Abstract
Background
The results of observational studies indicate a potential link between Helicobacter pylori infection and Sjogren’s syndrome (SS), but the causal relationship between them remains unknown. This study applied Mendelian randomization (MR) to evaluate this relationship.
Method
Genome-wide association study (GWAS) summary statistics on H. pylori infection [sample size=8735 (EBI, https://gwas.mrcieu.ac.uk/)] and SS [sample size=368,028 (cases=2495, controls=365533) (FinnGen, https://r9.finngen.fi/)] were analyzed. We used bidirectional MR to evaluate the association between H. pylori infection and SS and identify causation. The major MR analysis method was inverse-variance weighted (IVW) MR, supplemented by MR‒Egger and weighted median approaches. In addition, the stability and reliability of the results were tested using the retention method, heterogeneity test, and horizontal gene pleiotropy test.
Results
Evidence of the impact of H. pylori infection on SS risk was found in the IVW results [odds ratio (OR)=1.6705; 95% confidence interval (CI)=1.0966 to 2.5446; P=0.0168]. Evidence of the impact of SS on H. pylori infection risk was also found (OR=1.0158; 95% CI=1.0033 to 1.0285; P=0.0128).
Conclusion
The results of MR analysis support a causal association between H. pylori infection and SS and indicate that SS can lead to a greater risk of H. pylori infection. Our research will support the development of novel approaches for continued H. pylori and SS-related research and therapy that consider the genetic relationship between H. pylori infection and SS.
Similar content being viewed by others
Introduction
Helicobacter pylori (H. pylori) is a spiral-shaped gram-negative bacterium that frequently colonizes multiple sites in the body, such as the stomach and duodenum. According to the literature, the global prevalence of H. pylori infection in adults declined from 50–55% to 43% from 2014–2020, mostly attributed to improvements in living standards and hygiene conditions [1]. The current rates range from 50.8% in developing countries to 34.7% in developed countries [2]. H. pylori is clinically associated with various gastrointestinal diseases, such as gastric tumors, autoimmune gastritis, gastric mucosa-associated lymphoid tissue (MALT) lymphoma, and gastrointestinal ulcers [3]. In addition, there is a correlation between H. pylori infection and the occurrence of extragastrointestinal immune diseases such as rheumatic immune disease and autoimmune thyroid disease [4].
Sjogren's syndrome (SS) is a chronic inflammatory autoimmune disease characterized by lymphocyte proliferation and progressive damage to the exocrine glands. In addition to impaired function of the salivary and lacrimal glands, the clinical manifestations of SS may include multiple system and organ involvement, serum autoantibodies and hyperimmunoglobulinaemia [5]. The combined prevalence of SS in the global population is 60.82/100000, with women affected approximately ten times as often as men [6]. The prevalence of SS in Europe is approximately 0.23% [7]. At present, the exact aetiology and pathogenesis of SS are not clear, but it is generally believed that immune dysfunction is caused by various factors, such as genetic variants, viral infection, and abnormal sex hormone levels.
To explore the possible correlation between H. pylori infection and SS, many scholars have investigated the relationship between H. pylori and H. pylori-related antibodies in SS patients. Studies have shown that the average titre of anti-H. pylori serum antibodies in SS patients is significantly greater than that in SLE and RA patients [8]. Another study analyzed the levels of anti-H. pylori antibodies in 43 patients with SS and 95 controls, and reported that the levels of these antibodies were significantly increased in SS patients (34% vs. 10.5%, P=0.0001) [9]. Thus, the results of this study revealed that the infection rate of H. pylori infection in the SS group was significantly greater than that in the healthy population. Although many studies have shown that H. pylori infection promotes SS, other studies of the relationship between H. pylori infection and SS have reached the opposite conclusion. Theander et al. [10] reported that H. pylori seropositivity is not associated with the presence of immunological markers for SS, such as circulating autoantibodies or lip biopsies with abnormal focal scores. Interestingly, Ram et al. [11] reported that H. pylori infection rates are lower in patients with SS, suggesting a protective role, that is, that H. pylori infection is negatively correlated with the occurrence and development of SS.
The relationship between H. pylori infection and SS has been controversial. Furthermore, conclusions about causality cannot be drawn solely from the results of observational studies, as cohort and cross-sectional studies may have limitations such as limited sample sizes, difference in the racial compositions of cohorts, and the presence of other confounding factors.
