Abstract
This single-centre retrospective cohort study reports on the results of a descriptive (non-comparative) retrospective cohort study of early initiation of antivirals and combined monoclonal antibody therapy (mAbs) in 48 severely immunocompromised patients with COVID-19. The study assessed the outcomes and the duration of viral shedding. The patients started early combined therapy (ECT) a median of 2 days (interquartile range [IQR]: 1–3 days) after the diagnosis of SARS-CoV-2 infection. Except for 1 patient who died due COVID-19-related respiratory failure, patients had their first negative nasopharyngeal swab result after a median of 11 days (IQR: 6–17 days) after starting combined therapy. There were no reports of severe side effects. During a follow-up period of 512 days (interquartile range [IQR]: 413–575 days), 6 patients (12.5%) died and 16 (33.3%) were admitted to hospital. Moreover, 12 patients (25%) were diagnosed with SARS-CoV-2 reinfection a median of 245 days (IQR: 138–401 days) after starting combined treatment. No relapses were reported. Although there was no comparison group, these results compare favourably with the outcomes of severely immunocompromised patients with COVID-19 reported in the literature.
Similar content being viewed by others
Background
In severely immunocompromised patients, untreated COVID-19 is characterized by high rates of negative outcomes such as death and relapse [1]. Although early monotherapy with neutralizing monoclonal antibodies (mAbs) or antiviral agents against SARS-CoV-2 is recommended to prevent unfavourable outcomes in immunocompromised patients, the available studies suggest that complication rates remain high [2,3,4,5,6].
Therefore, COVID-19 remains a “sword of Damocles” for severely immunocompromised patients, and recent guidelines recommend longer treatment or additional courses of antivirals in patients with prolonged, symptomatic COVID-19 [7]. Several authors have reported the use of combined therapy in immunocompromised patients, but the incidence of unfavourable outcomes such as clinical worsening [4], relapse [6], and death [3, 4] remains high. Moreover, longer courses of treatment and combined therapy with 2 antivirals and mAbs could be unsafe. For example, both Gentile et al. [4] and Mikulska et al. [3] reported severe side effects in 9.1% of patients.
We previously proposed an algorithm for the treatment of outpatients with COVID-19 who were referred to a treatment centre for frail patients with COVID-19 in the Calabria region of Italy considering their comorbidities and vaccinal status [2].
In this study, we focused on severely immunocompromised COVID-19 patients over an extended follow-up period, aiming to explore the safety and efficacy of early combined therapy (ECT) involving both antivirals and monoclonal antibody therapy. Herein, we reported both clinical (i.e., death, hospital admission and relapse) and virological outcomes.
Methods
We conducted a single-centre, retrospective study including consecutive adult outpatients with major immunocompromising conditions (i.e., hematological malignancy, transplantation or treatment with anti-CD20 monoclonal antibodies and COVID-19) from January 1, 2022, to January 31, 2023. In accordance with the regional health system, these patients underwent third-generation antigenic swab testing for SARS-CoV-2 detection through local medical territorial services, including COVID-19-dedicated territorial physicians or patients’ primary care physicians (Fig. 1).
Flowchart illustrating the risk assessment for severe COVID-19 conducted by our facility for COVID-19 outpatients. Only patients who received early combined therapy (ECT) were included in this study. BMI: body mass index; eGFR: estimated glomerular filtration rate. RT-PCR: real-time polymerase chain reaction; mAbs: neutralizing monoclonal antibodies against SARS-CoV-2
After initial testing, patients deemed to be at low risk of severe COVID-19 were provided with over-the-counter medications (e.g., nonsteroidal anti-inflammatory drugs, inhaled corticosteroids) and were overseen by local medical territorial services, while those classified as having intermediate to high risk were referred to our specialized centre. Our facility focuses on providing tailored care for fragile COVID-19 patients [2]. SARS-CoV-2 infection diagnosis was confirmed using real-time polymerase chain reaction (RT-PCR, GeneFinder COVID-19 Plus RealAmp Kit, Elitech Group) targeting the RNA-dependent RNA polymerase (RdRP) gene, the envelope (E) gene, and the nucleocapsid (N) gene of SARS-CoV-2. According to literature, a cycle threshold (Ct) value lower than 35 indicated a confirmed positive test result [8]. For confirmed cases, a clinical assessment of the patient’s risk for progression to severe COVID-19 was conducted.
