Abstract
Background
Chronic kidney disease (CKD) is a major threat to the health of people of African ancestry. We assessed the prevalence and risk factors of CKD among adults in urban Cameroon.
Methods
This was a cross-sectional study of two months duration (March to April 2013) conducted at the Cité des Palmiers health district in the Littoral region of Cameroon. A multistage cluster sampling approach was applied. Estimated glomerular filtration rate (eGFR) was based on the Cockcroft-Gault (CG), the four-variable Modification of Diet in Renal Disease (MDRD) study and Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations. Logistic regression models were used to investigate the predictors of CKD.
Results
In the 500 participants with a mean age of 45.3 ± 13.2 years included, we observed a high prevalence of overweight and obesity (60.4 %), hypertension (38.6 %) and diabetes (2.8 %). The mean eGFR was 93.7 ± 24.9, 97.8 ± 24.9 and 99.2 ± 31.4 ml/min respectively with the MDRD, CG and CKD-EPI equations. The prevalence of albuminuria was 7.2 % while the prevalence of decreased GFR (eGFR < 60 ml/min) and CKD (any albuminuria and/or eGFR < 60 ml/min) was 4.4 and 11 % with MDRD, 5.4 and 14.2 % with CG, and 8.8 and 10 % with CKD-EPI. In age and sex adjusted logistic regression models, advanced age, known hypertension and diabetes mellitus, increasing body mass index and overweight/obesity were the predictors of albuminuria, decreased GFR and CKD according to various estimators.
Conclusion
There is a high prevalence of CKD in urban adults Cameroonian, driven essentially by the commonest risk factors for CKD.
Similar content being viewed by others
Background
Sub-Saharan Africa (SSA) countries are undergoing demographic and epidemiological transition with the double burden of non-communicable and infectious diseases [1]. The adoption of western lifestyles, mostly in urban areas, contributes to increase the prevalence of hypertension and diabetes mellitus in this setting [2–4]. The above factors are associated with glomerular diseases and constitute the main etiological factors for chronic kidney disease (CKD) in SSA [5, 6]. CKD is emerging as one of the major health threats, affecting 10 % of adults worldwide and contributing every year to millions of premature deaths [7, 8]. Few studies have been conducted on CKD epidemiology in SSA [9]. These studies mostly of low methodological quality have revealed huge disparities in the prevalence of CKD across SSA regions depending on the definition, method for assessing glomerular filtration rate (GFR) and targeted population [4, 9–14].
In central Africa, previous studies have reported a high prevalence of CKD which affects young adults in their productive years; being higher in high risk groups such as people with hypertension, diabetes mellitus, obesity or HIV infection [10–12]. The present report presents findings from a study on the prevalence and risk factors of CKD in urban Cameroon.
Methods
Study setting and design
We carried a cross-sectional study of two months duration from March to April 2013 in all health areas of the Cité des Palmiers health district in the Littoral region of Cameroon. The Cité des Palmiers health district is the second largest and populous health district in Douala, the economic Capital of Cameroon. It comprises eight health areas with an estimated population of 423,253 inhabitants in 2012. The population is diversified representing the different ethnic and social groups in the country, and comprises students, traders, civil servants, housewives, and low, middle and high income earners from private sectors. This study was approved by the Cameroon National Ethics Committee.
We used a multistage cluster sampling to recruit 500 participants as Sumaili et al. in Kinshasa [11], corresponding to 62–63 subjects per health area. Sampling stages included the health area (first stage), the neighbourhood (second stage) and individuals (third stage). Adults were informed through community leaders, posters, leaflets and words of mouth, and requested to report to the chieftainship. All adults who reported on the day of recruitment benefited from a sensitization campaign, followed by a random selection of study participants and data collection.
Data collection
Final year undergraduate medical students collected data between 8 a.m. to 12 a.m. for participants who provided a written informed consent. They used a pre-designed questionnaire to collect socio-demographic data (age, gender and occupation) and clinical information including personal history of existing conditions (hypertension, diabetes and gout), lifestyle characteristics (alcohol consumption and smoking), use of nephrotoxic agents (herbal medicines, foods addictive and street medicines), anthropometric measurements (weight, height and waist girth) and blood pressure variables. Blood pressure was measured according to the World Health Organization (WHO) guidelines [15] using an automated sphygmomanometer (OMRON HEM705CP, Omron Matsusaka Co, Matsusaka City, Mie-Ken, Japan) on the right arm with participants in a sitting position. All anthropometric measurements were performed three times and their average used in all analyses. In every participant, we drew 3 ml of whole blood from an antecubital vein into dry tubes for serum creatinine and collected mid-stream second morning urine for dipstick tests.