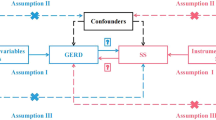
Mendelian randomization (MR) is a technique that uses genetic variation as an instrumental variable (IV) to assess whether the observed associations between exposure factors and outcomes are consistent with causal effects [12]. MR tests three assumptions: 1) genetic variation is associated with risk factors; 2) genetic variation is not associated with confounders; and 3) genetic variation affects outcomes only through risk factors [13]. Since genetic variation is not influenced by other factors, such as the external environment and social behavior, it is a stable exposure factor over time. Therefore, in observational studies, analytical bias can be minimized by avoiding the influence of confounders and reverse causality on correlation effects through MR methods. In recent years, MR methods have been widely used in studies assessing the causal relationship between exposure and outcome [14].
In clinical practice and research, a certain correlation between H. pylori infection and SS has been identified, but the causal relationship between H. pylori infection and SS remains unknown. Considering these findings, this study aimed to investigate the causal relationship between H. pylori infection and SS, using the data from a large-scale GWAS in a bidirectional MR design.
Materials and methods
Ethics
This study was reported according to the STROBE-MR guidelines [15], with data collected from public databases. No ethical approval was required for this study.
Study design
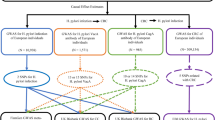
In this study, a bidirectional MR design was used to investigate the causal association between H. pylori infection and SS [14]. SNPs are used as IVs for MR studies to evaluate the causal effect of exposure variables [16]. The overall setup for the current MR study is shown in Fig. 1. The validity of MR analysis is subject to three core assumptions: ① relevance; ② independence; and ③ exclusion-restriction [13].
Data sources for anti-Helicobacter pylori IgG seropositivity and SS
Summary GWAS data for H. pylori infection were obtained from the publicly available European Bioinformatics Institute (EBI) database. Moreover, the genetic association of SS was available from FinnGen, comprising 2495 European cases and 365533 European controls. Detailed information about the GWAS data contained in this study is provided in Table 1.
Selection of genetic variants as instrumental variables(IVs)
In this study, MR analysis was performed on the principle that SNPs must be significantly correlated with exposure, and the loci of SNPs that were significantly associated with H. pylori infection or SS were selected according to a genome-wide threshold of P<5×10-8. Unfortunately, only a small number of SNPs were obtained for the IVs of H. pylori infection. To explore more relationships between H. pylori and SS and obtain more comprehensive results, we selected SNPs with a less strict significance of P<5x10-6 as suggested by previous studies [17, 18], and selected them as a second IV set to find more potential causal associations [19, 20].
Moreover, linkage disequilibrium (LD) analysis was performed to ensure independence between SNPs (LD, r2<0.001, clumping distance>10,000 kb) [13]. To ensure that the effect alleles belonged to the same allele, the exposure and outcome datasets were harmonized to eliminate SNPs with intermediate allele frequencies and ambiguous SNPs with mismatched alleles. Furthermore, to avoid the influence of weak IVs on the causal effect, the F-statistic of each selected IV had to be greater than 10. Finally, to ensure that IVs could affect the outcome only through exposure, SNPs associated with confounders were manually eliminated in PhenoScanner [21].
Statistical analysis
A two-sample MR method was used to evaluate the potential causal relationship between H. pylori infection and SS. Before the MR analysis, a pleiotropy residual sum and outlier (PRESSO) method was adopted to evaluate the horizontal polytropy of the data, which was corrected before effect assessment, to ensure the reliability of the MR analysis.
The inverse-variance weighted (IVW) method was the primarily method used in the MR analysis, as it provides consistent estimates of exposure‒outcome associations when the IVs are not pleiotropic [22].
Cochran’s Q statistic was applied to assess the heterogeneity across individual SNPs [23]. When the P value of Cochran's Q test was greater than 0.05, there was no heterogeneity among the SNPs. MR‒Egger and the weighted median were used in complementary analyses [24, 25]. Furthermore, sensitivity analysis was performed using the leave-one-out method, and the degree of influence of each SNP on causality was carefully evaluated after removing the final included SNPs one by one. All data analysis and statistical plots generation in this study were performed via the TwoSampleMR package (0.5.7), version R 4.3.1. Forest plots were created using the “forestplot” R package (version 3.1.1). The statistical results are expressed as odds ratios (ORs) and 95% confidence intervals (95% CIs), and P<0.05 was considered to indicate statistical significance.