Patients assessed to be at intermediate risk of developing severe COVID-19 were provided with a single-agent COVID-19 early therapy consisting of either antiviral regimen or mAbs. Only patients deemed to be at high risk of severe disease received ECT which included both mAbs and an antiviral regimen. This therapy was initiated simultaneously on the day of SARS-CoV-2 infection confirmation via molecular test. Only these patients were eligible for inclusion in this study.
According to the protocol [2], each patient received ECT against SARS-CoV-2 that included both mAbs (sotrovimab, casirivimab/imdevimab or tixagevimab/cilgavimab), selected for their likely effectiveness against the spread of local SARS-CoV-2 variants, and an antiviral agent (intravenous remdesivir for 3 days or 5 days of oral nirmatrelvir/ritonavir or molnupiravir) selected in accordance with their availability, feasibility of administering parenteral treatment, and potential for drug-drug interactions, as indicated by international guidelines [7]. Patients were actively monitored until the time of the first nasopharyngeal swab with a negative SARS-CoV-2 result. Outcomes occurring after viral clearance were recorded by performing follow-up phone calls between October 21, 2023, and November 3, 2023, extending the follow-up period to a median of 512 days (interquartile range [IQR]: 413–575 days) from ECT.
Results
The characteristics of the 48 patients included in the cohort are summarized in Table 1.
The majority of patients (44/48, 91.7%) were classified as having mild disease according to the severity scale provided by the National Institutes of Health (NIH) [7]. The remaining patients (4/48, 8.3%) were asymptomatic and tested positive through screening by territorial medical services due to their contact with a COVID-19 case. Therefore, none of these patients were provided with other medications approved for moderate to severe COVID-19 (e.g., systemic corticosteroids, tocilizumab) according to NIH guidelines [7]. The patients started ECT a median of 2 days (IQR: 1–3 days) after SARS-CoV-2 infection was diagnosed. Following the onset of symptoms, patients were referred to the centre for COVID-19 outpatients after a delay of 3 days (IQR: 2–5 days). At this time, confirmatory molecular testing was provided, and ECT was initiated (Fig. 2).
The median Ct values at confirmation of SARS-CoV-2 infection were 22 (IQR: 20–25), 20 (IQR: 16–24), and 20 (IQR: 17–23) for the genes RdRP, E, and N, respectively.
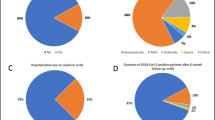
Only 2/48 (4.2%) patients were admitted to the hospital after receiving ECT due to COVID-19 before achieving viral clearance. Both patients had hematological malignancies and were admitted after 3 and 23 days form ECT initiation, respectively. Except for 1 of these patients who died due COVID-19-related respiratory failure after ECT comprising molnupiravir and tixagevimab/cilgavimab, patients had their first negative nasopharyngeal swab result after a median of 11 days (IQR: 6–17 days) after starting ECT. Among survivors, 13/47 (27.7%) were tested with molecular swab and achieved their first negative test results after a median of 18 days (IQR: 6–25 days) from ECT. Conversely, 34/47 (72.3%) patients underwent testing with a third-generation antigenic swab, with negative results obtained after a median period of 11 days (IQR: 6–14 days) from ECT (Fig. 3). No severe side effects were reported.
During the follow-up period subsequent to viral clearance, e5 patients (10.6%) died and 14 (29.9%) were admitted to hospital. Moreover, 12 patients (25.5%) were diagnosed with SARS-CoV-2 reinfection in a median of 245 days (IQR: 138–401 days) after starting ECT. No relapses were reported.