Dipstick tests were performed immediately after sample collection while blood specimens for serum creatinine were transported on ice-cooled containers to the biochemistry laboratory of the Douala General Hospital for processing. Urine dipstick tests were performed with CombiScreen 7SL PLUS 7 test strips (Analyticon Biotechnologies AG, D-35104 Lichentenfeis, Germany). Serum creatinine was measured with a kinetic modification of the Jaffé reaction using Human visual spectrophotometer (Human Gesellschaft, Biochemica und Diagnostica mbH, Wiesbaden, Germany) and Beckman creatinine analyzer (Beckman CX systems instruments, Anaheim, CA, USA). For any participant with positive dipstick [protein (at least traces), blood, leucocytes)], another urine sample was collected 2 to 3 weeks later to confirm the results. We excluded 11 (2.2 %) pregnant women and seven (1.4 %) participants with concomitant leucocyturia and urine nitrites.
Definitions and calculations
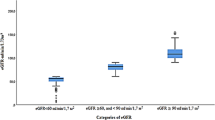
Estimated glomerular filtration rate (eGFR, mL/min) used the Cockcroft-Gault (CG), the four-variable Modification of Diet in Renal Disease (MDRD) study and Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations [16–18]. CKD was defined by a confirmed positive dipstick albuminuria (at least traces) and/or eGFR < 60 ml/min/1.73 m2 according to K/DIGO guidelines which were used to stage participants for CKD [19]. eGFR classification was the following: G1 (eGFR ≥ 90); G2 (eGFR 60–89); G3a (eGFR 45–59); G3b (eGFR 30–44); G4 (eGFR 15–29) and G5 (eGFR < 15). Albuminuria was classified as: A1 (negative); A2 (trace to 1+) and A3 (at least 2+). Decreased GFR corresponded to any eGFR < 60 ml/min regardless the equation used. The following formula was used to convert serum creatinine from Jaffé reaction (SCrJaffé) to standardized serum creatinine (SCrStandardized) to be used in MDRD and CKD-EPI formulas: SCrStandardized = 0.95*SCrJaffé – 0.10 [20, 21]. Hypertension was defined as a systolic (SBP) ≥140 mmHg and/or a diastolic blood pressure (DBP) ≥90 mmHg or being on antihypertensive drugs. Diabetes was defined as self-reported history of doctor diagnosed condition or use of glucose control agents.
Statistical analysis
Data analysis used SPSS® v.17 software for Windows® (SPSS Inc., Chicago, USA). We have reported the results as means and standard deviations, and counts and percentages. The Fisher exact test, Student t-test and Mann–Whitney U test were used to compare qualitative and quantitative variables across subgroups defined by sex, status for albuminuria, decreased GFR and CKD according to different GFR estimators. Age and sex adjusted logistic regression models were used to investigate the predictors of CKD. A p-value <0.05 was used to indicate statistically significant results.
Results
Baseline characteristics of the study population
The mean age was 45.3 years, similarly between men and women (p = 0.267), Table 1. Alcohol (68.9 vs. 37.8 %), tobacco use (12.7 vs. 0.4 %), and systolic blood pressure (137 vs. 128 mmHg) were higher in men compared to women (all p < 0.001). However, compared with men, women were more likely to have a history of hypertension (15.5 vs. 9.4 %, p = 0.041), to have high body mass index (BMI, 28.6 vs. 25.8 kg/m2, p < 0.001)), and to be overweight or obese (68.2 vs. 53.6 %, p = 0.001), Table 1. We observed a higher prevalence of CKD risk factors including hypertension (38.6 %), overweight and obesity (60.4 %), and longstanding use of herbal (57.8 %) and street (29.6 %) medicines in the study sample. As expected, serum creatinine was higher in men than women (p < 0.001) but women had a significantly higher mean estimated creatinine clearance by the CKD-EPI equation (103.5 vs. 95.5 ml/min, p = 0.005). Furthermore, estimated creatinine clearance was highest with CKD-EPI equation and lowest with the MDRD equation.
Staging of kidney function and prevalence of chronic kidney disease
The staging of kidney function according to various estimators is presented in Table 1. In general, there was no sex difference in the staging of kidney function, regardless of the estimator (all p > 0.206). None of the participants was in stage G5 regardless of the estimators used. Similar proportions of stages G3b and G4 were observed with CKD-EPI and MDRD estimators. The prevalence of albuminuria was 7.2 % while the prevalence of decreased GFR (eGFR < 60 ml/min) was 4.4, 5.4 and 8.8 % respectively based on GFR estimated from the CKD-EPI, MDRD and CG equations (Table 2). The prevalence of CKD (any albuminuria and/or eGFR < 60 ml/min) was 10.0, 11.0 and 14.2 % respectively for CKD-EPI, MDRD and CG equations (Table 3).