Results
Instrumental variable selection
Using a genome-wide threshold of P<5x10-6, rigorous screening was performed as previously described, resulting in the identification of 17 SNPs mediating the causal associations between H. pylori infection and SS. Moreover, 10 SNPs were ultimately obtained (P<5×10-8) for MR analysis of the causal association between SS and H. pylori infection. The F statistics of all IVs were greater than 10, suggesting that these IVs could generally be considered to provide sufficient information for MR studies (Supplementary Tables 1-3).
Causal effects of H. pylori infection on SS risk
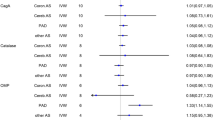
The causal estimates of this MR analysis are shown in Table 2 and Fig. 2. The ORs of H. pylori infection associated with SS for the three methods (IVW, weighted median, and MR‒Egger) were 1.6705 (95% CI: 1.0966-2.5446, P=0.0168), 1.9298 (95% CI: 1.0331-3.6047; P=0.0391), and 1.1493 (95% CI: 0.3035-4.3513; P=0.8403), respectively. The results of our study revealed that genetically predicted H. pylori infection was significantly associated with the risk of SS (IVW P<0.05).
Causal effects of SS on H. pylori infection risk
The reverse MR results were presented in Table 3 and Figure 3. The ORs of SS associated with H. pylori infection for the three methods (IVW, weighted median, and MR-Egger) were 1.0158 (95% CI: 1.0033–1.0285, P=0.0128), 1.0182 (95% CI: 1.0013–1.0353; P=0.0341), and 1.0256 (95% CI: 0.9924–1.0600; P=0.1697), respectively. The results demonstrated that genetically predicted SS led to a greater risk of H. pylori infection (IVW P<0.05).
Heterogeneity and sensitivity tests
Heterogeneity refers to the variability observed in the causal estimates obtained for each SNP. Low heterogeneity suggests increased reliability of MR estimates. In our study, the Cochran’s Q test demonstrated no evidence of heterogeneity among the IV estimates based on the individual variants (Tables 2, 3). Therefore, based on Cochran's Q test, we applied fixed effect IVW. Moreover, the leave-one-out analysis did not identify any SNP outliers, suggesting that our results were stable (Figs. 2C, 3C). In addition, the funnel plot displayed no evidence of asymmetry, indicating the absence of directional horizontal pleiotropy (Fig. 4).
Discussion
In this study, a bidirectional MR design was utilized to investigate the causal association between H. pylori infection and SS. To our knowledge, this study is the first to use MR methods for this purpose. Our data of this study revealed that there was a significant causal association between H. pylori infection and SS.
In recent years, studies have shown a correlation between H. pylori infection and SS. Studies have also revealed that SS patients have increased levels of anti-H. pylori serum antibodies than age-matched controls or patients with other connective tissue diseases [8, 9]. Similar results have been obtained in studies on anti-H. pylori antibodies in Italian SS patients (OR=15.67, 95% CI: 4.5–54.8; P < 0.001) [26].
Owing to the high degree of sequence homology between H. pylori and human heat shock protein (HSP), Aragona et al. hypothesized that H. pylori infection may trigger an autoimmune response to its HSP and proposed that the HSP60 produced by H. pylori may play a role in the pathogenesis of SS [27]. Thus, these results indicate that the hypothetical role of HSP60 in the development of the immune response in both primary SS and secondary SS seems to be closely linked to the prevalence of H. pylori infection.
SS patients commonly experience blood-related symptoms, including leukopenia, thrombocytopenia, and in some cases even severe thrombocytopenia. Autoimmune factors play a dominant role in the pathogenesis of thrombocytopenia caused by SS [28]. In 1998, Gasparin et al. [29] reported that H. pylori infection is associated with the occurrence of autoimmune thrombocytopenia. At present, the mechanism by which H. pylori infection leads to thrombocytopenia in SS patients is not yet clear. Kurata et al. suggested that there may be cross-reactivity between H. pylori and a certain platelet antigen component [30]. After H. pylori infection, certain components of the body are induced to transform into platelet cross antigens that are recognized by the body's immune system, suggesting that H. pylori infection may be associated with the occurrence of thrombocytopenia in SS patients.