The 5 patients who died after achieving viral clearance were older (median age: 77 years, IQR: 64–82 years) than those who survived (median age: 55 years, IQR: 44–71 years; p = 0.04, Mann–Whitney U test), and death occurred in a median of 276 days (154–397 days) after starting ECT. Among the patients who were hospitalised after achieving viral clearance following ECT (14/47, 29.8%), only 1 was admitted due to SARS-CoV-2 reinfection 236 days after the initial infection and recovered after receiving sotrovimab monotherapy. The other patients were admitted to hospital primarily for reasons related to their underlying diseases after a median of 222 days (IQR: 173–242 days). The prevalence of hematologic malignancies was significantly higher in hospitalised patients (13/14, 92.8%) than in those who did not require hospital admission (16/33, 48.5%; p = 0.008, Fisher’s exact test).
Discussion
We applied an innovative strategy aimed at preventing clinical worsening, including death, hospitalization, and relapse, with earlier initiation of combined therapy in severely immunocompromised outpatients with COVID-19. This approach was deemed necessary because neither vaccination alone [9] nor therapies approved for immunocompetent individuals are adequate to sufficiently shield these individuals from the risk of experiencing more severe outcomes [2,3,4,5,6].
In our cohort, a high incidence of adverse clinical outcomes was expected for several reasons. First, a relatively low percentage of our patients received vaccination, with 20.8% having not received any dose of the vaccine. Consequently, a high rate of severe progression of COVID-19 could have been observed in our population [10]. Second, our cohort demonstrated profound immunocompromise, as indicated by the low rate of response to vaccination, with only a third of patients testing positive for SARS-CoV-2 anti-spike antibodies before receiving ECT. This finding aligns with findings from other studies in the literature conducted on patients with hematological malignancies [11], solid organ transplant recipients [12], and those undergoing immunosuppressive therapy [13] which consistently reported incomplete responses to vaccination in these severely immunocompromised patient groups. Third, low Ct values were observed with RT-PCR on molecular swabs for SARS-CoV-2 RNA detection, with a median Ct value below 25 for all genes (RdRP, E, and N) at confirmation of SARS-CoV-2 infection. Importantly, despite pre-analytical factors such as swabbing technique and input volume for nucleic acid extraction as well as variations in assays and gene targets should be considered, as these factors may impact the reliability of Ct values [14], Ct values have been recognized as a surrogate for viral load [14,15,16] and it was suggested that Ct values may be associated with an increased risk of mortality [16]. Interestingly, Ct values at diagnosis may correlate with an increased likelihood of mortality in patients with hematological malignancies [17]. Fourth, alongside the immunocompromised status, our patients exhibited a notable burden of comorbidities, as assessed by the Charlson Comorbidity Index (CCI) with a median score of 5 (IQR: 3–8).This index serves as a clinically valuable tool for highlighting significant prognostic differences among patient subgroups sharing similar medical diagnoses [18]. This score has emerged as an effective tool for predicting mortality since the onset of the COVID-19 pandemic. Indeed, a metanalysis conducted by Tuty Kuswardhani et al. [19] found that a Charlson Comorbidity Index (CCI) score of 3 or higher was prognostically associated with mortality and a composite outcome including mortality, the need for critical care, severe disease presentation, and mechanical ventilation. Furthermore, each point increase in the CCI is associated with a 16% higher risk of mortality [19]. However, these estimations were derived from studies involving hospitalized patients with COVID-19, and the utility of CCI in predicting outcomes for COVID-19 immunocompromised outpatients has not been validated yet.
Although it is difficult to draw conclusions based on comparisons of different uncontrolled studies, our patients experienced better outcomes than untreated patients [1] and patients treated with monotherapy regimens [2]. In contrast, Lahouati et al. [20] reported rates of hospitalization (4.5%) and death (1.8%) similar to those reported in our study in severely immunocompromised patients, using early initiation of monotherapy after SARS-CoV-2 diagnosis. However, their study [20] was conducted during in the Omicron period, and our patients had a lower vaccination rate than their patients (20.8% vs. 3% were unvaccinated) and probably had more severe immunosuppression (66.7% vs. 16% tested negative for SARS-CoV-2 anti-spike antibody); therefore, our patients could have been expected to experience a worse outcome.
