Abstract
Background
Peritoneal dialysis (PD) is used as renal replacement therapy in patients with end-stage kidney disease. However, peritoneal membrane failure remains problematic and constitutes a critical cause of PD discontinuation. Recent studies have revealed the unique biological action of molecular hydrogen (H2) as an anti-oxidant, which ameliorates tissue injury. In the present study, we aimed to examine the effects of H2 on the peritoneal membrane of experimental PD rats.
Method
Eight-week-old male Sprague-Dawley rats were divided into the following groups (n = 8–11 each) receiving different test solutions: control group (no treatment), PD group (commercially available lactate-based neutral 2.5% glucose PD solution), and H2PD group (PD solution with dissolved H2 at 400 ppb). Furthermore, the influence of iron (FeCl3: 5 μM: inducer of oxidative cellular injury) in the respective PD solutions was also examined (Fe-PD and Fe-H2PD groups). The H2PD solution was manufactured by bathing a PD bag in H2-oversaturated water created by electrolysis of the water. Twenty mL of the test solutions were intraperitoneally injected once a day for 10 days. Parietal peritoneum samples and cells collected from the peritoneal surface following treatment with trypsin were subjected to analysis.
Results
In the PD group as compared to controls, a mild but significant sub-mesothelial thickening was observed, with increase in the number of cells in the peritoneal surface tissue that were positive for apoptosis, proliferation and vimentin, as seen by immunostaining. There were significantly fewer of such changes in the H2PD group, in which there was a dominant presence of M2 (CD163+) macrophages in the peritoneum. The Fe-PD group showed a significant loss of mesothelial cells with sub-mesothelial thickening, these changes being ameliorated in the Fe-H2PD group.
Conclusion
H2-dissolved PD solutions could preserve mesothelial cells and peritoneal membrane integrity in PD rats. Clinical application of H2 in PD could be a novel strategy for protection of peritoneal tissue during PD treatment.
Similar content being viewed by others
Background
Peritoneal dialysis (PD) can be used as home-based therapy for patients with end-stage kidney disease and, worldwide, has played an important role in patient rehabilitation over the last three decades [1, 2]. However, PD is not as robust as hemodialysis with regard to safety and long-term performance. Its Achilles’ heel includes progressive injury to the peritoneal membrane [3, 4] and development of encapsulating peritoneal sclerosis (EPS), which is the most severe complication of PD therapy [5,6,7].
Mesothelial cell injury is the first step in the development of peritoneal fibrosis, later leading to sclerosis [8, 9], with bio-incompatibility of the PD solution and toxic molecules playing a central role in the pathology [10]. These molecules include glucose and glucose degradation products (GDPs) in the PD solution [11,12,13], and exogenous oxidants, such as iron [14, 15]. Recent histological studies have reported that the use of neutral PD solutions with low GDPs are beneficial in ameliorating histological changes in the membrane [16, 17], although the risk of EPS remains an issue of serious concern, even in patients treated with neutral PD solutions [18, 19]. This highlights the need for development of more biocompatible PD solutions.
Recent studies have shown dihydrogen (H2) has a biological action as an anti-oxidative and anti-inflammatory molecule [20]. H2 dissolved in water, given orally or by intraperitoneal administration, can suppress oxidative or inflammatory injury in various types of animal models, by playing a role as modulator of the expression of various molecules, such as MAPK, MEK-1, NFκB, and caspase-3 and 12, and by upregulating Nrf-2, which could prevent oxidative injury and apoptosis [21]. Thus, addition of H2 to PD solutions could represent a unique clinical approach to protecting the mesothelial cells and peritoneal tissue of these patients [22, 23].
In the present study, we examined the effects of H2 in experimental rats treated with PD solution, to clarify whether adding H2 to PD solutions could preserve mesothelial cells and the peritoneal membrane, including in cases of enhanced oxidative injury.
