Abstract
Noroviruses are the second leading cause of death in children under the age of 5 years old. They are responsible for 200 million cases of diarrhoea and 50,000 deaths in children through the word, mainly in low-income countries. The objective of this review was to assess how the prevalence and genetic diversity of noroviruses have been affected by the introduction of rotavirus vaccines in Africa. PubMed, Web of Science and Science Direct databases were searched for articles. All included studies were conducted in Africa in children aged 0 to 5 years old with gastroenteritis. STATA version 16.0 software was used to perform the meta-analysis. The method of Dersimonian and Laird, based on the random effects model, was used for the statistical analyses in order to estimate the pooled prevalence’s at a 95% confidence interval (CI). Heterogeneity was assessed by Cochran’s Q test using the I2 index. The funnel plot was used to assess study publication bias. A total of 521 studies were retrieved from the databases, and 19 were included in the meta-analysis. The pooled norovirus prevalence’s for pre- and post-vaccination rotavirus studies were 15% (95 CI, 15–18) and 13% (95 CI, 09–17) respectively. GII was the predominant genogroup, with prevalence of 87.64% and 91.20% respectively for the pre- and post-vaccination studies. GII.4 was the most frequently detected genotype, with rates of 66.84% and 51.24% respectively for the pre- and post-vaccination studies. This meta-analysis indicates that rotavirus vaccination has not resulted in a decrease in norovirus infections in Africa.
Similar content being viewed by others
Introduction
Viral gastroenteritis is the main cause of morbidity and mortality worldwide, with norovirus and rotavirus being the predominant viral agents responsible for diarrheal disease globally [1]. Norovirus is a significant global health concern, causing an estimated 200 million cases of diarrhoea and 50,000 deaths in children under 5 worldwide, with low-income countries experiencing the most severe impact. In children, noroviruses contribute to 14% of gastroenteritis cases globally, with 17% and 12% of hospital admissions occurring in low- and middle-income countries, respectively [2, 3]. The geographical distribution of norovirus remains variable, with a prevalence of 15% in Latin America [2], 17% in Asia (China) [4], and a global estimate of 19% in Africa among children under 5 years old [5].
Norovirus, discovered in 1968 [6], belongs to the Caliciviridae family and is a small, non-enveloped, single-stranded RNA virus [7, 8]. Its genome of approximately 7.6 kilobase pairs comprises three open reading frames (ORF). ORF1 encodes six non-structural proteins, while ORF2 and ORF3 code for the main and minor capsid proteins, respectively [9,10,11]. The VP1 component consists of S and P domains, with the P domain further divided into P1 and P2 sub-domains [12]. The VP2 protein, along with VP1’s S domain, aids in the virus’s capsid stability and assembly, potentially facilitating host cell attachment [9, 11, 13, 14].
Noroviruses are classified into genogroups, genotypes and variants (strains or sub-genotypes) [15]. There are 8 P-groups (GI.P to GVII.P and GX.P) and 60 P-types based on the nucleotide diversity of the RdRp region of ORF1 [16]. They are also classified into 10 genogroups (GI-GX) and 49 genotypes based on the VP1 capsid. Human-infecting genogroups include GI, GII, GIV, GVIII, and GIX [16], with GI and GII being the most prevalent [17].
These viruses enter the body targeting intestinal enterocytes, causing replication [18] and increased production of TNF-α and IL-6, leading to intestinal mucosa alterations [19], and clinical symptoms like diarrhoea, vomiting, abdominal pain, fever, and dehydration [19,20,21,22]. Humans are the only reservoir of human noroviruses, primarily transmitted through the faecal-oral route and potentially via contaminated aerosols [23]. Currently, there are no licensed vaccines or antiviral drugs for norovirus prevention [18]. The World Health Organization (WHO) prioritized norovirus vaccine development in 2016, with candidate vaccines based on non-replicable viral pseudoparticles (VLPs) in development [24]. Prevention efforts focus on hygiene and compliance measures, while treatment involves symptom management due to the challenges in vaccine development posed by the virus’s rapid genetic evolution, limited understanding of its pathogenesis, and transient protective immunity against enteric pathogens [8, 18, 25] [26].