Compared with normal individuals, SS patients are more likely to be infected with H. pylori [31]. A meta-analysis revealed that 1958 participants (including 619 patients with SS) from nine studies met the inclusion criteria. The total infection rate of H. pylori was 53.83% (1054/1958). The study revealed that patients with SS had a significantly greater H. pylori infection rate than did control patients (OR=1.19, 95% CI: 1.01-1.41, P=0.033) [32].El Miedany et al. conducted relevant studies to determine the presence of clinical markers related to H. pylori infection in SS patients and their significance for the treatment of such patients [33]. The results revealed that certain risk factors, including age, disease duration, overall disease severity and C-reactive protein (CRP) levels, may be significantly associated with H. pylori infection in SS patients.
The role of H. pylori infection in the pathogenesis of immune diseases is not yet clear. Possible mechanisms include the activation of superantigens or polyclonal lymphocytes, molecular antigen imitation, epitope transmission, and bystander activation, all of which are believed to be related to immune dysregulation during infection [34]. The H. pylori strain encoding cytotoxin-associated gene A (CagA) has an enhanced ability to stimulate the secretion of proinflammatory cytokines, resulting in tissue injury, polarity, and host cell proliferation, thereby regulating the host immune response [35]. Therefore, H. pylori infection may be one factor that can trigger rheumatic immune disease.
Infection, including viral and bacterial infection, is considered a risk factor for SS. To date, some studies have suggested that dysbiosis of the oral microbiota may induce the occurrence and development of SS by promoting abnormal B lymphocytes activation and differentiation, leading to many lymphocytes infiltrating the salivary glands [36]. Given the presence of H. pylori in the host's oral cavity, it is also believed that SS may be associated with H. pylori infection.
The persistent presence of H. pylori in the gastric mucosa leads to chronic immune system activation, resulting in sustained cytokine signaling; infiltration of neutrophils, macrophages and lymphocytes into the gastric mucosa; and the production of antibodies and effector T cells [37]. Studies have shown that H. pylori infection induces a helper T-cell 1 (Th1) response, leading to the production of interleukin-2 (IL-2) and interferon-γ (IFN-γ) [38]. The IL-2 content in the lacrimal glands of SS patients is significantly greater than that in individuals without SS, indicating that IL-2 plays a major role in the pathological changes in lacrimal gland tissue and may be one of the main factors causing degeneration of lacrimal gland cells [39]. Other studies have shown that the salivary glands of SS patients are infiltrated with many plasmacytoid dendritic cells (pDCs), which mainly secrete IFN-γ, and that IFN-γ in the salivary glands of SS patients can induce dysfunction in salivary gland secretion [40].
H. pylori infection is associated with SS, also due to the common histological findings, such as exocrine gland destruction, lymphocyte infiltration and CD8+ T-cell activation [41, 42]. Irani et al. confirmed through immunohistochemistry that the level of H. pylori in patients with inflammatory lesions of the oral mucosa is greater than that in healthy individuals [43]. Moreover, H. pylori may interact with the surface of epithelial cells, directly causing cell damage or producing proinflammatory mediators [10]. SS patients with persistent oral lesions are more likely to be infected with H. pylori. Furthermore, a recent meta-analysis reported that of a total of 224 patients diagnosed with SS, 94 (41.96%) were infected with H. pylori. Multivariate analysis demonstrated that hypergammaglobulinemia could be an independent risk factor for H. pylori infection in patients with SS [44].
In summary, the H. pylori infection rate in SS patients was significantly higher than that in healthy individuals. Possible risk factors include age, duration of disease, overall disease severity, CRP, and hyperglobulinemia, etc. Moreover, H. pylori infection will facilitate the progress of SS. H. pylori infection causes chronic immune system activation, which produces a sustained cytokine signal that attacks the lacrimal glands, salivary glands, platelets, and more. Besides, the relationship between H. pylori infection and SS was explored in this study from a genetic perspective, and the results revealed a bidirectional causal relationship. Therefore, in the clinical management of SS, it is necessary to strengthen screening for H. pylori infection. In addition, H. pylori infection can exacerbate SS, and if necessary, anti-H. pylori treatment should be added to the treatment for SS. However, whether H. pylori eradication treatment can improve the clinical symptoms of SS still requires further rigorous large-scale, multicentre investigations of interfering factors.