Consistent with the results of our study, Orth et al. [5] found that combined treatment was both safe and effective in prevent prolonged viral shedding in immunocompromised patients, although their results refer to the Omicron wave. Interestingly, the initiation of combined therapy after 5 days after diagnosis was associated with prolonged viral shedding [5]. Triebelhorn et al. [21] recently reported that patients who did not fully respond to SARS-CoV-2 vaccination were more likely to be hospitalized, even when treated with monoclonal antibodies (mAbs). They observed higher hospitalization rates in patients receiving mAbs monotherapy compared to our cohort undergoing ECT (7.3% vs. 4.2%). Moreover, Gentile et al. [4] compared patients treated with combined treatment earlier and later and found that those treated later experienced worse outcomes. These results are consistent with our results, although the study by Gentile et al. [4] had a limited sample size, and both studies lacked a comparison group of patients treated early with monotherapy. Therefore, it is important to conduct appropriate controlled studies to assess whether ECT provides clinical benefits. Further studies should also investigate whether ECT prevents the emergence and accumulation of viral mutations, which is a major risk in immunocompromised individuals [2, 22] because in patients with prolonged symptomatic disease, the emergence of mutations can impair subsequent response to combined treatment. This question is important because the emergence of resistant viruses can lead to their eventual spread at population level [23].
This study has limitations that should be acknowledged. First, the small sample size may limit the generalizability of the findings and we were unable to identify variables associated with hospitalization or death from COVID-19, primarily due to the low occurrence of these outcomes in our cohort. However, the low rates of observed unfavourable outcomes may be attributed to the effectiveness of ECT. Nonetheless, these results are relevant because severely immunocompromised patients experience prolonged viral shedding, relapse, hospitalization, and death [1, 3, 5] despite receiving mAbs or antiviral therapy for SARS-CoV-2 infection [2,3,4, 6, 21]. Second, the absence of a control group hinders the ability to compare outcomes and assess the true efficacy of the combined therapy approach. However, the strength of this study lies in its focus on a specific population of severely immunocompromised patients, for whom there is a lack of established treatment protocols, especially in the early stages of COVID-19 [7]. Focusing on this high-risk group and evaluating the outcomes of ECT, we provide valuable insights into a previously understudied area of COVID-19 management. Third, while variations in the length of infection prior to the initiation of ECT among patients may indeed introduce confounding factors, they also reflect the real-world setting where patients medical care seeking is delayed before treatment. Importantly, in our centre, we prioritize minimizing the delay between diagnosis and treatment which was 2 days (IQR: 1–3 days) in this cohort severely immunocompromised outpatients with COVID-19. This proactive approach aims to promptly address the needs of this fragile patient population and optimize treatment outcomes.
Conclusions
In patients with severe immunosuppression, there is a strong rationale for prescribing combined anti-SARS-CoV-2 therapy as soon as possible after diagnosis to prevent prolonged viral replication and the consequent emergence of escaping mutants. The present results provide evidence that ECT appears to be safe and effective in preventing SARS-CoV-2 clinical complications, prolonged viral shedding, and relapse. However, the generalizability of our results may be limited because this is a retrospective, single-center, uncontrolled study. Therefore, prospective studies and controlled trials are urgently required to validate the “early and hard” approach for treating severely immunocompromised patients to inform guidelines from an evidence-based perspective.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Calderon-Parra J, Munez-Rubio E, Fernandez-Cruz A, Garcia-Sanchez MC, Maderuelo-Gonzalez E, Lopez-Dosil M, Calvo-Salvador M, Banos-Perez I, Valle-Falcones M, Ramos-Martinez A. Incidence, clinical presentation, relapses and outcome of severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection in patients treated with Anti-CD20 monoclonal antibodies. Clin Infect Dis. 2022;74(10):1786–94.