Correlates of albuminuria, decreased GFR and CKD
The distribution of baseline characteristics according to the presence of albuminuria, decreased GFR or CKD is shown in Tables 2 and 3. Advanced age and known hypertension and diabetes status were significantly associated with albuminuria, decreased GFR and CKD regardless the equation used meanwhile any hypertension was associated with decreased GFR and CKD. Decreased GFR and CKD estimated by CG were associated with increased BMI and overweight/obesity.
Age and sex adjusted predictors of albuminuria, decreased GFR and CKD
Age and sex adjusted predictors of albuminuria, decreased GFR and CKD are presented in Tables 4 and 5 separately for each of the estimators. Advanced age was consistently and positively associated with all these outcomes, with the magnitude of the effects per year increase in age being 4 % (95%CI: 1–7 %) for albuminuria, 11 % (7–16 %) to 17 % (11–23 %) for decreased GFR, and 6 % (4–9 %) to 9 % (7–12 %) for CKD. With the exception of decreased GFR and CKD estimated by the CG estimator, known hypertension status was also significantly and positively associated with all the outcomes; meanwhile existing diabetes was borderline associated with albuminuria [OR 3.05 (95%CI: 0.98–12.8), p = 0.055], and CKD based on MDRD [3.22 (0.99–10.45), p = 0.051] and CKD-EPI [3.36 (1.02–11.07), p = 0.046] equations. Increasing BMI was significantly and negatively associated with CG-defined decreased GFR and CKD, and positively with MDRD and CKD-EPI defined decreased GFR, but not CKD. As a consequence, overweight/obesity was associated with lower odd of CG defined decreased GFR [0.11 (0.05–0.26), p < 0.001] and CKD [0.30 (0.17–0.54), p < 0.001]. The small number of outcomes precluded expanded multivariable regression analysis.
Discussion
Our study assessed the prevalence and correlates of CKD in an urban adults Cameroonian population using the commonest estimators of kidney function. It revealed a high frequency of CKD and related risk factors in this population, with over one in ten participants having CKD regardless of whether CG, MDRD or CKD-EPI equations were used to estimate kidney function. This high prevalence of CKD appeared to be driven mostly by advanced age, hypertension, diabetes mellitus and adiposity.
Our study met the criteria of high quality applied in the meta-analysis by Stanifer et al., and revealed a higher prevalence of CKD in this setting, regardless the estimators used, in line with the findings of the meta-analysis and previous studies in Central and Western Africa region [9, 11, 14]. These results confirm the already suggested high burden of CKD in SSA setting. CKD prevalence rates in our setting approximate those reported in other low-to-middle income countries and implies that CKD is not affecting only high-income countries [22–25]. Furthermore, much higher prevalence rates have been reported in high risk groups such as hypertensive, diabetes, obese and HIV infected patients in SSA, inviting targeted and proactive screening of these patients [10]. However, lower prevalence rates have been observed in a country like Senegal, in spite of similar high frequency of CKD risk factors [4]. The discrepancy could be explained by the differences in CKD definition used as well as methods for assessing urinary albumin excretion and serum creatinine. The reported higher prevalence rate of CKD could be explained by the epidemiological transition; there is a dual burden of diseases in this setting characterized by the growing prevalence and the lower awareness, treatment adherence and control rates of non-communicable diseases, and the increase nephrotoxicity of drugs used in the treatment of communicable disease [1, 4, 11, 26–28].
Across estimators of GFR, the CG equation diagnosed more participants with decreased GFR and CKD while CKD-EPI and MDRD with ethnicity correction diagnosed about the same proportion of participants with both conditions, largely in line with existing and extensively discussed reports from previous studies [11, 13, 14]. The observed higher GFR estimated by CKD-EPI equation compared to others estimators could be related to the fact that this equation performed better than others especially at higher GFR [17, 18].
