disease, and heart failure. Future research may explore potential missing factors that may explain why these groups experience increased risk CKD progression but live longer. The search also identified most research on ethnic differences in CKD progression and mortality has been conducted in US populations and there is less research in other countries with a significant proportion of ethnic minority immigrant populations (e.g.: UK, Canada), as well as lower/middle income countries. Across all studies, comparisons were made mostly between Caucasians vs. African-Americans and Caucasians vs. Hispanic (in US studies), and Caucasians vs. Afro-Caribbean and Caucasian vs. South Asian (in UK studies). Fewer studies compared Caucasians to East Asians, Pacific Islanders or Native Americans. Furthermore, most studies did not distinguish between subgroups within an ethnic group (e.g.: Africans vs. Caribbean in the same ethnic group). There may be heterogeneity in findings within ethnic groups, as shown in a recent UK study that found risk of CKD progression was higher in Bangladeshis compared to Indians [36]. Similarly, identified studies did not distinguish or adjust for generational status or indigenous vs immigrant populations, though some existing studies may not have been captured through our searches.
Some studies did not adjust for important confounders of the association between ethnicity and CKD outcomes. Firstly, very few (n = 15) of the included studies adjusted for socioeconomic status. This is particularly important for US studies, where low socioeconomic status is closely linked to ethnicity and independently associated with health insurance and access to health care. Secondly, most studies on CKD progression did not account for competing risk of death, so differences in progression in these studies may have been at least partly due to differences in survival across ethnic groups. A limited number of studies (n = 18) assessed ethnic differences in both progression and mortality. These studies seemed to suggest Hispanics and East and South Asians experience increased risk of CKD progression as result of lower competing risk of death, and poorer evidence for significant differences in risk of mortality for African-Americans and Hispanics compared to Caucasians. However, further research is needed to confirm these findings. Thirdly, there were few studies exploring genetic risk factors for CKD progression and mortality. Some studies have suggested other genetic risk factors such as genes encoding non-muscle myosin heavy chain type II isoform A for ESKD in African-Americans [66], but there is a lack of genetic studies comparing risk of ESKD across different ethnic groups with pre-dialysis CKD. Future studies may also explore whether differences between ethnic groups hold across countries or whether they differ due to societal and health care reforms. Fourthly, some studies did not assess progression and mortality stratified by level of CKD severity, making it difficult to directly compare or identify if ethnic differences in progression and mortality vary at different stages of CKD. An updated systematic review and meta-analysis that goes beyond scoping to assess bias in these studies and pool together estimates from the different studies (where possible) may help explain some of the mixed findings and improve our understanding of the extent and key predictors of ethnic differences in CKD outcomes. Finally, there was lack of data on level of control of biologic factors such as blood pressure and glycemia, as well as limited data on medication and adherence, and how these vary across ethnic groups, all of which are important for differences in CKD progression and mortality. Future studies should aim to capture this data as much as possible.
Limitations
The review was based on a comprehensive search of the literature. However, it is possible that some relevant studies may have been missed as the search was restricted to studies that were published in English and published after 1992. Studies that examined CKD progression and outcomes for only one ethnic group and did not make comparisons with another ethnic group were also excluded. Furthermore, there was limited additional searching of grey literature, though we believe the majority of relevant studies will have been captured through the different databases and bibliography.
An important limitation of the scoping review approach is that papers are not critically appraised in detail and quality of the individual studies is therefore not assessed [10]. However, this scoping review was based on established methodology [10] and has mapped the existing literature on ethnic differences in CKD outcomes, identified gaps in the research and highlighted the need for further systematic reviews and additional primary research focusing on cardiovascular-related and other adverse outcomes for pre-dialysis CKD.
Clinical and policy implications of this scoping review
Increased risk of CKD progression in ethnic minority groups may be tackled through closer monitoring and management of renal comorbidities such as diabetes and cardiovascular disease, for example through proteinuria and blood pressure measurement, particularly in these high risk groups. There has been some evidence to suggest incentivisation programs, such as the Quality and Outcomes Framework programme in the UK, may help improve care for diabetics with CKD [67]. Interventions including the use of medications such as renin-angiotensin-aldosterone system blockers and patient-provider education interventions may also reduce risk of progression in high-risk groups [68]. In the UK, a national quality improvement programme has mapped laboratory data taken from all settings to derive graphs of kidney function over time. Declining kidney function is then flagged by a laboratory scientist and sent to primary care doctor for clinical review and referral, where necessary [69]. However, a better understanding of risk factors for CKD progression in high risk groups is needed to help develop more effective and targeted interventions.