Our MR study has several strengths. First, to our knowledge, this study is the first to use MR methods to assess the causal effects between H. pylori infection and SS. Second, MR explores the causal relationship between exposure and outcome through genetic data, which are unaffected by causal inversion and confounding factors. Third, MR uses genetic variation as IVs to mimic the design of randomized controlled trials. MR falls between observational studies and intervention trials, providing information on public health interventions in situations where randomized controlled trials may not be feasible.
However, this analysis also has several limitations. First, the majority of the samples used are from European populations. Although the use of a single population to study causal relationships can minimize population stratification bias, the findings of this study may not be applicable to other populations. Unfortunately, developing countries generally have higher rates of H. pylori infection than European countries; thus these results should be confirmed in other populations. Second, the diagnosis of H. pylori infection in the datasets was based on serum IgG antibodies testing. Finally, to ensure the inclusion of a sufficient number of SNPs that contribute to H. pylori infection, we used a more relaxed value (P<5x10-6) for the SNP selection cutoff. Previous studies have recommended this strategy [17] with the caveat that it might result in a slight bias in IVs.
Conclusion
This study explored the causal relationship between H. pylori infection and SS at the genetic level through two-sample MR analysis utilizing publicly available databases and large-scale GWAS. These results indicate that H. pylori infection increases the risk of developing SS and that SS can lead to a greater risk of H. pylori infection. However, the mechanism underlying H. pylori infection and SS are not clear. Data from GWAS with larger sample sizes are still needed to validate this relationship in the future. Most importantly, this study provides novel approaches for continued research and therapy for H. pylori and SS that consider the genetic relationship between H. pylori infection and SS.
Availability of data and materials
The datasets generated during analysis in the current study are available in the European Bioinformatics Institute (EBI) database[https://gwas.mrcieu.ac.uk/], and the FinnGen consortium[https://r9.finngen.fi/].
Abbreviations
- SS:
-
Sjogren's syndrome
- H.pylori :
-
Helicobacter pylori
- MALT:
-
Mucosa associated lymphoid tissue
- SLE:
-
Systemic lupus erythematosus
- RA:
-
Rheumatoid arthritis
- MR:
-
Mendelian randomization
- IV:
-
Instrumental variable
- GWAS:
-
Genome-wide association studies
- SNPs:
-
Single nucleotide polymorphisms
- EBI:
-
European Bioinformatics Institute
- LD:
-
Linkage disequilibrium
- PRESSO:
-
Pleiotropy residual sum and outlier
- IVW:
-
Inverse variance weighted
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- HSP:
-
Human heat shock protein
- CRP:
-
C-reactive protein
- Th1:
-
Helper T cell 1
- IL-2:
-
Interleukin-2
- IFN-γ:
-
Interferon-γ
- pDCs:
-
Plasmacytoid dendritic cells
- CagA:
-
Cytotoxin-associated gene A
References
Malfertheiner P, Camargo MC, El-Omar E, et al. Helicobacter pylori infection. Nat Rev Dis Primers. 2023;9(1):19 Published 2023 Apr 20.
Zamani M, Ebrahimtabar F, Zamani V, et al. Systematic review with meta-analysis: the worldwide prevalence of Helicobacter pylori infection. Aliment Pharmacol Ther. 2018;47(7):868–76.
Ranjbar R, Behzadi P, Farshad S. Advances in diagnosis and treatment of Helicobacter pylori infection. Acta Microbiol Immunol Hung. 2017;64(3):273–92.
Shamriz O, Shoenfeld Y. Infections: a double-edge sword in autoimmunity. Curr Opin Rheumatol. 2018;30(4):365–72.
Oliveira FR, Valim V, Pasoto SG, et al. 2021 recommendations of the Brazilian Society of Rheumatology for the gynecological and obstetric care of patients with Sjogren’s syndrome [published correction appears in Adv Rheumatol. 2022 Mar 20;62(1):8]. Adv Rheumatol. 2021;61(1):54.
