Abstract
Background
Usher syndrome (USH) is an autosomal recessive disorder characterized by hearing impairment and vision dysfunction due to retinitis pigmentosa. Phenotypic and genetic heterogeneities of this disease make it impractical to obtain a genetic diagnosis by conventional Sanger sequencing.
Methods
In this study, we applied a next-generation sequencing approach to detect genetic abnormalities in patients with USH. Two unrelated Chinese families were recruited, consisting of two USH afflicted patients and four unaffected relatives. We selected 199 genes related to inherited retinal diseases as targets for deep exome sequencing. Through systematic data analysis using an established bioinformatics pipeline, all variants that passed filter criteria were validated by Sanger sequencing and co-segregation analysis.
Results
A homozygous frameshift mutation (c.4382delA, p.T1462Lfs*2) was revealed in exon20 of gene USH2A in the F1 family. Two compound heterozygous mutations, IVS47 + 1G > A and c.13156A > T (p.I4386F), located in intron 48 and exon 63 respectively, of USH2A, were identified as causative mutations for the F2 family. Of note, the missense mutation c.13156A > T has not been reported so far.
Conclusion
In conclusion, targeted exome sequencing precisely and rapidly identified the genetic defects in two Chinese USH families and this technique can be applied as a routine examination for these disorders with significant clinical and genetic heterogeneity.
Similar content being viewed by others
Background
Usher syndrome (USH) is the most prevalent cause of hereditary deafness-blindness in humans [1]. It occurs in approximately 1 of 25,000 persons worldwide. USH is divided into clinical subtypes I, II, or III, based on severity and progression of vision and hearing loss [2]. Patients with Usher I have congenital deafness and begin to lose their vision early in life. They also exhibit difficulty balancing due to vestibular problems. Patients with Usher II exhibit congenital hearing loss that is mild-moderate in low tones and moderate to severe at higher frequencies. They generally have a normal vestibular system. Patients with Usher III are not born deaf, but experience a gradual loss of both their hearing and vision. Usher II is the most common form of USH, and may account for more than half of all patients with USH [3]. Such complex clinical heterogeneity often precludes an accurate clinical diagnosis. Hearing loss in USH is usually caused by impairment of inner ear structures such as hair cells, which transmit sound and motion signals to the brain. Vision loss results from the degeneration of photoreceptors in the retina [4].
Due to the genetic heterogeneity of USH, molecular diagnosis using traditional Sanger sequencing is quite laborious and expensive [5, 6]. To date, 12 genes (CDH23, CEP250, CIB2, CLRN1, DFNB31, GPR98, HARS, MYO7A, PCDH15, USH1C, USH1G and USH2A) have been reported to be responsible for Usher syndrome and three additional loci have been mapped. USH2A, the major pathogenic gene for Usher II, has 72 exons, the entire region of which is scattered with implicated mutations. Next-generation sequencing (NGS) technology has recently been demonstrated to be superior than Sanger for screening of hundreds of target genes at a given time [7]. In this study, we adopted an NGS-based capture technique to reveal genetic defects in two USH families and aimed to evaluate this method as a routine diagnostic tool for USH patients.
Methods
Patient recruitment
This study was performed in accordance with the tenets of the Declaration of Helsinki and the ethics committee of Taizhou Central Hospital, and written informedconsent was obtained from all study subjects. Two unrelated Chinese families, including two affected and four unaffected individuals, were enrolled. Clinical diagnosis of Usher syndrome was based on examination of audiologic function, visual acuity, fundus photography and optical coherence tomography (OCT). Typical initial symptoms of USH include hearing loss, vision acuity impairment, night blindness, and bone spicule-like pigmentation and attenuation of retinal vessels in fundus.
Sample preparation
Peripheral blood was collected from all subjects and used for DNA extraction using a kit (TIANGEN, Beijing) according to the manufacturer’s instructions. DNA yield was quantified with Nanodrop 2000 (Thermal Fisher Scientific, DE).
Targeted gene capture and sequencing
A solution-based sequence capture custom enrichment kit was used (GenCap, MyGenostics, Beijing), including 199 disease genes [8]. A minimum of 3 ug DNA was extracted per proband, and fragmented into 200–300 bp DNA libraries according to the manufacturer’s protocol. After end-repair and adapter ligation, target libraries were hybridized with the panel and enriched using the Illumina Exome Enrichment protocol. Purified and enriched libraries were then sequenced using an Illumina HiSeq 2000 Sequencer.
Bioinformatics analysis
After HiSeq 2000 re-sequencing, raw sequencing reads were first filtered by the Solexa QA package [9] and then by the cutadapt program (https://pypi.python.org/pypi/cutadapt). High quality reads were aligned to the reference genome sequence (hg19) by using Burrows-Wheeler Aligner and duplications were removed by using Sequence Alignment/Map tools [10]. Only unique aligned reads were kept for further analysis. Single-nucleotide variants (SNVs) and insertions or deletions (InDels) were identified by GATK software [11], and filtered to eliminate low quality SNVs or those with strand bias or proximity to InDels. Remaining variants were functionally annotated across the genome by ANNOVAR. The pathogenicity of nonsynonymous variants was then evaluated by four algorithms, PolyPhen2, SIFT, PANTHER and Pmut, as described previously [12].