Norovirus is responsible for sporadic cases and outbreaks of acute gastroenteritis in both children and adults, while rotavirus mainly affects neonates and children under 5 years of age. The WHO has recommended rotavirus vaccines in national immunization programs for children under 5 years old worldwide since 2007 [27, 28]. To date, four WHO-licensed vaccines (Rotarix, RotaTeq, Rotavac, and RotaSiil) effectively prevent rotavirus infections, notably reducing diarrhoea cases in children under 5 [29, 30]. The introduction of rotavirus vaccination is estimated to have reduced hospitalizations by 40% and annual deaths caused by rotavirus by 25%. However, anti-rotavirus vaccinations have shown lower effectiveness in developing countries, where acute gastroenteritis (AG) often leads to dehydration and malnutrition. Moreover, the widespread use of these vaccines has led to new challenges in combating gastroenteritis. Multiple studies across different countries have identified norovirus as the emerging primary cause of severe gastroenteritis in children following the global implementation of rotavirus vaccines. However, there is a scarcity of data and limited studies that have investigated this phenomenon in Africa [22, 31,32,33]. In this systematic review and meta-analysis, we focus on the evolution of norovirus prevalence and its genetic diversity before and after the implementation of rotavirus vaccines in Africa.
Methodology
Study search strategy
A literature search was conducted on electronic databases PubMed, Web of Science, and Science Direct using the following search terms: “Norovirus, Calicivirus, Norwalk virus, genotype, prevalence, epidemiology, rotavirus vaccines, gastroenteritis, children, diarrhoea, paediatric, and Africa” with no filters, language, or date restrictions. Boolean operators (AND, OR, NOT) were used to broaden or narrow searches during the literature review process. Two independent reviewers, DD and DO, screened titles and abstracts for relevance. The full-text articles were then obtained and assessed independently by these two reviewers for eligibility. Discrepancies were resolved by consensus, and if consensus couldn’t be reached, a third reviewer (AKO) was consulted. The reference lists of articles identified in the search were used to uncover additional literature.
Inclusion and exclusion criteria for studies
We applied the following eligibility criteria: (1) studies conducted in African countries, (2) involving children aged 0 to 5 years, (3) with a minimum of 20 samples, 5) stool samples, 6) both before and after the introduction of anti-rotavirus vaccines, and 7) with no restrictions on publication dates. The period of anti-rotavirus vaccine implementation in each country was considered to determine pre- and post-vaccination studies. Information regarding the type of rotavirus vaccine, vaccination coverage, and vaccine introduction dates per country was obtained from the WHO through the International Vaccine Access Center [34]. Studies based on data from gastroenteritis surveillance systems and cross-sectional studies were included. Eligibility for inclusion in the present review was extended to any research that utilized polymerase chain reaction (PCR) and/or sequencing techniques, regardless of publication language (English or French).
Systematic reviews, conference abstracts, meta-analyses, editorials, clinical trial studies, letters, case reports, in vitro experiments and all studies not meeting the above selection criteria were excluded. Studies involving non-human subjects were excluded. Serological studies and those with inadequate data sets were excluded from the review. Inadequate data referred to studies that did not specify essential information such as the number of samples in different sub-populations (e.g., children in hospital, outpatients, community, ambulatory, with severe diarrhoea, without diarrhoea), the number of genotyped samples, or the number of successfully genotyped samples, etc.
Study selection
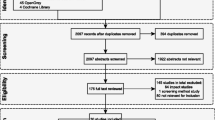
Upon completion of the search, the complete results from all databases were imported into a unique EndNote (version 20.2) library, and duplicates were subsequently removed. Reviewers initially screened studies by title and abstract in the EndNote library based on the inclusion criteria. Subsequently, the full text of relevant articles was retrieved if available online and subjected to the same screening process. A Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)-based study selection flowchart is shown in Fig. 1 [35].
Data extraction
The relevant data from the selected studies were extracted by the reviewer DD and tabulated in a Microsoft Excel 2019 spreadsheet specifically developed for this review. The following data were extracted from each article when available: the name of the first author, the year of publication, the duration of the study, the country, the size of the study population, the diagnostic methods, the prevalence of norovirus in the study population and that of the different genogroups and genotypes, the number of positive cases, the size of the genotyped samples, the year of introduction of the rotavirus vaccine in the country where the study was conducted, the design of the study (cross-sectional study, sentinel surveillance study, etc.).
Quality assessment: determining publication bias
To explore potential publication bias and test the assumption of symmetry among the included studies, the symmetry of the funnel plot was constructed. Egger’s objectivity estimation test was used with a p < 0.05 as evidence for the existence or not of publication bias [36].