Conclusions
Scoping reviews are a relatively novel method of systematically assessing a wide range of literature in a particular field, in order to identify important gaps in the literature, and inform more targeted systematic reviews or further studies. This is the first synthesis of the extensive body of literatures on ethnic differences in CKD progression and mortality. The findings of this review suggest higher rates of RRT in ethnic minority groups may be partly due to increased risk of progression and reduced mortality in these groups (compared to Caucasians), though more evidence is needed for African-American and Hispanic ethnicity. The review highlights the need for further studies using similar approaches and adjusting for the same confounders, which would improve our understanding of disease progression and mortality in people with CKD.
Availability of data and materials
Not applicable.
Abbreviations
- APOL 1:
-
Apolipoprotein L1
- CKD:
-
Chronic kidney disease
- ESKD:
-
End-stage kidney disease
- GFR:
-
Glomerular filtration rate
- HIV:
-
Human Immunodeficiency virus
- HR:
-
Hazard ratio
- IQR:
-
Interquartile range
- RRT:
-
Renal replacement therapy
- UK:
-
United Kingdom
- US:
-
United States
References
Jager K, Fraser SD. The ascending rank of CKD in the global burden of disease study. Nephrol Dial Transplant. 2017;32(suppl_2):ii121–8.
Hill NR, Fatoba ST, Oke JL, Hirst JA, O’Callaghan CA, Lasserson DS, Hobbs FDR. Global prevalence of chronic kidney disease- a systematic review and meta-analysis. PLoS One. 2016;11(7):e0158765.
Astor BC, Matsushita K, Gansevoort RT, van der Velde M, Woodward M, Levey AS, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int. 2011;79:1331–40.
Taal MW, Brenner BM. Predicting initiation and progression of chronic kidney disease: developing renal risk scores. Kidney Int. 2006;70:1694–705.
McClellan WM, Flanders WD. Risk factors for progressive chronic kidney disease. J Am Soc Nephrol. 2003;14:S65–70.
Ansell D, Feehally J., Feest, T.G., Tomson C, Williams AJ, Warwick G. UK Renal Registry Report 2007. UK Renal Registry, Bristol, UK. 10th Annual Report of the Renal Association, 2007. http://www.renalreg.com/Reports/2007.html.
USRDS. Renal Data System (USRDS) Annual Data Report. Bethesda: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2008.
Caskey FJ. Renal replacement therapy: can we separate the effects of social deprivation and ethnicity? Kidney Int Suppl. 2013;3:246–9.
Barbour SJ, Schachter M, Er L, Djurdjev O, Levin A. A systematic review of ethnic differences in the rate of renal progression in CKD patients. Nephrol Dial Transplant. 2010;25(8):2422–30.
Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32.
Ethnicity and Health. Pan American Health Organization/World Health Organization. 132nd Session of the Executive Committee. Washington, D.C., USA, 23–27 June 2003. Available from: https://eur03.safelinks.protection.outlook.com/?url=http%3A%2F%2Fwww1.paho.org%2Fenglish%2Fgov%2Fce%2Fce132-16-e.pdf&data=01%7C01%7Ch.o.hounkpatin%40soton.ac.uk%7C9ed5af6fb530413bc1b708d7a33cfc4b%7C4a5378f929f44d3ebe89669d03ada9d8%7C0&sdata=Va7b40jyQjROcMD7282AwhAr9M%2BnwaigPJqxwiOzrmc%3D&reserved=0 (Accessed 6th February 2020).
Powe NR, Melamed ML. Racial disparities in the optimal delivery of chronic kidney disease care. Med Clin North Am. 2005;89(3):475–8.
Norris K, Mehrotra R, Nissenson AR. Racial differences in mortality and end-stage renal disease. Am J Kidney Dis. 2008;52(2):205–8.
Crews DC, Liu Y, Boulware LE. Disparities in the burden, outcomes and care of chronic kidney disease. Curr Opin Nephrol Hypertens. 2014;23(3):298–305.
Horowitz B, Miskulin D, Zager P. Epidemiology of hypertension in CKD. Adv Chronic Kidney Dis. 2015;22(2):88–95.
Harding K, Mersha TB, Vassalotti JA, Webb FA, Nicholas SB. Current state and future trends to optimize the care of chronic kidney disease in African Americans. Am J Nephrol. 2017;46(2):176–86.
Cass A, Cunningham J, Hoy W, Snelling P, Wang Z. Exploring the pathways leading from disadvantage to end-stage renal disease for indigenous Australians. Soc Sci Med. 2004;58(4):767–85.
