Abstract
Background
To clarify the differences in diaphragm thickness between male and female participants in healthy young adults with ultrasonography using the mean intima media thickness (IMT) method and to investigate the relationship between diaphragm thickness and respiratory pressure.
Methods
Twenty-nine healthy individuals (16 females and 13 males) participated in the study. Diaphragm thickness was measured at total lung capacity (TLC) and at functional residual capacity (FRC) in each participant. We measured the diaphragm thickness using a method for mean intima media thickness. Moreover, change ratio of diaphragm thickness was calculated with the diaphragm thickness at TLC and FRC.
Results
Mean diaphragm thicknesses at FRC in males were significantly narrower than those in females (p < 0.001). The change ratio of diaphragm thickness was significantly augmented in males compared with that in females (p < 0.001). There was a positive correlation between the change ratio of diaphragm thickness and pulmonary function data and respiratory muscle strength in healthy young adults.
Conclusions
The change ratio of diaphragm thickness using the IMT method can be accurately performed with a high degree of reproducibility by clinical laboratory technicians and may be a useful indicator for evaluating diaphragm muscle strength.
Similar content being viewed by others
Background
Diaphragm movement and function can be assessed through various methods, including chest X-ray, CT, and MRI scans; pulmonary function test; evaluation of maximum respiratory pressure; and electromyography. Diaphragm ultrasonography is a cost-beneficial method that enables easy assessment of diaphragm thickness; moreover, it is a noninvasive bedside tool that can assess diaphragm function and movement [1]. At the bedside, we can measure diaphragm thickness using the B-mode with a linear probe, and diaphragm movement using M-mode with a convex probe, via ultrasonography [2, 3]. Recently, many reports have demonstrated associations among respiratory muscle strength, neuromuscular disorders, and chronic obstructive pulmonary disease assessed using ultrasonography [4, 5]. However, few studies have been published regarding the difference in diaphragm thickness between the sexes.
The respiratory pressure test is a useful method to assess respiratory muscle strength in clinical practice [6]. The evaluation of respiratory pressure is often used to determine muscle weakness in patients with muscular dystrophy, assess disease severity, and postulate prognosis [7]. Several studies were performed to define normative values for maximal respiratory pressure in children and adults, considering factors such as age, sex, height, and body weight [8, 9]; however, there are no reports regarding the relationship between diaphragm thickness with ultrasonography using the mean intima media thickness (IMT) method and maximal respiratory pressure in healthy young adults.
This study aimed to determine diaphragm thickness with ultrasonography using the IMT method in male and female healthy young adults. Additionally, we investigated the relationship between diaphragm thickness and maximal respiratory pressure.
Methods
Participants
This study was a prospective observational study conducted from October 2016 to March 2019. The study was approved by the Institutional Review Board of the Kagawa Prefectural University of Health Sciences, and informed consent was obtained from the participants before the study. Additionally, the study was conducted in accordance with the Declaration of Helsinki. First, 11 healthy participants (8 females and 3 males) aged 21–22 years were enrolled to investigate data on the intra-analyst and inter-analyst variabilities in order to evaluate the accuracy of the mean diaphragm thickness (MDT) using the IMT method. Subsequently, other 29 healthy participants (16 females, 13 males) aged 19–34 years were enrolled to evaluate the DMT thickness and relationship between diaphragm thickness and maximal respiratory pressure. The participants were either students or staff at Kagawa Prefectural University of Health Sciences and Tottori University. Body weight and height were measured, and The Du Bois and Du Bois body surface area (BSA) [10] and body mass index (BMI) were calculated for each participant. Participants with obesity (BMI > 25), neuromuscular disease, cardiorespiratory disease, or chronic illness were excluded.
Observer training
The 1st and 2nd observers, who were students at Kagawa Prefectural University of Health Sciences and conducted the ultrasonography, were provided training ultrasonographic measurements of the diaphragm thickness by MO in 2 sessions, each session lasting 15 min.