Methods
Treatment protocol
Male Sprague-Dawley rats aged 8–10 weeks old were housed under controlled environmental conditions (temperature 22 ± 1.5 °C; humidity 55% ± 5%; 12 hourly dark: light cycle with lights on at 7 a.m.) with free access to water and standard pellet food (0.8% NaCl; Nihon CLEA Japan, Inc., Tokyo, Japan).
All procedures in this study were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the study protocols were approved by the Animal Committee of Fukushima Medical University (approval number: 25,017).
The rats were divided into three groups: control group (n = 9; no treatment), PD group (n = 11; treatment with a commercially available 2.5% glucose peritoneal dialysis solution for intraperitoneal use), and the H2PD group (n = 8; treatment with the same intraperitoneal solution as the PD group, but with addition of 400 ppb of dissolved H2). Furthermore, in order to enhance the oxidative stress resulting from the PD solution, the influence of the addition of iron (FeCl3: 5 μM) in the PD solution was also examined in another set of rats divided into Fe-PD (n = 8) and Fe-H2PD groups (n = 8).
All groups were treated for ten weekdays, while being fed standard pellet food. Body weight was measured at the beginning and end of the experiment.
Twenty mL of the respective test solutions were intraperitoneally injected into the lower abdomen of rats in the different groups using a 20 gauge needle, once a day for 10 days.
Hydrogen gas loading into the PD solution and loss after loading
Hydrogen gas (H2) loading into the PD solution was performed by bathing the bag in H2-enriched electrolyzed water generated using an electrolyzer (Nihon Trim, Osaka, Japan), as reported elsewhere [22]. Details of the process are shown in Fig. 1.
Procedure for producing H2-dissolved peritoneal dialysis solution by the bathing method. a Time-course of changes in dissolved H2 levels in electrolyzed water (*) and the solution in the PD bag (#). b Time-course of changes in dissolved H2 levels in the solution in the PD bag when placed in air. Water was electrolyzed by Nafion (Synthetic polymer membrane; DuPont, Wilmington, DE) to generate highly dissolved H2 water, with the concentration of H2 exceeding 1.5 ppm. Then, the PD bag was bathed in the water to allow H2 to shift into the PD solution by diffusion (a). Dissolved hydrogen is lost rapidly after loading once it is exposed to room air (b). Thus, the H2-dissolved PD solution was subjected to experiments within 1 h of 12-h bathing, to ensure a high level of H2 in the PD solution (>400 ppm)
Collection of peritoneal tissue and sample analysis
All animals were sacrificed on the final day of the study (15th day). Pentobarbital (50 mg/kg) was administered intraperitoneally for euthanasia. Then, the abdominal cavity was opened and peritoneal tissue samples were carefully collected from the abdominal parietal wall. The samples were fixed in 10% buffered formalin, embedded in paraffin and serially sectioned at a thickness of 2.5 μm. Only the centers of the tissue samples were used for histological analysis, the edges being removed after the samples were fixed in formalin. Rat peritoneal histological examination was conducted under light microscopy with Masson staining and immunohistochemistry staining. Half of each sample was also used for collection of peritoneal cells. For this, peritoneal tissues were soaked in trypsin solution and incubated at 37 °C for 50 min. Then, the suspended cells were treated with Trizol (Thermo Fisher Scientific, Waltham, MA). The extracted nucleic acids were used for real-time polymerase chain reaction (RT-PCR) testing using the two-step RT-PCR kit (Bio-Rad, Hercules, CA) and Agilent array (Takara Bio, Kusatsu, Shiga, Japan).
Immunohistochemical analysis was performed using monoclonal antibodies against the mesenchymal marker vimentin (Santa Cruz Biotechnology Inc., Dallas, TX), proliferative marker Ki-67 (Novus Biologicals, CO), apoptosis marker M30 CytoDeath (PEVIVA, Sundbyberg, Sweden), total macrophage CD68 mouse anti-rat monoclonal (ED1) antibody (LSBio, Seattle, WA), M1 macrophage CD80 mouse monoclonal antibody (Origene Technologies Inc., Rockville, MD), and M2 macrophage CD163 mouse monoclonal antibody (Leica Biosystems, Nussloch, Germany).