Qin B, Wang J, Yang Z, et al. Epidemiology of primary Sjögren’s syndrome: a systematic review and meta-analysis. Ann Rheum Dis. 2015;74(11):1983–9.
Brito-Zerón P, Baldini C, Bootsma H, et al. Sjögren syndrome. Nat Rev Dis Primers. 2016;2:16047.
Showji Y, Nozawa R, Sato K, Suzuki H. Seroprevalence of Helicobacter pylori infection in patients with connective tissue diseases. Microbiol Immunol. 1996;40(7):499–503.
Saghafi M, Abdolahi N, Orang R, Hatef MR, Molseghi MH. Helicobacter Pylori Infection in Sjögren’s Syndrome: Co-incidence or Causality? Curr Rheumatol Rev. 2019;15(3):238–41.
Theander E, Nilsson I, Manthorpe R, Jacobsson LT, Wadström T. Seroprevalence of Helicobacter pylori in primary Sjögren’s syndrome. Clin Exp Rheumatol. 2001;19(6):633–8.
Ram M, Barzilai O, Shapira Y, et al. Helicobacter pylori serology in autoimmune diseases - fact or fiction? Clin Chem Lab Med. 2013;51(5):1075–82.
Birney E. Mendelian randomization. Cold Spring Harb Perspect Med. 2022;12(4):a041302.
Emdin CA, Khera AV, Kathiresan S. Mendelian Randomization. JAMA. 2017;318(19):1925–6.
Burgess S, Scott RA, Timpson NJ, Davey Smith G, Thompson SG. EPIC- InterAct Consortium. Using published data in Mendelian randomization: a blueprint for efficient identification of causal risk factors. Eur J Epidemiol. 2015;30(7):543–52.
Skrivankova VW, Richmond RC, Woolf BAR, et al. Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization: The STROBE-MR Statement. JAMA. 2021;326(16):1614–21.
Davey Smith G, Holmes MV, Davies NM, Ebrahim S. Mendel’s laws, Mendelian randomization and causal inference in observational data: substantive and nomenclatural issues. Eur J Epidemiol. 2020;35(2):99–111.
Zhang X, Shi Y, Li T, et al. Causal association between helicobacter pylori and atherosclerosis: a two-sample Mendelian randomization. BMC Cardiovasc Disord. 2024;24(1):161 Published 2024 Mar 15.
Luo F, Zhou P, Ran X, Gu M, Zhou S. No evident causal association between Helicobacter pylori infection and colorectal cancer: a bidirectional mendelian randomization study. Sci Rep. 2023;13(1):18544.
Zou XL, Wang S, Wang LY, et al. Childhood Obesity and Risk of Stroke: A Mendelian Randomisation Analysis. Front Genet. 2021;12:727475 Published 2021 Nov 17.
Huang JY, Labrecque JA. From GWAS to PheWAS: the search for causality in big data. Lancet Digit Health. 2019;1(3):e101–3.
Kamat MA, Blackshaw JA, Young R, et al. PhenoScanner V2: an expanded tool for searching human genotype-phenotype associations. Bioinformatics. 2019;35(22):4851–3.
Burgess S, Butterworth A, Thompson SG. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol. 2013;37(7):658–65.
Hemani G, Bowden J, Davey Smith G. Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum Mol Genet. 2018;27(R2):R195–208.
Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512–25.
Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet Epidemiol. 2016;40(4):304–14.
Caporali R, Epis O, Negrini R, Scirè CA, Solcia E, Montecucco C. Salivary gland lymphocytic infiltrates and Helicobacter pylori serology in anti-SSA/Ro positive patients in Italy. Clin Exp Rheumatol. 2003;21(2):266–7.
Aragona P, Magazzù G, Macchia G, et al. Presence of antibodies against Helicobacter pylori and its heat-shock protein 60 in the serum of patients with Sjögren’s syndrome. J Rheumatol. 1999;26(6):1306–11.
Asmussen K, Andersen V, Bendixen G, Schiødt M, Oxholm P. A new model for classification of disease manifestations in primary Sjögren’s syndrome: evaluation in a retrospective long-term study. J Intern Med. 1996;239(6):475–82.