Jadawji C, Crasto W, Gillies C, Kar D, Davies MJ, Khunti K, Seidu S, et al. Prevalence and progression of diabetic nephropathy in south Asian, white European and African Caribbean people with type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2019;21:658–73.
Chen W, Bushinsky DA. Addressing racial disparity in the progression of chronic kidney disease: prescribe more fruits and vegetables? Am J Nephrol. 2018;47:171–3.
Agarwal R, Bunaye Z, Bekele DM, Light RP. Competing risk factor analysis of end-stage renal disease and mortality in chronic kidney disease. Am J Nephrol. 2008;28(4):569–75.
Alves TP, Hulgan T, Wu P, Sterling TR, Stinnette SE, Rebeiro PF, et al. Race, kidney disease progression, and mortality risk in HIV-infected persons. Clin J Am Soc Nephrol. 2010;5(12):2269–75.
Babayev R, Whaley-Connell A, Kshirsagar A, Klemmer P, Navaneethan S, Chen SC, et al. Association of race and body mass index with ESRD and mortality in CKD stages 3-4: results from the kidney early evaluation program (KEEP). Am J Kidney Dis. 2013;61(3):404–12.
Choi AI, Rodriguez RA, Bacchetti P, Bertenthal D, Hernandez GT, O'Hare AM. White/black racial differences in risk of end-stage renal disease and death. Am J Med. 2009;122(7):672–8.
Crews DC, Banerjee T, Wesson DE, Morgenstern H, Saran R, Burrows NR, Williams DE. Powe NR; Centers for Disease Control and Prevention chronic kidney disease surveillance team. Race/ethnicity, dietary acid load, and risk of end-stage renal disease among US adults with chronic kidney disease. Am J Nephrol. 2018;47(3):174–81. https://doi.org/10.1159/000487715.
Derose SF, Rutkowski MP, Crooks PW, Shi JM, Wang JQ, Kalantar-Zadeh K, et al. Racial differences in estimated GFR decline, ESRD, and mortality in an integrated health system. Am J Kidney Dis. 2013;62(2):236–44.
Hall YN, Choi AI, Chertow GM, Bindman AB. Chronic kidney disease in the urban poor. Clin J Am Soc Nephrol. 2010;5(5):828–35.
Hsu CY, Lin F, Vittinghoff E, Shlipak MG. Racial differences in the progression from chronic renal insufficiency to end-stage renal disease in the United States. J Am Soc Nephrol. 2003;14(11):2902–7.
Hsu CC, Kao WH, Coresh J, Pankow JS, Marsh-Manzi J, Boerwinkle E, Bray MS. Apolipoprotein E and progression of chronic kidney disease. JAMA. 2005;293(23):2892–9.
Hunsicker L, Adler S, Caqqiula A, England B, Greene T, Kusek J, et al. Predictors of the progression of renal disease in the modification of diet in renal disease study. Kidney Int. 1997;51:1908–19.
Jones-Burton C, Seliger SL, Brown J, Stackiewicz L, Hsu VD, Fink JC. Racial variations in erythropoietic response to epoetin alfa in chronic kidney disease and the impact of smoking. Nephrol Dial Transpl. 2005;20(12):2739–45.
Lewis EF, Claggett B, Parfrey PS, Burdmann EA, McMurray JJV, Solomon SD, et al. Race and ethnicity influences on cardiovascular and renal events in patients with diabetes mellitus. Am Heart J. 2015;170(2):322–9 e324.
Lucas GM, Lau B, Atta MG, Fine DM, Keruly J, Moore RD. Chronic kidney disease incidence, and progression to end-stage renal disease, in HIV-infected individuals: a tale of two races. J Infect Dis. 2008;197(11):1548–57.
Parsa A, Kao WH, Xie D, Astor BC, Li M, Hsu CY, et al. (2013). APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med. 2013;369(23):2183–96.
Yang W, Xie D, Anderson AH, Joffe MM, Greene T, Teal V, et al. Association of kidney disease outcomes with risk factors for CKD: findings from the chronic renal insufficiency cohort (CRIC) study. Am J Kidney Dis. 2014;63(2):236–43.
Dreyer G, Hull S, Mathur R, Chesser A, Yaqoob MM. Progression of chronic kidney disease in a multi-ethnic community cohort of patients with diabetes mellitus. Diabet Med. 2013;30(8):956–63.
Mathur R, Dreyer G, Yaqoob MM, Hull SA. Ethnic differences in the progression of chronic kidney disease and risk of death in a UK diabetic population: an observational cohort study. BMJ Open. 2018;8:e020145. https://doi.org/10.1136/bmjopen-2017-020145.