Data processing and analysis
Data from extracted from selected studies were entered into Microsoft Excel 2019 and imported into Stata software version 16.0 (Stata Corp., College Station, TX, USA) for statistical analysis [37, 38]. Microsoft Excel was used to construct frequency histograms illustrating the distribution of norovirus genotypes in children aged 0 to 5 years old in Africa. The following formulae were used to determine the prevalence:
-
Prevalence of norovirus-positive cases in the study population = number of positive cases in/total number of samples tested.
-
Genogroup prevalence = total number of positive cases of the genogroup concerned/total number of genogroups detected.
-
Genotype prevalence = number of positive cases of the genotype concerned/total number of samples tested positive in the study or total number of genotyped samples.
The metaprop command in Stata software (Stata Corp., College Station, TX, USA) version 16.0 was used to perform the meta-analysis [37, 38]. DerSimonian and Laird’s random effects meta-analysis model [39] was used to generate pooled (combined) overall and subgroup prevalence estimates at 95% confidence intervals. These pooled prevalence’s are represented by forest plot graphs with 95% confidence intervals. Cochran’s Q test was used to determine the statistical heterogeneity index -I2 [37]. I2 values of 25%, 50%, and 75%, were considered low, moderate, and high levels of heterogeneity, respectively. Subgroup statistical analysis was performed for the pre- and post-vaccination studies and p-value < 0.05 was considered statistically significant [17].
Results
Literature search (analysis of primary data)
The search yielded 521 papers (PubMed: 167, Web of Sciences: 176 and Science Direct: 192). Studies identification included duplicates removing, title and abstract screening, and full-text screening leading to the selection of 19 articles for the systematic review and meta-analysis (Fig. 1). All the studies included in the meta-analysis were conducted between 2005 and 2022.
Characteristics of the studies included in the systematic review and meta-analysis
Out of the 19 studies in the meta-analysis, 6 were from sentinel surveillance programs and networks for rotavirus and/or norovirus. Two of the three studies excluded from the meta-analysis did not perform genotypic characterisation of noroviruses [40, 41], and one could not be classified as a pre- or post-vaccination study [31]. The meta-analysis covered 14 African countries, with 6 (31.58%) studies from East Africa, 6 (31.58%) from West Africa, 3 (15.80) from Central Africa, 2 (10.52) from Southern Africa and 2 from North Africa. Tables 1 and 2 provide a summary of the characteristics of the studies included in the systematic review and meta-analysis.
Prevalence of norovirus
Among the 19 articles in the meta-analysis, 9,505 samples from children aged 0–5 years with gastroenteritis were analysed. The cumulative prevalence of norovirus in children across all pre- and post-vaccination studies was 14% (95% CI, 12–17), with the forest plot (Fig. 2) indicating statistically significant heterogeneity (I2 = 91.87%, p < 0.001).
Forest plot of pooled norovirus prevalence in children aged 0–5 years with gastroenteritis in Africa. Legend The size of each grey square indicates the weight of the study contributing to the pooled prevalence estimate. The line running horizontally through each grey square refers to a 95% confidence interval with the mean effect at the centre (the point estimate of prevalence for each study). The dotted blue line represents the mean estimate of the pooled prevalence of norovirus. The dark blue diamond represents the 95% confidence interval of the pooled norovirus prevalence estimate. The length of the confidence interval is proportional to the size of the study sample and therefore to the precision of the estimate
Distribution of norovirus-positive cases by zone in Africa
According to the distribution of norovirus infections by African zone, the highest prevalence was observed in Central Africa, with a value of 18% (95% CI, 6–29). North Africa had a prevalence of 17% (95% CI, 15–20). West Africa, with a prevalence of 16% (95 CI, 12–20). East Africa, with 11% (95 CI, 8–14) and South Africa, 11% (95 CI, 8–13), recorded the lowest prevalence of norovirus infections (Fig. 3).
Subgroup statistical analyses
Norovirus prevalence in hospitalised and non-hospitalised children in Africa
Of the 19 studies included in this meta-analysis, 14 recruited only hospitalised children and 4 worked on samples from non-hospitalised children. One study that recruited both hospitalised and non-hospitalised children could not be classified because it did not specify the number of norovirus-positive cases in these two respective subgroups.