Ultrasound imaging and analysis
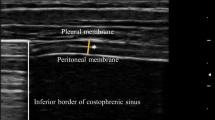
Using an ultrasound machine (ARIETTA 60, Hitachi, Chiba, Japan), measurements were taken with the 7.5 MHz linear array transducer placed in the 9th or 10th intercostal space between anterior and midaxillary lines in the zone of apposition. The participants were placed in the supine position for the measurement [11]. Only one focus-level measurement was chosen, and the depth was at diaphragm level. MDT was measured using IMT method both at total lung capacity (TLC) and at functional residual capacity (FRC) [12]. The mean IMT method is often used for measuring plaques automatically in the carotid artery. We calculated a mean of three points using IMT software: the center of the diaphragm thickness, and two surrounding points on each side (1 cm from the center of the diaphragm thickness) (Fig. 1).
Representative ultrasound of the diaphragm. Diaphragm at total lung capacity (a) and functional residual capacity (b). The diaphragm (D, white bidirectional arrows) is the 3-layered structure situated deep to the intercostal (IC) muscles spanning two ribs; it is identified as the last set of parallel lines, with the pleural and peritoneal membranes overlying the less echogenic muscle. We calculated a mean of three points using the IMT software: the center of the diaphragm thickness, and two surrounding points on either side (each 10 mm from the center of the diaphragm thickness)
Subsequently, we calculated the change ratio of diaphragm thickness (expressed in percentage) using the following formula: MDT at TLC minus MDT at FRC divided MDT at TLC [(MDTtlc—MDTfrc)/MDTtlc] × 100.
Measurement of pulmonary function test and maximal respiratory pressure
A multifunctional spirometer (Autospiro AS507, Minato Medical Science, Osaka, Japan) was used to evaluate respiratory function. Before recording respiratory pressure data, the spirometric parameters of forced vital capacity (FVC) (%) and forced expiratory volume in one second (FEV1)/FVC (%) were evaluated with participants in the sitting position. Respiratory pressure examinations, as well as the spirometric examination, were also conducted with the participants in the sitting position. First, to measure exhaled air from the participant’s mouth, they had to hold a mouthpiece between their teeth. Second, they held the cylinder between their teeth using light pressure, and a clip was placed on their nose to prevent air leaking out during the respiratory tests. Finally, we recorded maximal respiratory pressure to assess respiratory muscles strength. We measured maximum expiratory pressure (MEP) or maximum inspiratory pressure (MIP) by asking participants to breath in/out as deeply as they could and breath out/in sharply and quickly. The MEP and MIP were recorded three times, and maximal end-expiratory and end-inspiratory potential data were chosen for further analysis.
Statistical analysis
We compared the age, weight, height, BMI, BSA, mean diaphragm thickness, change ratio of the mean diaphragm thickness, and maximal end-expiratory and end-inspiratory potentials between males and females. We used the Welch t test for these analyses. Analyses were performed using GraphPad 6 (GraphPad Software, La Jolla, CA, USA). Pearson’s correlation coefficient was used to assess correlation between diaphragm measurements and the maximal respiratory potentials or anthropometric measurements. A p value < 0.05 was considered statistically significant. Moreover, we analyzed intraobserver and interobserver variabilities in the change ratio of diaphragm thickness. The intraobserver variability was assessed by estimation of the intraclass correlation coefficient using the 11 observations in the same subject by the 1st observer. Interobserver variability was tested between observations made by the 1st and 2nd observers in the same subjects.
Results
Intraobserver and interobserver variability
The intraobserver correlation coefficient of the change ratio in diaphragm thickness in observer 1 was 0.856 (95% confidence interval [CI] 0.492–0.961) with a p value of 0.002. The interobserver correlation coefficient of the change ratio in diaphragm thickness between the 1st and 2nd observers was 0.736 (95% CI 0.079–0.928) with a p value of 0.023.
Baseline characters
Participant baseline characteristics by sex are shown in Table 1. Sixteen participants (57%) were female, with a mean age of 24 (interquartile range [IQR] 19–34) years, mean weight of 51 (IQR 41–58) kg, and mean height of 158 (IQR 151–165) cm. The mean BMI of the female participants was 20 (IQR 18–24) kg/m2. The height, weight, and BMI of males were higher than those of females. There were no significant differences with respect to sex in age, vital capacity, and FEV1.