Expanded validation
DNA samples of the parents of two probands were used to validate all likely pathogenic variants by using conventional Sanger sequencing, and to perform familial segregation analyses whenever possible. Purified PCR products were cycle-sequenced on an ABI 3500 Genetic Analyzer (Applied Biosystems, CA). The results of Sanger sequencing were analyzed by Mutation Surveyor (Softgenetics, PA).
Results
Clinical findings
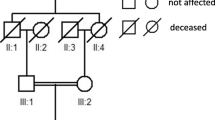
The two families enrolled in this study had USH sporadic cases, and two individuals (F1-I-1, F1-I-2) in family F1 were consanguineous (Fig. 1). The on-set age of both probands are about 15 years old. In family F1, the proband (F1-II-1) was 50 years old and suffered night blindness since adolescence. His visual deterioration had been progressive and at the time of the study he was nearly completely blind. He had no vestibular system problems. Analysis of pure tone audiogram testing showed bilateral moderate to severe hearing loss, moderate at low sound frequencies sloping to severe at higher tested frequencies. Tympanometry examination demonstrated the normal functions of middle ear and eardrum. Upon ophthalmologic examination, fundus photography showed significant bone-spicule pigmentation in the retinal periphery, attenuated retinal arteries and waxy pallor of the optic nerve, all typical signs of retinitis pigmentosa. Results of the OCT test clearly displayed extremely thin and disorganized inner and outer photoreceptor segments (Fig. 2). Collectively, this clinical evidence suggested a diagnosis of Usher II for this proband. The patient, F2-II-6 from family F2 was only 20 years old and exhibited symptoms similar to that of proband F1-II-1 (data not shown). However, the severity of his symptoms was relatively mild, likely due to his younger age.
Clinical examination. a Pure tone audiogram showed bilateral downward-sloping moderate to severe hearing loss in one patient with Usher syndrome (F1-II-1); b Tympanometry examination demonstrated the normal functions of middle ear and eardrum in the F1-II-1 proband; c Fundoscopy of the F1-II-1 patient revealed symptoms typical of retinitis pigmentosa. d OCT of the F1-II-1 patient showed that retinal thickness was diminished
Candidate mutations identified by targeted exome sequencing
We performed targeted exome sequencing of two proband samples from the USH families. The average sequencing depths and coverage of the target regions were 300 reads and 98.0 %, respectively. The coverage of targeted exons for >10 reads was 90.7 % and >20 reads was 81.6 %. After processing of raw data through the BWA program, variants were called and annotated using GATK. We identified numerous non-synonymous variants by the GATK program. Any variants that were reported in the HapMap 28 and the SNP release of the 1000 Genome Project with a MAF > 0.05 were removed. To evaluate the pathogenicity of the variants, we used bioinformatics tools to analyze potential impacts on the function or structure of the encoded proteins. By employing this stepwise analyses approach, we obtained a list of candidate mutations for the two probands. Finally, there were only one homozygous and two heterozygous variants in USH2A in these two pedigrees, respectively.
Expanded familial validation and Sanger sequencing confirmation
Results from targeted exome sequencing of samples from the two probands indicated that they probably had defects in the USH2A gene. To validate the sequencing results, we used Sanger sequencing to screen the same mutation sites in their parents’ DNA. In family F1, we confirmed a heterozygous frameshift mutation (c.4382delA, p.T1462Lfs*2) in exon 20 of USH2A. In family F2, the unaffected father was found to have a heterozygous splice site mutation (IVS47 + 1G > A) in intron 48 of USH2A and the unaffected mother had a novel heterozygous mutation (c.13156A > T) in exon 63 of USH2A. The affected proband, F2-II-6, harbored both compound heterozygous USH2A mutations (IVS47 + 1G > A, c.13156A > T) from his parents (Fig. 3).
Analysis of USH2A mutations
The frameshift mutation (c.4382delA, p.T1462Lfs*2) and the splicing site mutation (IVS47 + 1G > A) found here has been previously reported in a previous study [8]. The missense variant (c.13156A > T) has not been reported in the 1000 Genomes database or in any other single nucleotide polymorphism database. This mutation caused substitution of phenylalanine for isoleucine at amino acid position 4386 and was speculated by prediction software to effect protein functions. In this study, the pathogenicity of missense was then evaluated by four algorithms, PolyPhen2, SIFT, PANTHER and Pmut. All these four algorithms showed the p.I4386F was damaging. It is located in a highly conserved domain of USH2A, when compared to other species (Fig. 3). In summary, we successfully identified causative mutations in two USH families, by combining Sanger sequencing and co-segregation analysis.