For the 14 studies that recruited hospitalised patients, a total of 7130 samples were tested compared with 1855 for non-hospitalised cases. The prevalence’s in these two subgroups of hospitalised and non-hospitalised children were 13.64% (973/7130) and 11.48% (213/1855) respectively.
Prevalence of norovirus before and after the introduction of rotavirus vaccines
Six studies included in this meta-analysis were post-vaccination studies against 13 pre-vaccination studies. The pooled prevalence of norovirus in children with gastroenteritis for pre-vaccination studies was 15% (95% CI, 15–18), and for post-vaccination studies, it was 13% (95% CI, 09–17). There was significant and important heterogeneity (Fig. 3) between the pre- (p < 0.001; I2 = 92.29%) and post-vaccination studies (p < 001; I2 = 89.53%).
Diversity of norovirus genogroups in Africa
In the 19 studies included into this meta-analysis, 1277 samples tested positive for norovirus with successful genotyping of 1205. The overall prevalence of GII was 11.20% (135/1205), making it the predominant genogroup with a prevalence of 88.38% (1065/1205). GI.GII co-infection was observed at a prevalence of 0.33% (4/1205), while the GIX genogroup had the lowest reported prevalence (0.08%, 1/1205) in children with gastroenteritis. The cumulative prevalence of the genogroups during pre- and post-vaccination periods are provided in Table 3.
Rotavirus vaccine and norovirus genotypic diversity in Africa
In 13 pre-vaccination studies, 955 norovirus-positive samples were identified, with 677 genotyped and 570 successfully analysed. In 6 post-vaccination studies, out of 250 positive samples, 201 were genotyped. The GII.4 genotype was most prevalent in both groups, with 66.84% in pre-vaccination and 51.24% in post-vaccination studies. Other significant genotypes included GII.3 (4.40%), GII.6 (3.80%), GI.3 (3.51%), GII.16 (3.00%), GII.2 (2.63%), GII.7 (1.61%), GII.21 (1.41%), GI.1 (1.40%), GI.5 and GII.17 (1.23% each) in the pre-vaccination studies and GII.6 (8.95%), GII.12 and GII.3 (5.47% each), GII.17 (4.97%), GII.10 and GII.12 (3.99% each), GII.17 (4.97%), GII.7 (2.98%), GI.3 and GII.13 (2.48% each) in the post-vaccination studies (Fig. 4). The GI.1, GI.2, GI.4, GI.7, GI.11, GI.14, GII.1, GII.11 and GII.15 found pre-vaccination were absent post-vaccination.
Distribution of recombinant noroviruses
In the present study, recombinant norovirus infections were observed in four studies (Angola, Malawi, Senegal and Nigeria) [42, 47, 48, 57]. Recombinants reported included inter-genogroups (GI.3_GII.3 and GII.4_GI.5) and intra-genogroups (GII.4_GII.6 and GII.7_GII.14). The combined prevalence was 0.2% (1/570) for each of the four recombinants.
Rotavirus and Norovirus co-infection in children
In this meta-analysis, only 8 studies including one post-vaccination study, assessed rotavirus infections in norovirus-infected children, with the combined prevalence of norovirus-rotavirus co-infection at 22% (95% CI, 15–29). The highest co-infection rate (36%, 95% CI, 15–65) was in Nigeria, the lowest (12%, 95% CI, 06–21) in Cameroon. Figure 5 illustrates a forest plot of these co-infection cases.
Prevalence and genetic diversity of noroviruses in asymptomatic children in Africa
Prevalence of noroviruses in asymptomatic children in Africa
Of the 19 studies included in this meta-analysis, 5 recruited case controls. Controls were children aged between 0 and 5 years without gastroenteritis and without clinical manifestations, asymptomatic. Of the 5 studies that included controls, 3 were pre-vaccination studies and 2 were post-vaccination studies. For the 5 studies, the total number of children with gastroenteritis (symptomatic children) was 3,798 compared with 1,689 for asymptomatic children. The prevalence of norovirus (Table 4) in these two subpopulations of symptomatic and asymptomatic children was 12.5% (478/3798) and 8.70% (147/1689) respectively.