For quantitative analysis, the number of positive cells in the peritoneal tissue sample was counted in five randomly selected fields. The positive cells in each picture were counted in relation to per surface length. The surface length of peritoneum was measured by the free software, ImageJ. The average surface length of peritoneum was 220 μm, which corresponds to 9 pixels in the software. We randomly selected 5 fields for image quantification. With regard to analysis of shed cells, the surface length of the peritoneal membrane with/without mesothelial cell covering was measured by ImageJ in 5 randomly taken pictures, and the proportion of uncovered area was calculated by the following formula: (uncovered length/total surface length observed).
RT-PCR analysis was performed using probe sets from the Bio Rad CFX96 system (Bio Rad Laboratories Inc., Hercules, CA). Gene-specific primers for glyceraldehyde-3-phosphate dehydrogenase (GAPDH): forward GGCACAGTCAAGGCTGAGAATG, reverse ATGGTGGTGAAGACGCCAGTA); Vascular endothelial growth factor (VEGF): forward ATCATGCGGATCAAACCTCACC, reverse GGTCTGCATTCACATCTGCTATGC; B-cell lymphoma 2 (BCL2): forward CCTGTGGATGACTGAGTACCTGAAC, reverse CAGAGTCTTCAGAGACAGCCAGGA; Smooth muscle actin A (αSMA): forward GCTCTGTAAGGCGGGCTTTG, reverse ACGAAGGAATAGCCACGCTCA; E-cadherin: forward CAGGAT TACAAGTTCCCGCCA, reverse CACTGTCCGCTG CCTTCA; BCL-2-like protein 4 (BAX): forward CTGCAGAGGATGATTGCTGA, reverse GATCAGCTCGGGCACTTTAG, BCL-2-associated death promoter (BAD): forward CAGTGATCTGCTCCACATTC, reverse TCCAGCTAGGATGATAGGAC; Vimentin: forward AATTGCAGGAGCTGAATGAC, reverse AATGACTGCAGGGTGCTCTC; SNAIL: forward GCTCCTTCCTGGTCAGGAAG, reverse GGCTGAGGTACTCCTTATTAC; and Cytokeratin: forward GAGGAGACCAAAGGCCGTTAC, reverse GAGGAGAATTGAGAGGATGAGGA, were used for amplification of specific complementary (cDNA)s with the iScript one-step RT-PCR kit, also from Bio Rad. The relative expression levels of each messenger RNA (mRNA) were normalized to GAPDH mRNA levels.
Cluster analysis (Pearson’s correlation coefficient) of the results of the Agilent array was performed using a microarray data analysis tool (Filgen Inc., Nagoya, Japan).
Statistical analysis
Statistical analyses were performed using Sigma plot version 12 (Hulinks, Tokyo, Japan). All results were expressed as the mean ± standard error. Comparisons of groups were performed using one-way ANOVA and the Tukey post-hoc test. Values of p < 0.05 were considered to indicate statistical significance.
Results
Histological examinations: Masson and immunohistochemistry staining in PD and H2PD groups
Representative findings of histological examinations are shown in Fig. 2. Mesothelial cells and sub-mesothelial layers were observed by Masson staining. As compared to the control group, PD group rat peritoneum showed cuboidal changes in the cells on the surface of the membrane, with mononuclear cells infiltrating the surface as well as in the sub-mesothelial layer. The H2PD group showed relatively flatter cells on the surface. In terms of immunohistochemical staining, such as for vimentin, Ki-67, and M30 CytoDeath, positive cells were mainly observed in the surface of the peritoneum.