Diaphragm evaluation using ultrasonography
The MDT at FRC, MDT at TLC, and change ratio of diaphragm thickness are listed in Table 2. The MDTs at FRC and TLC in the male participants were 1.2 (IQR 0.6–1.5) and 3.8 (IQR 2.5–5.5), respectively. The MDTs at FRC and TLC in female participants were 1.5 (IQR 1.3–2.1) and 3.7 (IQR 2.3–5.2), respectively. The mean change ratio of the diaphragm thickness was 67.2% (IQR 58.2–77.6) in males and 57.2% (IQR 40.0–72.1) in females. Although the MDT at FRC in males was significantly narrower than in females (1.2 ± 0.3 mm vs 1.5 ± 0.2, p < 0.001), the change ratio of diaphragm thickness in males was larger than that in females (67.2 ± 6.2% vs 57.2 ± 7.8%, p < 0.001). There were no significant differences in the MDT at TLC with respect to sex.
Respiratory muscle strength evaluation
Table 3 shows the mean maximal respiratory pressures in male and female participants. The mean MEPs were 88.2 (IQR 49.7–113.6) cmH2O and 56.8 (IQR 32.3–87.0) cmH2O and mean MIPs were 79.0 (IQR 44.9–116.1) cmH2O and 81.1 (IQR 43.5–129.6) cmH2O in male and female participants, respectively. While there were no significant differences in MIPs, the MEPs were higher in males than in females.
Association between the change ratio of diaphragm thickness and anthropometric data or pulmonary function test
Subsequently, we assessed the correlation between the change ratio of diaphragm thickness and anthropometric data or pulmonary function test or maximal respiratory mouth pressure. Significant positive correlations were found only in males between the change ratio of diaphragm thickness and FVC (r = 0.57, p = 0.04), the change ratio of the diaphragm thickness and MIP (r = 0.56, p = 0.05), and the change ratio of the diaphragm thickness and MEP (r = 0.71, p = 0.007) (Fig. 2). Based on Fig. 2, the change ratio of the diaphragm thickness seems to be associated with FVC (r = 0.54, p = 0.10) and MIP (r = 0.35, p = 0.19).
Correlation between the change ratio of diaphragm thickness and other measures in males and females. Measures included % forced vital capacity, maximum inspiratory pressure (MIP) or maximum expiratory pressure (MEP) for males (a, c, e), and females (b, d, f). There was a significant positive correlation between the change ratio of diaphragm thickness and % forced vital capacity or respiratory mouth pressures in males, but not in females
Discussion
In this study, we determined normal reference values for diaphragm thickness in healthy young adults with ultrasonography using the IMT method to assess respiratory muscle strength; additionally, we analyzed the relationship between diaphragm thickness and maximal respiratory mouth pressure by classifying the participants by sex. Our study revealed a positive correlation between the change ratio of diaphragm thickness and respiratory muscle strength in young adult.
A diaphragm measurement methodology using the IMT method has never been reported. Several previous reports without the IMT method show that ultrasonography measurements of diaphragm thickness have a high degree of reproducibility. In the study by Dhungana et al. [1], the coefficient of reproducibility in DMT at FRC was high (0.986 and 0.987 for intra-observer and interobserver variabilities, respectively). Although our coefficient of reproducibility in the change ratio of diaphragm thickness using the IMT method showed significant reproducibility, the coefficient value in our study was lower than that in a previous report. Dhungana et al. [1] reported that diaphragm thickness was measured by observers after an initial training of three sessions, each lesson lasting 30 min. It seemed that the coefficient of reproducibility in the previous study could be augmented with more training sessions and lesson time when compared to that in this study.
Previous studies have demonstrated various methods to observe diaphragm thickness with ultrasonography [1, 13]. Regarding the studies on diaphragm thickness with ultrasonography, Boon et al. reported a lower limit of normal diaphragm thickness with ultrasonography placed over one of the most caudal intercostal spaces of 1.4 mm in participants between 33 and 84 years of age [3]. De Bruin et al., using ultrasonography to place the right hemidiaphragm at the apposition zone, reported a mean normal diaphragm thickness at TLC for participants aged 7–12 years of 3.5 mm [14]. Our study revealed that the lower limit of the diaphragm thickness at FRC in males and females was 1.2 mm and 1.5 mm, respectively. Additionally, the mean normal diaphragm thickness at TLC in males was 3.8 mm. These results were approximately consistent with previous studies [3, 14].