Genetic diversity of noroviruses in asymptomatic children in Africa
Of the 147 norovirus positive samples, GI represented 12.92% (19/147) and recombinant GI.GII with a prevalence of 0.68% (1/147). GII was by far the most important genogroup, with a prevalence of 86.40% (127/147). Figure 7 shows the prevalence of the different norovirus genogroups in children aged 0–5 years without gastroenteritis. GII was the predominant genogroup in the pre- and post-vaccination studies, with prevalence’s of 84.35% (97/115) and 93.75 (30/32) respectively. GI was observed with proportions of 14.78% (17/115) and 6.25% (2/32) respectively in the pre- and post-vaccination studies.
For genotype detection, 59 were admitted for genotyping and 55 were successfully assigned
In the pre-vaccination studies, 5 (GI.5, GI.7, GII.4, GII.16 and GII.21) and 12 (GI.3, GI.7, GII.1, GII.2, GII.3, GII.4, GII.6, GII.10, GII.13, GII.14, GII.16 and GII.21) were observed. GII.4 was the most frequently detected genotype, with prevalence’s of 54.17% (13/24) and 38.71% (12/31) respectively for the pre- and post-vaccination studies.
In the pre-vaccination studies, genotypes GII.21 (25%), GI.5 (8.33%) and GI.7 (8.33%) were the most frequently observed after GII.4. In the post-vaccination studies, GII.6, GII.13 and GII.14, with a prevalence of 9.68% each, were most frequently detected after GII.4 (Fig. 8).
Evaluation of publication bias
The results of the graph showed a fairly low publication bias, as indicated by the distribution of studies in Fig. 9. Egger’s objectivity test confirmed the low level of publication bias in the studies selected for this meta-analysis.
Funnel diagram for assessing study publication bias. Legend: Each point represents a study. The subjective evaluation of this diagram gives an indication of the heterogeneity of the studies. The y-axis represents the precision (inverse of the variance) expressed here at a ratio of 1/1000. The x axis represents the standardised effect transformed into a log (estimate divided by its standard error)
Discussion
Norovirus gastroenteritis is prevalent in low- and middle-income countries, with poor socio-economic conditions and sanitation [2]. In this meta-analysis, 9505 symptomatic gastroenteritis cases were examined across 19 studies. The pooled norovirus detection rate in children (0–5 years old) was 14% (95% CI, 12–17). These results align with global estimates of 14% (95% CI, 11–16) of norovirus infection in children with acute gastroenteritis [2, 3] and are close to the 15% reported in Latin America [58]. However, our findings contrast with Ahmed et al. [3] meta-analysis who found norovirus prevalence of 18% (95% CI, 15–20) in children under 5 years old. Other meta-analyses focusing on children under 5 in Africa and Asia (China) reported norovirus prevalence of 19.25% (95% CI, 14.4–23.5) [5], and 17.39% (95% CI, 17.32–17.47) [4], respectively. Additionally, a study in low- and middle-income countries found a 17% prevalence (95% CI, 17–18) of norovirus among hospitalised children under 5 years [2]. The discrepancies in norovirus prevalence between various studies could be attributed to differences in detection method sensitivity, some are more sensitive than others [59], geographical areas [49], and duration of study periods [5]. Notably, a higher burden of norovirus diarrhoea during the dry season has been observed [60]. In our meta-analysis, substantial heterogeneity was observed among the included studies, indicating significant variation. In addition to the sensitivity of the different detection methods used by the included studies, the geographical area and the length of the study periods, other reasons could explain this heterogeneity. These include the limited number of sampling sites found in the included studies, the short sampling period, and the small sample sizes for most of the included studies. The limited sample sizes make it impossible to capture broad information on the genetic diversity and national circulation of noroviruses. The context in which the studies were conducted, and the definition of cases of diarrhoea or gastroenteritis are other factors that could explain the heterogeneity observed between the studies included in this meta-analysis. Policies on the management of gastroenteritis differ from country to country. Most children suffering from gastroenteritis do not routinely undergo tests to establish aetiology, except in cases of spontaneous studies and severe gastroenteritis.
The pooled prevalence’s of norovirus for the pre- and post-vaccination studies were 15% (95% CI, 15–18) and 13% (95% CI, 13–17) respectively. For pre-vaccine studies, our observations were similar to those of O’Ryan et al. [61] who reported a pooled prevalence of 15% (95% CI: 12–19) for their rotavirus pre-vaccine studies. The pooled post-vaccination prevalence found in this meta-analysis tends towards the 16% (95% CI: 12–22) reported by O’Ryan et al. [61]. In this study, we observed a change in the prevalence of norovirus from 15% (95% CI, 15–18) in the pre-vaccination period to 13% (95% CI, 13–17) in the post-vaccination period. However, given the heterogeneity of the studies included, this does not allow us to conclude that there has been a real reduction in the prevalence of norovirus following the introduction of rotavirus vaccines in Africa.