Regardless of the estimators used to assess CKD, advanced age, hypertension, diabetes mellitus and adiposity were the risk factors of CKD observed in this study as reported elsewhere [4, 11–13]. These are well known clinical and socio-demographic risk factors for CKD occurrence and progression to end stage renal disease (ESRD) [19]. Moreover, hypertension and diabetes mellitus are associated with glomerular diseases and constitute the main etiological factors for CKD in SSA [5, 6]. These findings invite appropriate management of such factors and an array of actions to tackle them as well as implementation of sensitization campaign to raise awareness, increase treatment adherence and improve control rate. This is important to reduce the growing prevalence of ESRD patients in this lower middle income country where social security programs are inexistent, and where patients with CKD are referred late to nephrologists [29, 30]. Furthermore, in Cameroon for instance, and in spite of government’s subsidies, patients with ESRD on renal replacement therapy must pay the equivalent of US$ 12 per dialysis session [US$ 1248 per annum, which is almost the gross national income per capita of US$ 1270 in 2013] in addition to the costs of caring for comorbidities [29–31].
Strengths and limitations
The present study has some limitations including the semi-quantitative assessment of urinary albumin excretion using dipsticks, the non-validation of any of the equations used in SSA populations and the lack of three months control of positive findings to confirm the chronicity of renal injury as recommended by the KDIGO guidelines [19]. However, previous studies from Ghana and South Africa have found a high agreement between ethnicity corrected MDRD and CKD-EPI equations, supporting their use in this setting [13, 14]. Moreover, by conducting this study in only one urban health district of the country, there is little opportunity of assessing variations in the prevalence of CKD across the gradient of urbanization in the country. Lastly, the study was likely underpowered to reliably investigate the determinants of the disease. However, this study to our knowledge is the first to use a multistage cluster sampling to provide community-based data on the epidemiology of kidney disease in the country with the three estimators of kidney function. The inclusion of participants from a cosmopolite urban health district likely captures the diversity of the national population with our results likely reflecting the national urban prevalence of CKD.
Conclusions
This study revealed that more than one in ten participants presented with CKD regardless the estimators used. This sizable prevalence of CKD, similar to those reported in developed countries, is driven essentially by the well-known clinical and socio-demographic risk factors for CKD. Actions are needed both to prevent further increase in the prevalence of CKD and to improve the detection and appropriate management of those with risk factors of the disease.
Abbreviations
- A:
-
Albuminuria
- BMI:
-
Body mass index
- CG:
-
Cockroft-Gault
- CKD:
-
Chronic kidney disease
- CKD-EPI:
-
Chronic kidney disease epidemiology collaboration
- DBP:
-
Diastolic blood pressure
- eGFR:
-
Estimated glomerular filtration rate
- MDRD:
-
Modification of Diet in Renal Disease
- SBP:
-
Systolic blood pressure
- SD:
-
Standard deviation
References
Marshall SJ. Developing countries face double burden of disease. Bull World Health Organ. 2004;82(7):556.
Mbanya JC, Ngogang J, Salah JN, Minkoulou E, Balkau B. Prevalence of NIDDM and impaired glucose tolerance in a rural and an urban population in Cameroon. Diabetologia. 1997;40(7):824–9.
Mbanya JC, Minkoulou EM, Salah JN, Balkau B. The prevalence of hypertension in rural and urban Cameroon. Int J Epidemiol. 1998;27(2):181–5.
Seck SM, Doupa D, Gueye L, Ba I. Chronic kidney disease epidemiology in northern Senegal: a cross-sectional study. Iran J Kidney Dis. 2014;8(4):286–91.
Naicker S. End-stage renal disease in sub-Saharan Africa. Ethn Dis. 2009;19(1 Suppl 1):S1-13-15.
Kaze FF, Ashuntantang G. Halle MP. Outcomes of Non-Tunneled Non-Cuffed Hemodialysis Catheters in Patients on Chronic Hemodialysis in a Resource Limited Sub-Saharan Africa Setting. Ther Apher Dial: Kengne AP; 2014.
Welborn TABA, Rubinstein AH, Dollery CT, Fraser TR. Serum-insulin in essential hypertension and in peripheral vascular disease. Lancet. 1966;1:1336–7.
Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–128.
Stanifer JW, Jing B, Tolan S, Helmke N, Mukerjee R, Naicker S, et al. The epidemiology of chronic kidney disease in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(3):e174–81.
Sumaili EK, Cohen EP, Zinga CV, Krzesinski JM, Pakasa NM, Nseka NM. High prevalence of undiagnosed chronic kidney disease among at-risk population in Kinshasa, the Democratic Republic of Congo. BMC Nephrol. 2009;10:18.
Sumaili EK, Krzesinski JM, Zinga CV, Cohen EP, Delanaye P, Munyanga SM, et al. Prevalence of chronic kidney disease in Kinshasa: results of a pilot study from the Democratic Republic of Congo. Nephrol Dial Transplant. 2009;24(1):117–22.