Measuring respiratory mouth pressure can contribute to the assessment of respiratory muscle strength in neuromuscular disease. The mean respiratory muscle strength values have been previously reported [7, 15, 16]. The mean MIP and MEP for participants aged > 18 years were 106 and 148 cmH2O in males and 73 and 93 cmH2O in females, respectively [16]. Gibson et al. collected data from a small group of 10 healthy Caucasian women, obtaining an MEP of 67–140 cmH2O and MIP of 35–95 cmH2O, respectively [17]. Our results are similar to these results. Additionally, Heinzmann-Filho et al., despite the lack of statistical differences, found higher respiratory muscle strength values for boys than girls [6]. Our results also showed a higher MEP in males than in females. As for MIP, no significant difference was found between the sexes. To assess the MEP in young adults, we should evaluate the measurement data according to sex.
The change ratio of the diaphragm thickness appears to be a useful indicator to evaluate morphological changes of the diaphragm and differentiation based on sex. Previous studies on diaphragm thickness with ultrasonography based on sex in healthy infants and children indicated no significant differences between males and females [18]. In an adult study, Boussuges et al. found weak correlations between diaphragmatic excursion and body weight or height [19], and significant correlations between diaphragmatic measurements and FEV1 [5, 20]. Additionally, there was a significant relationship between MEP and body weight [6]. In this study, a positive correlation was observed between the change ratio of diaphragm thickness and respiratory muscle strength and/or FVC in healthy young adults. This result suggests that the change ratio of the diaphragm thickness correlates with breathing effort. One reason for why there was no significant positive correlation between the change ratio and respiratory muscle strength may be because of the small number of females, which may have affected the statistical analysis of the results.
Our study has some limitations. First, the sample size was small, and we only assessed young adults. A larger sample size with various age groups is necessary to draw more definitive conclusions. Second, a diaphragm measurement methodology using the IMT method has never been reported; therefore, we could not compare the diaphragm thickness results using the IMT method with previous data. Further studies are required to determine the optimal diaphragm assessment method with ultrasonography.
Conclusions
In conclusion, the result of our study indicated that the measurement of the change ratio of diaphragm thickness using the IMT method can be accurately performed with a high degree of reproducibility. Normal reference values based on sex for diaphragm thickness and change ratio of the diaphragm thickness, evaluated by B-mode sonography in healthy young adults, were determined in this study. A positive correlation was found between the change ratio of diaphragm thickness and respiratory muscle strength in young adults; thus, the change ratio of diaphragm thickness using the IMT method may be a useful indicator for evaluating diaphragm muscle strength.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- BMI:
-
Body mass index
- BSA:
-
Body surface area
- FEV:
-
Forced expiratory volume
- FRC:
-
Functional residual capacity
- FVC:
-
Forced vital capacity
- IMT:
-
Intima media thickness
- IQR:
-
Interquartile range
- MDT:
-
Mean diaphragm thickness
- MEP:
-
Maximum expiratory pressure
- MIP:
-
Maximum inspiratory pressure
- TLC:
-
Total lung capacity
References
Dhungana A, Khilnani G, Hadda V, Guleria R. Reproducibility of diaphragm thickness measurements by ultrasonography in patients on mechanical ventilation. World J Crit Care Med. 2017;6:185–9.
Beck J, Weinberg J, Hamnegard CH, Spahija J, Olofson J, Grimby G, et al. Diaphragmatic function in advanced Duchenne muscular dystrophy. Neuromuscul Disord. 2006;16:161–7.
Boon AJ, Sekiguchi H, Harper CJ, Strommen JA, Ghahfarokhi LS, Watson JC, et al. Sensitivity and specificity of diagnostic ultrasound in the diagnosis of phrenic neuropathy. Neurology. 2014;83:1264–70.
Sarwal A, Walker FO, Cartwright MS. Neuromuscular ultrasound for evaluation of the diaphragm. Muscle Nerve. 2013;47:319–29.