However, some studies conducted in low- and middle-income countries have found an increase in the pattern of norovirus infections after the introduction of rotavirus vaccines [31, 33, 61]. The slight decrease in norovirus infections observed in our study could be explained by the positive effect of raising awareness of norovirus prevention and control through networks (such as NoroNet and CaliciNet) and paediatric viral gastroenteritis surveillance programmes set up in the various African countries. This meta-analysis shows that the widespread use of rotavirus vaccines does not appear to have a real positive impact on the norovirus infection pattern in Africa. For the pre-vaccination studies, the combined prevalence’s for GII and GI were 87.64% and 11.94% respectively. These observations are consistent with previous studies conducted in Asia, Europe, Latin America, and Africa, which reported prevalence ranging from 72.1 to 97.8% for GII, and from 3.02 to 27.90% for GI [53, 62,63,64]. In post-vaccination studies, the combined proportions of GII and GI genogroups were 91.20% and 8.40% respectively. These results are similar to those of previous studies conducted in children under 5 years in Africa [31, 65].
Norovirus GII is the predominant norovirus genogroup worldwide [65, 66]. Our findings align with this observation, suggesting that GII predominance may be associated with its higher viral load in patient stools compared to GI [63]. Such a high concentration of GII would therefore enhance contagiousness. The ability of GII to escape the immune system, its affinity for histological blood group antigens (HBGA) and its ability to bind to cell receptors [63], are other factors that could explain the predominance of this genotype.
In this study, a wide genotypic diversity of noroviruses was observed. A total of 9 GI genotypes (GI.1–5,7,9,11, and GI.14) and 18 GII genotypes (GII.1–4, GII.6–17, GII.20 and GII.21) were identified in the pre-vaccination studies. In the post-vaccination studies, 3 genotypes (GI.3,5,9) of GI and 14 genotypes (GII.2–4, GII.6,7,9,10, GII.12–14, GII.16,17,20,21) of GII were detected. The genotypic diversity observed in this analysis is consistent with the results of other studies carried out in Africa and other parts of the world [4, 5, 63, 67,68,69]. Genotypic diversity is a well-documented feature of norovirus epidemiology [68, 70]. Indeed, noroviruses, like all RNA viruses, are naturally diverse [31]. Other factors such as hygiene, sanitation, and socio-economic conditions, are thought to contribute to the genetic diversity of noroviruses [71]. The distribution of the different genotypes shows that GII.4 is by far the predominant genotype in both pre- and post-vaccination studies, with combined prevalence of 66.84% and 51.24% respectively. These results are similar to those of previous studies conducted in Africa, which reported GII.4 prevalence of 54.1% [68] and 65.2% [67]. Other recent meta-analysis reported similar prevalence of 59.3% [65] and 52% [66].
For post-vaccination studies, our results are comparable to those of a meta-analysis on studies published between 2015 and 2020, with combined prevalence of 50.81% for GII.4 [5]. However, the prevalence of GII.4 observed in our study is lower than that (89.2%) of a study conducted in Brazil [72]. In Africa and other parts of the world, GII.4 has been shown to be the predominant genotype of all norovirus genotypes [5, 65, 66]. Our observation supports the conclusion that GII.4 has dominated over all non-GII.4 genotypes over the last two decades [21, 73]. The predominance of GII.4 is attributed to its high mutational capacity [74], driven by specific recombination [75]. GII.4 can accumulate mutations, periodically replacing antigenic variants [70], fostering the emergence of new variants capable of evading immune responses developed against previous ones [76].