Van Den Beukel TO, De Goeji MCM, Dekker FW, Siegert CEH, Halbesma N. Differences in progression to ESRD between black and white patients receiving predialysis care in a universal health care system. Clin J Am Soc Nephrol. 2013;8(9):1540–7.
Fischer MJ, Hsu JY, Lora CM, Ricardo AC, Anderson AH, Bazzano L, et al. CKD progression and mortality among Hispanics and non-Hispanics. J Am Soc Nephrol. 2016;27(11):3488–97.
Grams ME, Yang W, Rebholz CM, Wang X, Porter AC, Inker LA, et al. Risks of adverse events in advanced CKD: the chronic renal insufficiency cohort (CRIC) study. Am J Kidney Dis. 2017;70(3):337–46.
Go AS, Yang J, Tan TC, et al. Contemporary rates and predictors of fast progression of chronic kidney disease in adults with and without diabetes mellitus. BMC Nephrol. 2018;19:146.
Hebert LA, Kusek JW, Greene T, Agodoa LY, Jones CA, Levey AS, et al. Effects of blood pressure control on progressive renal disease in blacks and whites. Modification of diet in renal disease study group. Hypertension. 1997;30(3 Pt 1):428–35.
Jolly SE, Navaneethan SD, Schold JD, Arrigain S, Sharp JW, Jain AK, Schreiber MJ, Simon JF, Nally JV. Chronic kidney disease in an electronic health record problem list: quality of care, ESRD, and mortality. Am J Nephrol. 2014;39(4):288–96.
Kovesdy CP, Anderson JE, Derose SF, Kalantar-Zadeh K. Outcomes associated with race in males with nondialysis-dependent chronic kidney disease. Clin J Am Soc Nephrol. 2009;4(5):973–8.
Menon V, Wang X, Sarnak MJ, Hunsicker LH, Madero M, Beck GJ, et al. Long-term outcomes in nondiabetic chronic kidney disease. Kidney Int. 2008;73(11):1310–5.
Salifu MO, Shah S, Iqbal MH, Nabi M, Hayat A, Whaley-Connell AT, et al. Effect of ethnicity on the progression of diabetic kidney disease independent of glycemic control. Am J Nephrol. 2009;30:261–7.
Ali O, Mohiuddin A, Mathur R, Dreyer G, Hull S, Yaqoob MM. A cohort study on the rate of progression of diabetic chronic kidney disease in different ethnic groups. BMJ Open. 2013;3(2):e001855.
Earle KA, Porter KK, Ostberg J, Yudkin JS. Variation in the progression of diabetic nephropathy according to racial origin. Nephrol Dial Transplant. 2001;16(2):286–90.
Hull S, Dreyer G, Badrick E, Chesser A, Yaqoob M. The relationship of ethnicity to the prevalence and management of hypertension and associated chronic kidney disease. BMC Nephrol. 2011;12(1):41.
Barbour SJ, Er L, Djurdjev O, Karim MM, Levin A. Differences in progression of CKD and mortality amongst Caucasian, oriental Asian and south Asian CKD patients. Nephrol Dial Transplant. 2010;25(11):3663–72.
Koppiker N, Feehally J, Raymond N, Abrams KR, Burden AC. Rate of decline in renal function in indo-Asians and whites with diabetic nephropathy. Diabet Med. 1998;15:60–5.
Pallayova M, Rayner H, Taheri S, Dasgupta I. Is there a difference in progression of renal disease between south Asian and white European diabetic adults with moderately reduced kidney function? J Diabetes Complicat. 2015;29(6):761–5.
Peralta CA, Shlipak MG, Fan D, Ordonez J, Lash JP, Chertow GM, Go AS. Risks for end-stage renal disease, cardiovascular events, and death in Hispanic versus non-Hispanic white adults with chronic kidney disease. J Am Soc Nephrol. 2006;17(10):2892–9.
Samuel SM, Palacios-Derflingher L, Tonelli M, Manns B, Crowshoe L, Ahmed SB, et al. Association between first nations ethnicity and progression to kidney failure by presence and severity of albuminuria. Can Med Assoc J. 2014;186(2):E86–94.
Cardarelli F, Bellasi A, Veledar E, Vaccarino V, Raggi P. Impact of race and chronic kidney disease on 1-year outcome in patients undergoing percutaneous coronary interventions: a single tertiary center experience. Am Heart J. 2008;155(6):1027–32.