Gasbarrini A, Franceschi F, Tartaglione R, Landolfi R, Pola P, Gasbarrini G. Regression of autoimmune thrombocytopenia after eradication of Helicobacter pylori. Lancet. 1998;352(9131):878.
Kurata Y. The future expected therapeutic approaches for ITP patients. Nihon Rinsho. 2003;61(4):664–9.
Radić M, Martinović Kaliterna D, Bonacin D, Morović Vergles J, Radić J. Correlation between Helicobacter pylori infection and systemic sclerosis activity. Rheumatology (Oxford). 2010;49(9):1784–5.
Chen Q, Zhou X, Tan W, Zhang M. Association between Helicobacter pylori infection and Sjögren syndrome: A meta-analysis. Medicine (Baltimore). 2018;97(49):e13528.
El Miedany YM, Baddour M, Ahmed I, Fahmy H. Sjogren’s syndrome: concomitant H. pylori infection and possible correlation with clinical parameters. Joint Bone Spine. 2005;72:135–41.
Kuhn KA, Pedraza I, Demoruelle MK. Mucosal immune responses to microbiota in the development of autoimmune disease. Rheum Dis Clin North Am. 2014;40(4):711–25.
Ebrahimi A, Soofizadeh B, Ebrahimi F, et al. Relationship between Helicobacter pylori cytotoxin-associated gene A protein with clinical outcomes in patients with rheumatoid arthritis. Immunol Lett. 2019;211:49–52.
Rusthen S, Kristoffersen AK, Young A, et al. Dysbiotic salivary microbiota in dry mouth and primary Sjögren’s syndrome patients. PLoS One. 2019;14(6):e0218319.
Blaser MJ, Atherton JC. Helicobacter pylori persistence: biology and disease[J]. J Clin Invest. 2004;113(3):321–33.
Hasni S, Ippolito A, Illei GG. Helicobacter pylori and autoimmune diseases[J]. Oral Dis. 2011;17(7):621–7.
Coll J, Tomás S, Vilella R, Corominas J. Interleukin-2 receptor expression in salivary glands of patients with Sjögren’s syndrome. J Rheumatol. 1995;22(8):1488–91.
Carvajal P, Aguilera S, Jara D, et al. hsa-miR-424-5p and hsa-miR-513c-3p dysregulation mediated by IFN-γ is associated with salivary gland dysfunction in Sjögren’s syndrome patients. J Autoimmun. 2023;138:103037.
Fisher BA, Brown RM, Bowman SJ, Barone F. A review of salivary gland histopathology in primary Sjögren’s syndrome with a focus on its potential as a clinical trials biomarker. Ann Rheum Dis. 2015;74(9):1645–50.
Mezache L, Magro C, Hofmeister C, Pichiorri F, Sborov D, Nuovo GJ. Modulation of PD-L1 and CD8 Activity in Idiopathic and Infectious Chronic Inflammatory Conditions. Appl Immunohistochem Mol Morphol. 2017;25(2):100–9.
Irani S, Monsef Esfahani A, Bidari Zerehpoush F. Detection of Helicobacter pylori in Oral Lesions. J Dent Res Dent Clin Dent Prospects. 2013;7(4):230–7.
He Y, Hu L, Qiu W, Zhu L, Zhu X, Hong M. Clinical characteristics and risk factors of Helicobacter pylori infection-associated Sjogren’s syndrome. Immun Inflamm Dis. 2023;11(10):e994.
Acknowledgments
We want to acknowledge the European Bioinformatics Institute and FinnGen consortium for providing related GWAS summary data.
Funding
This study was supported by the National Natural Science Foundation of China (NO. 82360441), projects funded by the Science and Technology Department of Jilin Province (No.20200201492JC and YDZJ202201ZYTS161).
Author information
Authors and Affiliations
Contributions
Dinglu Cui: Data curation, Software, Writing – original draft. Rongxian An: Writing – original draft. Lei Li: Writing – original draft. Long Jiang: Writing – original draft. Chunshan Jiang: Writing – review & editing. Jingchun Jin: Supervision, Writing – review & editing.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Cui, D., An, R., Li, L. et al. Causal association between Helicobacter pylori infection and Sjogren’s syndrome: a bidirectional Mendelian randomization analysis. BMC Infect Dis 24, 782 (2024). https://doi.org/10.1186/s12879-024-09678-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09678-2