In the present meta-analysis, genotypes GII.3 (4.40%), GII.6 (3.80%), GI.3 (3.51%), GII.16 (3.00%), GII.2 (2.63%), GII.7 (1.61%), GII.21 (1.41%), GI.1 (1.40%), GI.5 and GII.17 (1.23% each), were the most important genotypes in the pre-vaccination studies after GII.4. In the post-vaccination studies, genotypes GII.6 (8.95%), GII.12 and GII.3 (5.47% each), GII.17 (4.97%), GII.10 and GII.12 (3.99% each), GII.17 (4.97%), GII.7 (2.98%), GI.3 and GII.13 (2.48% each) were the most frequently detected after GII.4. These observations are similar to those of previous studies conducted in Africa and other parts of the world [5, 31, 33, 72, 77]. Non-GII.4 norovirus genotypes have a limited number of variants that can persist for decades [70]. This would explain their low prevalence compared to GII.4. The distribution of genotypes shows an emergence of GII.17, GII.6, GI.10, GII.12, GII.2, GII.13, GII.7, GI.9, and GII.3 genotypes after the introduction of rotavirus vaccines in Africa. This observation is similar to those of previous studies conducted worldwide, which have reported the emergence of GII.6, GII.17, GII.2, GII.3 [5], GII.2 [78,79,80], and GII.6 [81].
The emergence or re-emergence of genotypes GII.17, GII.6, GI.10, GII.12, GII.2, GII.13, GII.7, GI.9, and GII.3 and the predominance of GII.4, GI.3, GII.16, GII.7, GII.21, GI.1 and GI.5, observed in this study, shows the need to strengthen genomic surveillance of noroviruses in Africa. Genotypes GII.4, GI.3, GII.16, GII.7, GII.21, GI.1 and GI.5 could be the genotypes to target in the development of future norovirus vaccines.
In asymptomatic children, the pooled prevalence of norovirus infection was 8.70%. The pooled prevalence observed in this meta-analysis shows that asymptomatic children could play an important role in the transmission pattern of noroviruses in Africa. In this meta-analysis, genogroups GII and GI were observed at prevalence’s of 86.40% and 12.92% in asymptomatic children. As in symptomatic children, GII was by far the predominant genotype in asymptomatic children.
In the pre-vaccination studies, 5 (GI.5, GI.7, GII.4, GII.16 and GII.21) and 12 (GI.3, GI.7, GII.1, GII.2, GII.3, GII.4, GII.6, GII.10, GII.13, GII.14, GII.16 and GII.21) were observed. GII.4 was the most frequently detected genotype, with prevalence’s of 54.17% and 38.71% respectively for the pre- and post-vaccination studies. As with symptomatic children, a high genetic diversity of noroviruses was observed in asymptomatic children. In addition to the contexts in which the studies were carried out [5, 60], the geographical areas [49] and the different norovirus detection methods used by the different studies [59], the genetic diversity observed in this meta-analysis could also be explained by genetic variations linked to the mutation and recombination phenomena affecting the norovirus.
The results of this study will contribute to understanding the impact of rotavirus vaccines on norovirus epidemiology in Africa. This meta-analysis made it possible to estimate the evolution of the different norovirus genotypes circulating in Africa before and after the advent of rotavirus vaccines. This information could help to better guide norovirus prevention strategies, particularly with regard to the genotypes to target, in the development of future norovirus vaccines. The results of this meta-analysis could therefore help in the development of vaccines that will be more effective in the real socio-economic context of the African region. The results of this study also serve as a reminder of the need to step up sentinel surveillance against noroviruses in Africa.
Limitations of the study
Heterogeneity between the studies included in this meta-analysis could have an effect on the overall grouped prevalence or those of the subgroups. This heterogeneity could be related to differences in the spatio-temporal and epidemiological patterns of the studies included in this meta-analysis. Also, although seasonality of norovirus infections is important for effective healthcare planning, in our study we were unable to establish seasonality because it was inconsistent across individual studies. This is another limitation of this study.
Conclusion
This meta-analysis provided interesting, up-to-date data and information on the evolution of the prevalence, molecular epidemiology, and genetic diversity of noroviruses in children aged 0 to 5 years old after the introduction of universal rotavirus vaccination program in Africa. Our study confirmed the important role played by noroviruses in gastroenteritis in young children both before and after the introduction of rotavirus vaccines in Africa. The advent of rotavirus vaccines in Africa has not shown any real positive impact on norovirus infections. While waiting for norovirus vaccines to become available, it will be necessary to strengthen paediatric gastroenteritis control systems and strategies in Africa.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Thorne L, Nalwoga A, Mentzer AJ, de Rougemont A, Hosmillo M, Webb E, Nampiija M, Muhwezi A, Carstensen T, Gurdasani D, et al. The First Norovirus Longitudinal Seroepidemiological Study from Sub-saharan Africa reveals high seroprevalence of diverse genotypes Associated with host susceptibility factors. J Infect Dis. 2018;218(5):716–25.