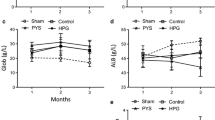
There were significant differences in the thickness of the sub-mesothelial layer among the three groups (46.6 ± 2.6 μm in the control, 52.9 ± 4.6 in the PD, and 50.7 ± 5.6 in the H2PD group; control vs. PD: p < 0.05) (Fig. 3a).
Quantitative analysis of peritoneal thickness and immunohistochemical staining of the peritoneum in PD and H2PD groups. Peritoneal thickness (a), and immunostainings of mesenchymal marker: vimentin (b), proliferative marker: Ki-67 (c), apoptosis marker: M30 CytoDeath (d), and total macrophage marker: CD68+, M1 macrophage: CD80+, and M2 macrophage: CD163+ (e), and ratio of M1 /M2 (positive cells number per field) (f). * p < 0.05
A significant increase in the number of vimentin-positive cells were observed in the PD group compared with the control and H2PD groups (5.9 ± 0.6 positive cells/field in the control, 17.7 ± 1.4 in the PD, and 8.7 ± 2.8 in the H2PD group; PD vs. control and H2PD groups: p < 0.05, respectively) (Fig. 3b).
Ki-67-positive cells were significantly increased in the PD group as compared with the control and H2PD groups (1.2 ± 0.0 positive cells /field in the control, 5.9 ± 1.2 in the PD, and 1.6 ± 0.3 in the H2PD group; PD vs. control and H2PD groups: p < 0.05, respectively) (Fig. 3c).
M30 CytoDeath-positive staining was significantly increased in the PD group as compared with the control and H2PD groups (0.3 ± 0.0 positive cells/field in the control, 5.0 ± 1.0 in the PD, and 1.2 ± 0.5 in the H2PD group: PD vs. control and H2PD groups: p < 0.05, respectively) (Fig. 3d).
There were no significant differences in total macrophage infiltration (C68), and M1 macrophages (CD80) in the peritoneum among the three groups. However, there was a significant difference in the infiltration of M2 macrophages (C163) (0.4 ± 0.1 positive cells/field in the control, 1.4 ± 0.3 in the PD, and 2.2 ± 1.2 in the H2PD group; control vs. H2PD: p < 0.05) (Fig. 3e). There were statistically significant differences in the ratios of M1/M2 macrophage infiltration in the peritoneum between the PD and H2PD groups (2.9 ± 0.7 in the PD and 1.1 ± 0.4 in the H2PD group, p < 0.05) (Fig. 3f).
Real-time PCR in PD and H2PD groups
In the PD group, there was a significant increase in gene expression of BAD as compared with the control group. There were tendencies for increased expression of genes, such as aSMA and vimentin, in the PD group as compared with the other groups, although the differences between them were not statistically significant (Fig. 4).
Agilent array in PD and H2PD groups
Whole gene expression, performed using a Microarray data analysis tool, indicated that there were differences in total gene expression levels by 8.7% between PD and control groups, and by 3.7% between H2PD and PD groups, respectively (Fig. 5).
Comparisons of Fe-PD and Fe-H2PD groups
Representative findings of Masson and immunohistochemical staining in the Fe-PD and Fe-H2PD groups are shown in Fig. 6a. In the Fe-PD group, severe shedding of mesothelial cells was observed, and the cells remaining on the peritoneal surface included a mix of cuboid and aggregated cells. The findings were similar in the Fe-H2PD group, although the extent of shedding was less than in the Fe-PD group.