Hayes J, Kalantar-Zadeh K, Lu JL, Turban S, Anderson JE, Kovesdy CP. Association of Hypo- and hyperkalemia with disease progression and mortality in males with chronic kidney disease: the role of race. Nephron Clin Pract. 2012;120(1):C8–C16.
Jolly SE, Burrows NR, Chen SC, Li S, Jurkovitz CT, Norris KC, Shlipak MG. Racial and ethnic differences in mortality among individuals with chronic kidney disease: results from the kidney early evaluation program (KEEP). Clin J Am Soc Nephrol. 2011;6(8):1858–65.
Navaneethan SD, Schold JD, Arrigain S, Jolly SE, Jain A, Schreiber MJ Jr, et al. Low 25-hydroxyvitamin D levels and mortality in non-dialysis-dependent CKD. Am J Kidney Dis. 2011;58(4):536–43.
Wetmore JB, Sankaran S, Jones PG, Reid KJ, Spertus JA. Association of decreased glomerular filtration rate with racial differences in survival after acute myocardial infarction. Clin J Am Soc Nephrol. 2011;6(4):733–40.
Hutchison CA, Burmeister A, Harding SJ, Basnayake K, Church H, Jesky MD, et al. Serum polyclonal immunoglobulin free light chain levels predict mortality in people with chronic kidney disease. Mayo Clin Proc. 2014;89(5):615–22.
Fedewa SA, McClellan WM, Judd S, Gutierrez OM, Crews DC. The association between race and income on risk of mortality in patients with moderate chronic kidney disease. BMC Nephrol. 2014;15:9.
Mehrotra R, Kermah D, Fried L, Adler S, Norris K. Racial differences in mortality among those with CKD. J Am Soc Nephrol. 2008;19(7):1403–10.
Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol. 2004;15(5):1307–15.
Kovesdy CP, Quarles LD, Lott EH, Lu JL, Ma JZ, Molnar MZ, et al. Survival advantage in black versus white men with CKD: effect of estimated GFR and case mix. Am J Kidney Dis. 2013;62(2):228–35.
Newsome BB, McClellan WM, Coffey CS, Allison JJ, Kiefe CI, Warnock DG. Survival advantage of black patients with kidney disease after acute myocardial infarction. Clin J Am Soc Nephrol. 2006;1(5):993–9.
Conley J, Tonelli M, Quan H, Manns BJ, Palacios-Derflingher L, Bresee LC, et al. Association between GFR, proteinuria, and adverse outcomes among white, Chinese, and south Asian individuals in Canada. Am J Kidney Dis. 2012;59(3):390–9.
Kao WH, Klag MJ, Meoni LA, Reich D, Berthier-Schaad Y, Li M, et al. MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nat Genet. 2008;40:1185–92.
NHS Digital. Quality and Outcomes Framework, Achievement, prevalence and exceptions data. Available from: https://digital.nhs.uk/data-and-information/publications/statistical/quality-and-outcomes-framework-achievement-prevalence-and-exceptions-data/2017-18 [Accessed 10 Dec 2019].
Narva AS. Reducing the burden of chronic kidney disease among American Indians. Adv Chronic Kidney Dis. 2008;15:168–73.
Gallagher H, Methven S, Casula A, Thomas N, Tomson CRV, Caskey FJ, et al. A programme to spread eGFR graph surveillance for the early identification, support and treatment of people with progressive chronic kidney disease (ASSIST-CKD): protocol for the stepped wedge implementation and evaluation of an intervention to reduce late presentation for renal replacement therapy. BMC Nephrol. 2017;18:131.
Acknowledgements
The authors acknowledge Kidney Research UK for funding this study. HH was funded by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care (CLAHRC) Wessex at Southampton NHS Hospitals Foundation Trust. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Funding
This study was funded by Kidney Research UK (KRUK) and NIHR CLAHRC. The funding supported research time. KRUK and NIHR CLAHRC were not involved in the design of the project’s protocol and analysis plan, the collection or analyses. The funders had no input into the interpretation or publication of the study results.
Author information
Authors and Affiliations
Contributions
All listed authors (HH, GD, SF, AB, PJ, RH) contributed to the concept and design of the study. HH and RH reviewed articles to determine inclusion. HH drafted the article. All authors (HH, GD, SF, AB, PJ, RH) read, provided feedback and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval was not required for this study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Appendix 1.
Search strategies. Appendix 2. Evidence tables.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hounkpatin, H.O., Fraser, S.D.S., Honney, R. et al. Ethnic minority disparities in progression and mortality of pre-dialysis chronic kidney disease: a systematic scoping review. BMC Nephrol 21, 217 (2020). https://doi.org/10.1186/s12882-020-01852-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-020-01852-3