Discussion
Usher syndrome (USH) is the most common cause of hereditary deafness–blindness of humans. The typical symptoms are hearing loss and visual dysfunction, with differing severities in each subtype [13, 14]. Clinical and genetic heterogeneity of USH makes it difficult to accurately diagnose this disease at a molecular level by conventional direct sequencing. Targeted exome sequencing is an efficient and low-cost technique for sequencing hundreds of potential target genes at one time. In this study, we employed a comprehensive strategy to screen USH patients for mutation in large group of target genes responsible for inherited retinal degeneration. We performed target exome sequencing on two probands and successfully identified causative mutations by combining familiar validation and co-segregation analyses.
In these two Chinese USH families, mutations in the USH2A gene were identified to be possibly disease-causing. The missense heterozygous SNV (c.13156A > T) was a novel mutation located in a highly conserved region in USH2A. The protein encoded by the USH2A gene, usherin, is critical for proper development of the inner ear and retina, which helps explain the incidence of disease-associated hearing and vision loss. Our findings expanded the spectrum of known USH2A mutations and reflected the significant implication of USH2A in Usher syndrome (Table 1).
Conclusions
The technology we employed in this study will increase the accuracy of efficiency by which patients with retinal dystrophy such as USH, and retinitis pigmentosa receive diagnosis and prognosis. For those patients and their family members who are at high risk of disease, an accurate and timely clinical diagnosis can help them obtain personalized therapy and professional advice on concerns relating to marriage and procreation.
Abbreviations
- USH:
-
Usher syndrome
- SNVs:
-
Single-nucleotide variants
- Indels:
-
Insertions or deletions
- OCT:
-
Optical coherence tomography
- PCR:
-
Polymerase chain reaction
- NGS:
-
Next-generation sequencing
References
Boughman JA, Vernon M, Shaver KA. Usher syndrome: definition and estimate of prevalence from two high-risk populations. J Chronic Dis. 1983;36(8):595–603.
Besnard T, Vache C, Baux D, Larrieu L, Abadie C, Blanchet C, et al. Non-USH2A mutations in USH2 patients. Hum Mutat. 2012;33(3):504–10.
Bonnet C, El-Amraoui A. Usher syndrome (sensorineural deafness and retinitis pigmentosa): pathogenesis, molecular diagnosis and therapeutic approaches. Curr Opin Neurol. 2012;25(1):42–9.
Petit C. Usher syndrome: from genetics to pathogenesis. Annu Rev Genomics Hum Genet. 2001;2:271–97.
Nakanishi H, Ohtsubo M, Iwasaki S, Hotta Y, Usami S, Mizuta K, et al. Novel USH2A mutations in Japanese Usher syndrome type 2 patients: marked differences in the mutation spectrum between the Japanese and other populations. J Hum Genet. 2011;56(7):484–90.
Nakanishi H, Ohtsubo M, Iwasaki S, Hotta Y, Mizuta K, Mineta H, et al. Identification of 11 novel mutations in USH2A among Japanese patients with Usher syndrome type 2. Clin Genet. 2009;76(4):383–91.
Bras J, Guerreiro R, Hardy J. Use of next-generation sequencing and other whole-genome strategies to dissect neurological disease. Nat Rev Neurosci. 2012;13(7):453–64.
Huang XF, Xiang P, Chen J, Xing DJ, Huang N, Min Q, et al. Targeted exome sequencing identified novel USH2A mutations in Usher syndrome families. PLoS One. 2013;8(5), e63832.
Cox MP, Peterson DA, Biggs PJ. SolexaQA: At-a-glance quality assessment of Illumina second-generation sequencing data. BMC Bioinformatics. 2010;11:485.
Li H, Durbin R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics (Oxford, England). 2010;26(5):589–95.
DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43(5):491–8.
Jin ZB, Mandai M, Yokota T, Higuchi K, Ohmori K, Ohtsuki F, et al. Identifying pathogenic genetic background of simplex or multiplex retinitis pigmentosa patients: a large scale mutation screening study. J Med Genet. 2008;45(7):465–72.
Dreyer B, Brox V, Tranebjaerg L, Rosenberg T, Sadeghi AM, Moller C, et al. Spectrum of USH2A mutations in Scandinavian patients with Usher syndrome type II. Hum Mutat. 2008;29(3):451.
van Wijk E, Pennings RJ, te Brinke H, Claassen A, Yntema HG, Hoefsloot LH, et al. Identification of 51 novel exons of the Usher syndrome type 2A (USH2A) gene that encode multiple conserved functional domains and that are mutated in patients with Usher syndrome type II. Am J Hum Genet. 2004;74(4):738–44.
Acknowledgements
We appreciate and acknowledge all patients and family members for their participation in this study. Written consent was obtained from the patient or their relative for publication of study.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
HGS and JH conceived the idea, HGS, HB, YCP, HYX, JXS and JH collected the samples and performed the experiments, HGS and JH performed data analyses, HGS and JH wrote the manuscript. All authors have read and approved the final manuscript.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Shu, HR., Bi, H., Pan, YC. et al. Targeted exome sequencing reveals novel USH2A mutations in Chinese patients with simplex Usher syndrome. BMC Med Genet 16, 83 (2015). https://doi.org/10.1186/s12881-015-0223-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12881-015-0223-9