Comparison of Fe-PD and Fe-H2PD groups. Representative findings of Masson and immunohistochemical staining (CD68) (a), and quantitative analysis of peritoneal morphology and immunohistochemical staining of the peritoneum in the respective groups (b-g) are shown. Peritoneal thickness (b), ratio of shed cells in the peritoneal surface (c), immunostainings of mesenchymal marker: vimentin (d), apoptosis marker: M30 CytoDeath (e), proliferative marker: Ki-67 (f), and total macrophage marker: CD68+, M1 macrophage: CD80+, and M2 macrophage: CD163+ (e), respectively. * p < 0.05
Significant differences were found in the thickness of the sub-mesothelial layer between the groups (60.9 ± 3.1 μm in the Fe-PD, and 53.3 ± 5.7 in the Fe-H2PD group; p < 0.05) (Fig. 6b), and the proportion of shed cells on the peritoneal surface (28.9 ± 7.1% in the Fe-PD, and 2.4 ± 1.6% in the Fe-H2PD group; p < 0.05) (Fig. 6c). There were no significant differences in vimentin-positive cells (Fig. 6d), although there were statistically significant differences in M30 CytoDeath-positive cells (0.7 ± 0.1 and 0.9 ± 0.2 positive cells/field in the Fe-HD and Fe-H2PD groups, respectively; p < 0.05) (Fig. 6e), and in Ki-67-positive cells (0.2 ± 0.1 and 0.5 ± 0.1 positive cells/field in the Fe-PD and Fe-H2PD groups, respectively; p < 0.05) between the groups (Figs. 6f).
There were no significant differences in the infiltration of all types of macrophages (C68), M1 macrophages (CD80), and M2 macrophages (CD163) in the peritoneum between the two groups (Fig. 6g).
Discussion
In the present study, we examined the potential of an H2-containing PD solution (400 ppb) in the protection of peritoneal mesothelial cells and peritoneal tissue in experimental PD rats. We employed a commercially available low-GDP, neutral PD solution for the study, and also studied a solution with FeCl3, to enhance oxidative stress-induced tissue injury.
We observed mild but significant sub-mesothelial thickening in the PD group as compared to controls. Notably, analysis of the PD group indicated characteristic morphological changes in the mesothelial cells, in the form of cuboidal formation and nuclear aggregation. Further, there was increased immunostaining suggestive of apoptosis, proliferation, and vimentin in the peritoneal surface tissue. On the other hand, there were significantly fewer changes in the H2PD group, which instead exhibited a dominant presence of M2 type macrophages. The Fe-PD group exhibited significant loss of mesothelial cells and sub-mesothelial thickening, although these changes were ameliorated in the Fe-H2PD group.
In this study, there were unique findings in the PD group, namely simultaneous increases in proliferation and apoptosis in the surface cells. Although the exact mechanism of this finding remains to be elucidated, we suggest the following hypothesis: the mesothelial cells are constantly exposed to PD solution, which is potentially bio-incompatible. The mesothelial cells seem to be in both a damaged “pre-apoptotic” state and in a state of compensatory proliferation in order to restore the membrane. The balance between the two opposite states is probably crucial for preservation of peritoneal integrity. In fact, the disruption in balance caused by oxidative stress secondary to FeCl3 resulted in severe mesothelial loss, along with an increase in accompanying membrane thickness (Fig. 6b and c).
Of note in the present study, morphological changes were found in the surface cells of the peritoneum in the PD group, in the form of cuboidal changes in the cells. There was a significant increase in vimentin staining in the PD group, as compared to the control and H2PD groups, which may indicate phenotypic alteration of mesothelial cells, resulting in epithelial-mesenchymal transition (EMT). However, unexpectedly, the expression of genes that modulate EMT was not different between the PD and H2PD groups. Hence, it remains unclear whether the same or different mesothelial cells presented with apoptosis and proliferation. This needs to be addressed in future studies.
With regard to PCR analysis, we chose EMT and its related genes, and anti-apoptotic and apoptotic genes, because we originally hypothesized that H2 could ameliorate activation of the process of EMT and the oxidative cellular injury resulting from exposure to PD solutions. We expected differences in gene expressions between the PD and H2PD groups, e.g. increases in SNAIL, vimentin, aSMA and VEGF, and decreases in ECADHERIN and CYTOKERATIN in the former group, and increases in BAX and BAD, and decreases in BCL2 in the latter group. However, unexpectedly, there were no differences in gene expressions between the two groups (Fig. 4). Therefore, we suppose other potential mechanisms for the effect of H2 on membrane protection.