Sumaili EK, Nseka NM, Lepira FB, Krzesinski JM, Makulo JR, Bukabau JB, et al. Screening for proteinuria and chronic kidney disease risk factors in Kinshasa: a World Kidney Day 2007 study. Nephron Clin Pract. 2008;110(4):c220–8.
Matsha TE, Yako YY, Rensburg MA, Hassan MS, Kengne AP, Erasmus RT. Chronic kidney diseases in mixed ancestry south African populations: prevalence, determinants and concordance between kidney function estimators. BMC Nephrol. 2013;14:75.
Eastwood JB, Kerry SM, Plange-Rhule J, Micah FB, Antwi S, Boa FG, et al. Assessment of GFR by four methods in adults in Ashanti, Ghana: the need for an eGFR equation for lean African populations. Nephrol Dial Transplant. 2010;25(7):2178–87.
Chalmers J, MacMahon S, Mancia G, Whitworth J, Beilin L, Hansson L, et al. 1999 World Health Organization-International Society of Hypertension Guidelines for the management of hypertension. Guidelines sub-committee of the World Health Organization. Clin Exp Hypertens. 1999;21(5–6):1009–60.
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41.
Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145(4):247–54.
Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro 3rd AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12.
K/DIGO. K/DIGO Guidelines: Definition and classification. Kidney Int Suppl. 2013;3(1):19–62.
Rule AD, Bailey KR, Schwartz GL, Khosla S, Lieske JC, Melton 3rd LJ. For estimating creatinine clearance measuring muscle mass gives better results than those based on demographics. Kidney Int. 2009;75(10):1071–8.
Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, et al. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53(4):766–72.
Iseki K. Chronic kidney disease in Japan. Intern Med. 2008;47(8):681–9.
Rajapurkar MM, John GT, Kirpalani AL, Abraham G, Agarwal SK, Almeida AF, et al. What do we know about chronic kidney disease in India: first report of the Indian CKD registry. BMC Nephrol. 2012;13:10.
de Lima AO, Kesrouani S, Gomes RA, Cruz J, Mastroianni-Kirsztajn G. Population screening for chronic kidney disease: a survey involving 38,721 Brazilians. Nephrol Dial Transplant. 2012;27 Suppl 3:iii135–8.
Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–47.
Ataklte F, Erqou S, Kaptoge S, Taye B, Echouffo-Tcheugui JB, Kengne AP. Burden of undiagnosed hypertension in Sub-Saharan Africa: a systematic review and meta-analysis. Hypertension. 2015;65(2):291.
Katte JC, Dzudie A, Sobngwi E, Mbong EN, Fetse GT, Kouam CK, et al. Coincidence of diabetes mellitus and hypertension in a semi-urban Cameroonian population: a cross-sectional study. BMC Public Health. 2014;14:696.
Kalyesubula R, Perazella MA. Nephrotoxicity of HAART. AIDS Res Treat. 2011;2011:562790.
Kaze FF, Kengne AP, Choukem SP, Dzudie A, Halle MP, Dehayem MY, et al. Dialysis in Cameroon. Am J Kidney Dis. 2008;51(6):1072–4.
Halle MP, Kengne AP, Ashuntantang G. Referral of patients with kidney impairment for specialist care in a developing country of sub-Saharan Africa. Ren Fail. 2009;31(5):341–8.
The World Bank. Cameroon. http://data.worldbank.org/country/cameroon.
Acknowledgements
We thank the Cité des Palmiers health district community for participating in the study. We also thank the administrative and traditional leaders of this community for their support during the study as well as the biochemistry laboratory technicians of the Douala General Hospital. No funding was received for this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
FFK: Conception, design, collection and supervision of clinical and biological data, interpretation of data, and drafting of the manuscript; MPH: Conception, design, collection and supervision of clinical data and drafting of the manuscript; HTM: collection of clinical and biological data, and critical revision of the manuscript; GA: Critical revision of the manuscript; HF: Critical revision of the manuscript; JN: Acquisition and validation of the biological data, and critical revision of the manuscript; APK: Analysis and interpretation of data, and drafting of the manuscript. All authors read and approved the final manuscript.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Kaze, F.F., Halle, MP., Mopa, H.T. et al. Prevalence and risk factors of chronic kidney disease in urban adult Cameroonians according to three common estimators of the glomerular filtration rate: a cross-sectional study. BMC Nephrol 16, 96 (2015). https://doi.org/10.1186/s12882-015-0102-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-015-0102-9