Scaglione V, Rotundo S, Marascio N, De Marco C, Lionello R, Veneziano C, Berardelli L, Quirino A, Olivadese V, Serapide F, et al. Lessons learned and implications of early therapies for coronavirus disease in a territorial service centre in the Calabria region: a retrospective study. BMC Infect Dis. 2022;22(1):793.
Mikulska M, Sepulcri C, Dentone C, Magne F, Balletto E, Baldi F, Labate L, Russo C, Mirabella M, Magnasco L, et al. Triple combination therapy with 2 antivirals and monoclonal antibodies for persistent or relapsed severe Acute Respiratory Syndrome Coronavirus 2 infection in immunocompromised patients. Clin Infect Dis. 2023;77(2):280–6.
Gentile I, Foggia M, Silvitelli M, Sardanelli A, Cattaneo L, Viceconte G. Optimizing COVID-19 treatment in immunocompromised patients: early combination therapy with remdesivir, nirmatrelvir/ritonavir and sotrovimab. Virol J. 2023;20(1):301.
Orth HM, Flasshove C, Berger M, Hattenhauer ST, Biederbick KD, Mispelbaum R, Klein U, Stemler J, Fisahn M, Doleschall AD et al. Early combination therapy of COVID-19 in high-risk patients. Infection 2023.
Brosh-Nissimov T, Ma’aravi N, Leshin-Carmel D, Edel Y, Ben Barouch S, Segman Y, Cahan A, Barenboim E. Combination treatment of persistent COVID-19 in immunocompromised patients with remdesivir, nirmaltrevir/ritonavir and tixegavimab/cilgavimab. J Microbiol Immunol Infect 2023.
COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. https://www.covid19treatmentguidelines.nih.gov/. Accessed May 1, 2024.
Singanayagam A, Patel M, Charlett A, Lopez Bernal J, Saliba V, Ellis J, Ladhani S, Zambon M, Gopal R. Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020. Euro Surveill 2020, 25(32).
Lee A, Wong SY, Chai LYA, Lee SC, Lee MX, Muthiah MD, Tay SH, Teo CB, Tan BKJ, Chan YH, et al. Efficacy of covid-19 vaccines in immunocompromised patients: systematic review and meta-analysis. BMJ. 2022;376:e068632.
Antinori A, Bausch-Jurken M. The Burden of COVID-19 in the Immunocompromised patient: implications for vaccination and needs for the future. J Infect Dis. 2023;228(Suppl 1):S4–12.
Lim SH, Stuart B, Joseph-Pietras D, Johnson M, Campbell N, Kelly A, Jeffrey D, Turaj AH, Rolfvondenbaumen K, Galloway C, et al. Immune responses against SARS-CoV-2 variants after two and three doses of vaccine in B-cell malignancies: UK PROSECO study. Nat Cancer. 2022;3(5):552–64.
Prendecki M, Thomson T, Clarke CL, Martin P, Gleeson S, De Aguiar RC, Edwards H, Mortimer P, McIntyre S, Mokreri D, et al. Immunological responses to SARS-CoV-2 vaccines in kidney transplant recipients. Lancet. 2021;398(10310):1482–4.
Prendecki M, Clarke C, Edwards H, McIntyre S, Mortimer P, Gleeson S, Martin P, Thomson T, Randell P, Shah A, et al. Humoral and T-cell responses to SARS-CoV-2 vaccination in patients receiving immunosuppression. Ann Rheum Dis. 2021;80(10):1322–9.
Poon KS, Wen-Sim Tee N. Caveats of reporting cycle threshold values from severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) qualitative polymerase chain reaction assays: a molecular Diagnostic Laboratory Perspective. Clin Infect Dis. 2021;73(9):e2851–2.