Recent studies have revealed a significant role of tissue macrophages in orchestrating the healing process in wound tissue, i.e., M1 macrophages have inflammatory actions, and M2 have remodeling/healing actions in damaged tissues [24]. In this study, there were significant differences in infiltration of M2 macrophage sub-populations in the peritoneum between the PD and H2PD groups, with M2 being dominant in the H2PD group. This may indicate enhanced healing of peritoneal tissue in the H2PD group. However, the question as to whether H2 can facilitate macrophage functional switching in order to reduce tissue injury needs to be clarified in future.
Improved biocompatibility of PD solutions is the most crucial factor in preserving peritoneal integrity and ensuring successful long-term PD. Although low-GDP neutral PD solutions are supposedly biocompatible, as compared to conventional acidic solutions with high GDPs [16, 17], the present results indicate that the neutral solution is still somewhat bio-incompatible. Cluster analysis, which revealed an 8.7% difference in gene expression profiles between the PD and control groups, may well support this notion. We suppose that high glucose may, at least partly, play a crucial role as an oxidant, as reported elsewhere [11,12,13], which indicates room for improvement in the biocompatibility of the present standard solutions. Studies on clinical approaches to peritoneal protection have been limited so far [25,26,27].
To date, no critical adverse effects of H2 have been reported in humans, making it seem like a very good candidate for clinical application, provided there is scientific rationale for its use. We previously produced an H2-dissolved hemodialysis solution and observed an improvement in hypertension, as well as decreases in plasma levels of MCP-1 and MPO in chronic hemodialysis patients [28,29,30]. We believe that H2-containing PD solutions could be a candidate as novel PD solutions with improved biocompatibility, and our results support the significance of H2PD clinical trials in the future. In future clinical trials, the risks of flammable H2 gas need to be taken into account. However, the explosive concentration of H2 is close to 4% (40 × 103 ppm), while the levels of H2 in PD solution bags are less than 0.4 ppm, and H2 levels of water for bathing manufactured by the present method are 1.6 ppm at maximum (Fig. 1a). Therefore, we believe it is safe to conduct clinical trials using this system.
Conclusion
H2-dissolved PD solutions could preserve mesothelial cells and the peritoneal membrane in experimental PD rats. Clinical application of H2 in PD could lead to creation of a novel strategy for the protection of peritoneal tissues during PD treatment.
Abbreviations
- CD163+:
-
M2
- CD80 +:
-
M1
- ED1, CD68:
-
Macrophages
- EMT:
-
epithelial mesenchymal transition
- EPS:
-
encapsulating peritoneal sclerosis
- GDP:
-
glucose degradation products
- H2 :
-
dihydrogen, molecular hydrogen
- MAPK:
-
mitogen-activated protein kinase
- MCP-1:
-
monocyte chemotactic and activating factor
- MEK:
-
MAPK/ERK kinase
- MPO:
-
myeloperoxidase
- NFkB:
-
nuclear factor-kappa B
- Nrf2:
-
NF-E2 related factor 2
- PD:
-
Peritoneal dialysis
- Ppb:
-
parts per billion
- Ppm:
-
parts per million
References
Popovich RP, Moncrief JW, Nolph KD, Ghods AJ, Twardowski ZJ, Pyle WK. Continuous ambulatory peritoneal dialysis. 1978. J Am Soc Nephrol. 1999;10(4):901–10.
Oreopoulos DG, Robson M, Izatt S, Clayton S, deVeber GA. A simple and safe technique for continuous ambulatory peritoneal dialysis (CAPD). Trans Am Soc Artif Intern Organs. 1978;24:484–9.
Honda K, Hamada C, Nakayama M, Miyazaki M, Sherif AM, Harada T, Hirano H. Impact of uremia, diabetes, and peritoneal dialysis itself on the pathogenesis of peritoneal sclerosis: a quantitative study of peritoneal membrane morphology. Clin J Am Soc Nephrol. 2008;3(3):720–8.