Scheibe N, Sosnowski N, Pinkhasik A, Vonderbank S, Bastian A. Sonographic evaluation of diaphragmatic dysfunction in COPD patients. Int J Chron Obstruct Pulmon Dis. 2015;10:1925–30.
Heinzmann-Filho JP, Vasconcellos Vidal PC, Jones MH, Donadio MV. Normal values for respiratory muscle strength in healthy preschoolers and school children. Respir Med. 2012;106:1639–46.
Spiesshoefer J, Herkenrath S, Henke C, Langenbruch L, Schneppe M, Randerath W, et al. Evaluation of respiratory muscle strength and diaphragm ultrasound: normative values, theoretical considerations, and practical recommendations. Respiration. 2020;99:369–81.
Reiter M, Totzauer A, Werner I, Koessler W, Zwick H, Wanke T. Evaluation of inspiratory muscle function in a healthy Austrian population–practical aspects. Respiration. 2006;73:590–6.
Gopalakrishna A, Vaishali K, Prem V, Aaron P. Normative values for maximal respiratory pressures in an Indian Mangalore population: a cross-sectional pilot study. Lung India. 2011;28:247–52.
DuBois D, DuBois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;17:863–71.
Goligher EC, Fan E, Herridge MS, Murray A, Vorona S, Brace D, et al. Evolution of diaphragm thickness during mechanical ventilation. Impact of inspiratory effort. Am J Respir Crit Care Med. 2015;192:1080–8.
Terminology, Diagnostic Criteria Committee, Japan Society of Ultrasonics in Medicine. Standard method for ultrasound evaluation of carotid artery lesions. J Med Ultrason (2001). 2009;36:219–26.
Coiffard B, Riegler S, Sklar MC, Dres M, Vorona S, Reid WD, et al. Diaphragm echodensity in mechanically ventilated patients: a description of technique and outcomes. Crit Care. 2021;25:64.
De Bruin PF, Ueki J, Bush A, Khan Y, Watson A, Pride NB. Diaphragm thickness and inspiratory strength in patients with Duchenne muscular dystrophy. Thorax. 1997;52:472–5.
Szeinberg A, Marcotte JE, Roizin H, Mindorff C, England S, Tabachnik E, et al. Normal values of maximal inspiratory and expiratory pressures with a portable apparatus in children, adolescents, and young adults. Pediatr Pulmonol. 1987;3:255–8.
Wilson SH, Cooke NT, Edwards RH, Spiro SG. Predicted normal values for maximal respiratory pressures in Caucasian adults and children. Thorax. 1984;39:535–8.
Pines A, Kaplinsky N, Olchovsky D, Rozenman J, Frankl O. Pleuro-pulmonary manifestations of systemic lupus erythematosus: clinical features of its subgroups. Prognostic and therapeutic implications. Chest. 1985;88:129–35.
El-Halaby H, Abdel-Hady H, Alsawah G, Abdelrahman A, El-Tahan H. Sonographic evaluation of diaphragmatic excursion and thickness in healthy infants and children. J Ultrasound Med. 2016;35:167–75.
Boussuges A, Gole Y, Blanc P. Diaphragmatic motion studied by m-mode ultrasonography: methods, reproducibility, and normal values. Chest. 2009;135:391–400.
Noda Y, Sekiguchi K, Kohara N, Kanda F, Toda T. Ultrasonographic diaphragm thickness correlates with compound muscle action potential amplitude and forced vital capacity. Muscle Nerve. 2016;53:522–7.
Acknowledgements
Not applicable.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
MO designed the study, managed and analyzed the data, performed statistical analyses, and wrote the manuscript. TH designed the study and revised the manuscript. TO, TI, KO, SK, and SW critically revised the manuscript. YM discussed the interpretations of the data and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of the Kagawa Prefectural University of Health Sciences. Informed consent was obtained from all patients for being included in the study. Additionally, written informed consent was obtained from all subjects.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Oguri, M., Okanishi, T., Ikeguchi, T. et al. Influence of gender on diaphragm thickness using a method for determining intima media thickness in healthy young adults. BMC Med Imaging 22, 26 (2022). https://doi.org/10.1186/s12880-022-00748-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12880-022-00748-y