Juanola-Falgarona M, Penarrubia L, Jimenez-Guzman S, Porco R, Congost-Teixidor C, Varo-Velazquez M, Rao SN, Pueyo G, Manissero D, Pareja J. Ct values as a diagnostic tool for monitoring SARS-CoV-2 viral load using the QIAstat-Dx(R) respiratory SARS-CoV-2 panel. Int J Infect Dis. 2022;122:930–5.
Hagman K, Hedenstierna M, Widaeus J, Arvidsson E, Hammas B, Grillner L, Jakobsson J, Gille-Johnson P, Ursing J. Correlation of SARS-CoV-2 nasopharyngeal CT values with viremia and mortality in adults hospitalized with COVID-19. Open Forum Infect Dis. 2022;9(9):ofac463.
Santarelli IM, Manzella DJ, Gallo Vaulet ML, Rodriguez Fermepin M, Crespo Y, Toledo Monaca S, Dobarro M, Fernandez SI. Cycle threshold predicted mortality in a cohort of patients with hematologic malignancies infected with SARS-CoV-2. Rev Argent Microbiol. 2023;55(3):246–50.
Charlson ME, Carrozzino D, Guidi J, Patierno C. Charlson Comorbidity Index: a critical review of Clinimetric Properties. Psychother Psychosom. 2022;91(1):8–35.
Tuty Kuswardhani RA, Henrina J, Pranata R, Anthonius Lim M, Lawrensia S, Suastika K. Charlson comorbidity index and a composite of poor outcomes in COVID-19 patients: a systematic review and meta-analysis. Diabetes Metab Syndr. 2020;14(6):2103–9.
Lahouati M, Cazanave C, Labadie A, Gohier P, Guirle L, Desclaux A, Gigan M, Malvy D, Pedeboscq S, Xuereb F, et al. Outcomes of targeted treatment in immunocompromised patients with asymptomatic or mild COVID-19: a retrospective study. Sci Rep. 2023;13(1):15357.
Triebelhorn J, Schneider J, Spinner CD, Iakoubov R, Voit F, Wagner L, Erber J, Rothe K, Berthele A, Pernpeintner V et al. Clinical and immunological outcomes of SARS-CoV-2-infected vaccine responders, vaccine non-responders, and unvaccinated patients evaluated for neutralizing monoclonal antibody treatment at a single German tertiary care center: a retrospective cohort study with prospective follow-up. Infection 2024.
Wada D, Nakamori Y, Maruyama S, Shimazu H, Saito F, Yoshiya K, Kuwagata Y. Novel treatment combining antiviral and neutralizing antibody-based therapies with monitoring of spike-specific antibody and viral load for immunocompromised patients with persistent COVID-19 infection. Exp Hematol Oncol. 2022;11(1):53.
Sanderson T, Hisner R, Donovan-Banfield I, Hartman H, Lochen A, Peacock TP, Ruis C. A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes. Nature 2023.
Funding
This work was supported by the Regione Calabria (no. COVID19@UMG POR Calabria-FESR/FSE 2014–2020 D.D.R.C. n. 4584 del 4/5/2021- Azione 10.5.12).
Author information
Authors and Affiliations
Contributions
Conceptualisation and methodology: SR, CT; recruitment: SR, LB, SG, VLG, RL; data curation: SR, LB, SG; original draft preparation: SR, LB, SG, VLG, RL, AR, EMT, CT; supervision: AR, EMT, CT. All authors have read and agreed to the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the ethics committee of the Calabria Region (no. COVID19@UMG POR Calabria-FESR/FSE 2014–2020 D.D.R.C. n. 4584 del 4/5/2021- Azione 10.5.12) and was conducted in accordance with the Declaration of Helsinki and the Good Clinical Practice guidelines. Written informed consent was obtained from all participants for the administration of combined therapy and the use of anonymized patient data for research purposes.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rotundo, S., Berardelli, L., Gullì, S. et al. Early initiation of combined therapy in severely immunocompromised patients with COVID-19: a retrospective cohort study. BMC Infect Dis 24, 564 (2024). https://doi.org/10.1186/s12879-024-09466-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09466-y