Krediet RT, Struijk DG. Peritoneal changes in patients on long-term peritoneal dialysis. Nat Rev Nephrol. 2013;9(7):419–29.
Kawaguchi Y, Saito A, Kawanishi H, Nakayama M, Miyazaki M, Nakamoto H, Tranaeus A. Recommendations on the management of encapsulating peritoneal sclerosis in Japan, 2005: diagnosis, predictive markers, treatment, and preventive measures. Perit Dial Int. 2005;25(Suppl 4):S83–95.
Korte MR, Sampimon DE, Betjes MG, Krediet RT. Encapsulating peritoneal sclerosis: the state of affairs. Nat Rev Nephrol. 2011;7(9):528–38.
Nakayama M, Terawaki H. Multidisciplinary clinical strategies for encapsulating peritoneal sclerosis in peritoneal dialysis: update from Japan. Int J Urol. 2014;21(8):755–61.
Zhou Q, Bajo MA, Del Peso G, Yu X, Selgas R. Preventing peritoneal membrane fibrosis in peritoneal dialysis patients. Kidney Int. 2016;90(3):515–24.
Yung S, Chan TM. Intrinsic cells: mesothelial cells -- central players in regulating inflammation and resolution. Perit Dial Int. 2009;29(Suppl 2):S21–7.
Perl J, Nessim SJ, Bargman JM. The biocompatibility of neutral pH, low-GDP peritoneal dialysis solutions: benefit at bench, bedside, or both? Kidney Int. 2011;79(8):814–24.
Ishibashi Y, Sugimoto T, Ichikawa Y, Akatsuka A, Miyata T, Nangaku M, Tagawa H, Kurokawa K. Glucose dialysate induces mitochondrial DNA damage in peritoneal mesothelial cells. Perit Dial Int. 2002;22(1):11–21.
Lee HB, MR Y, Song JS, Ha H. Reactive oxygen species amplify protein kinase C signaling in high glucose-induced fibronectin expression by human peritoneal mesothelial cells. Kidney Int. 2004;65(4):1170–9.
Hung KY, Liu SY, Yang TC, Liao TL, Kao SH. High-dialysate-glucose-induced oxidative stress and mitochondrial-mediated apoptosis in human peritoneal mesothelial cells. Oxidative Med Cell Longev. 2014;2014:642793.
Breborowicz A, Polubinska A, Kupczyk M, Wanic-Kossowka M, Oreopoulos DG. Intravenous iron sucrose changes the intraperitoneal homeostasis. Blood Purif. 2009;28(1):53–8.
Breborowicz M, Polubinska A, Tam P, Wu G, Breborowicz A. Effect of iron sucrose on human peritoneal mesothelial cells. Eur J Clin Investig. 2003;33(12):1038–44.
Tsukamoto M, Ishibashi Y, Takazawa Y, Komemushi Y, Kume H. Normal peritoneal histology after ten years of peritoneal dialysis in a contemporary Japanese patient. Perit Dial Int. 2013;33(4):463–4.
Hamada C, Honda K, Kawanishi K, Nakamoto H, Ito Y, Sakurada T, Tanno Y, Mizumasa T, Miyazaki M, Moriishi M, et al. Morphological characteristics in peritoneum in patients with neutral peritoneal dialysis solution. J Artif Organs. 2015;18(3):243–50.
Tanno Y, Yokoyama K, Hosoya T. Laparoscopic approach for the evaluation of peritoneal injury. Kidney Int. 2012;82(2):244–5.
Nakayama M, Miyazaki M, Honda K, Kasai K, Tomo T, Nakamoto H, Kawanishi H. Encapsulating peritoneal sclerosis in the era of a multi-disciplinary approach based on biocompatible solutions: the NEXT-PD study. Perit Dial Int. 2014;34(7):766–74.
Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S, Ohta S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13(6):688–94.
Ichihara M, Sobue S, Ito M, Hirayama M, Ohno K. Beneficial biological effects and the underlying mechanisms of molecular hydrogen - comprehensive review of 321 original articles. Med Gas Res. 2015;5:12.
Terawaki H, Hayashi Y, Zhu WJ, Matsuyama Y, Terada T, Kabayama S, Watanabe T, Era S, Sato B, Nakayama M. Transperitoneal administration of dissolved hydrogen for peritoneal dialysis patients: a novel approach to suppress oxidative stress in the peritoneal cavity. Med Gas Res. 2013;3(1):14.
Terawaki H, Nakano H, Zhu WJ, Nakayama M. Successful treatment of encapsulating peritoneal sclerosis by hemodialysis and peritoneal lavage using dialysate containing dissolved hydrogen. Perit Dial Int. 2015;35(1):107–12.
Novak ML, Koh TJ. Macrophage phenotypes during tissue repair. J Leukoc Biol. 2013;93(6):875–81.
Lu Y, Shen H, Shi X, Feng S, Wang Z, Shi Y. Hydrogen sulfide ameliorates high-glucose toxicity in rat peritoneal mesothelial cells by attenuating oxidative stress. Nephron Exp Nephrol. 2014;126(3):157–65.
Yang L, Wu L, Du S, Hu Y, Fan Y, Ma J. 1,25(OH)2D3 inhibits high glucose-induced apoptosis and ROS production in human peritoneal mesothelial cells via the MAPK/P38 pathway. Mol Med Rep. 2016;14(1):839–44.
Wakabayashi K, Hamada C, Kanda R, Nakano T, Io H, Horikoshi S, Tomino Y. Oral Astaxanthin supplementation prevents peritoneal fibrosis in rats. Perit Dial Int. 2015;35(5):506–16.
Nakayama M, Kabayama S, Terawaki H, Nakayama K, Kato K, Sato T, Ito S. Less-oxidative hemodialysis solution rendered by cathode-side application of electrolyzed water. Hemodial Int. 2007;11(3):322–7.
Nakayama M, Nakano H, Hamada H, Itami N, Nakazawa R, Ito S. A novel bioactive haemodialysis system using dissolved dihydrogen (H2) produced by water electrolysis: a clinical trial. Nephrol Dial Transplant. 2010;25(9):3026–33.
Terawaki H, Zhu WJ, Matsuyama Y, Terada T, Takahashi Y, Sakurai K, Kabayama S, Miyazaki M, Itami N, Nakazawa R, et al. Effect of a hydrogen (H2)-enriched solution on the albumin redox of hemodialysis patients. Hemodial Int. 2014;18(2):459–66.
Acknowledgements
Our special thanks Ms. Ohashi and Ms. Hashimoto for their technical assistance with the experiments.
Funding
The study was supported by a fund from the Japan Science and Technology Agency (JST) Revitalization promotion program (H25 II-205: 2013–2015); Chief investigator: MN.
Availability of data and materials
Not applicable.
Ethics approval
All the study procedures and protocols were approved by the Animal Committee of Fukushima Medical University (approval number: 25,017).
Author information
Authors and Affiliations
Contributions
MN designed and supervised the entire study and finalized the manuscript. WZ, KW, and AG carried out the experimental study. WZ, KW, and MN wrote the draft manuscript and created the figures. AMS, SK, and SI contributed to study conception and analysis and interpretation of data. All the authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Consent for publication
Not applicable.
Competing interests
None.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Nakayama, M., Zhu, WJ., Watanabe, K. et al. Dissolved molecular hydrogen (H2) in Peritoneal Dialysis (PD) solutions preserves mesothelial cells and peritoneal membrane integrity. BMC Nephrol 18, 327 (2017). https://doi.org/10.1186/s12882-017-0741-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-017-0741-0