Mans J. Norovirus infections and Disease in Lower-MiddleandLow-Income Countries, 1997–2018. Viruses 2019, 11(4).
Ahmed SM, Hall AJ, Robinson AE, Verhoef L, Premkumar P, Parashar UD, Koopmans M, Lopman BA. Global prevalence of norovirus in cases of gastroenteritis: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14(8):725–30.
Wei N, Ge J, Tan C, Song Y, Wang S, Bao M, Li J. Epidemiology and evolution of Norovirus in China. Hum Vaccin Immunother. 2021;17(11):4553–66.
Afework DT, Shumie MK, Endalew GF, Adugna AG, Tarekegn BG. Pooled prevalence and genetic diversity of norovirus in Africa: a systematic review and meta-analysis. Virol J. 2022;19(1):115.
Adler JL, Zickl R. Winter vomiting disease. J Infect Dis. 1969;119(6):668–73.
Kapikian AZ, Wyatt RG, Dolin R, Thornhill TS, Kalica AR, Chanock RM. Visualization by immune electron microscopy of a 27-nm particle associated with acute infectious nonbacterial gastroenteritis. J Virol. 1972;10(5):1075–81.
Robilotti E, Deresinski S, Pinsky BA. Norovirus. Clin Microbiol Rev. 2015;28(1):134–64.
Hardy ME. Norovirus protein structure and function. FEMS Microbiol Lett. 2005;253(1):1–8.
Glass PJ, White LJ, Ball JM, Leparc-Goffart I, Hardy ME, Estes MK. Norwalk virus open reading frame 3 encodes a minor structural protein. J Virol. 2000;74(14):6581–91.
Campillay-Véliz CP, Carvajal JJ, Avellaneda AM, Escobar D, Covián C, Kalergis AM, Lay MK. Human norovirus proteins: implications in the replicative cycle, Pathogenesis, and the host Immune Response. Front Immunol. 2020;11:961.
Prasad BV, Hardy ME, Dokland T, Bella J, Rossmann MG, Estes MK. X-ray crystallographic structure of the Norwalk virus capsid. Science. 1999;286(5438):287–90.
Tan M, Huang P, Meller J, Zhong W, Farkas T, Jiang X. Mutations within the P2 domain of norovirus capsid affect binding to human histo-blood group antigens: evidence for a binding pocket. J Virol. 2003;77(23):12562–71.
Vongpunsawad S, Venkataram Prasad BV, Estes MK. Norwalk Virus Minor Capsid Protein VP2 associates within the VP1 Shell Domain. J Virol. 2013;87(9):4818–25.
Zheng DP, Ando T, Fankhauser RL, Beard RS, Glass RI, Monroe SS. Norovirus classification and proposed strain nomenclature. Virology. 2006;346(2):312–23.
Chhabra P, de Graaf M, Parra GI, Chan MC, Green K, Martella V, Wang Q, White PA, Katayama K, Vennema H, et al. Updated classification of norovirus genogroups and genotypes. J Gen Virol. 2019;100(10):1393–406.
Li Y, Xue L, Gao J, Cai W, Zhang Z, Meng L, Miao S, Hong X, Xu M, Wu Q, et al. A systematic review and meta-analysis indicates a substantial burden of human noroviruses in shellfish worldwide, with GII.4 and GII.2 being the predominant genotypes. Food Microbiol. 2023;109:104140.
Estes MK, Ettayebi K, Tenge VR, Murakami K, Karandikar U, Lin SC, Ayyar BV, Cortes-Penfield NW, Haga K, Neill FH et al. Human Norovirus Cultivation in Nontransformed Stem Cell-Derived Human Intestinal Enteroid Cultures: Success and Challenges. Viruses 2019, 11(7).
Newman KL, Leon JS. Norovirus immunology: of mice and mechanisms. Eur J Immunol. 2015;45(10):2742–57.
Cöl D, Biçer S, Ciler Erdağ G, Giray T, Gürol Y, Yilmaz G, Küçük Ö, Vitrinel A. Annual report on norovirus in children with acute gastroenteritis in 2009 and their genotypes in Turkey. Infez Med 2013, 21(4):261–269.
Mugyia AE, Ndze VN, Akoachere JTK, Browne H, Boula A, Ndombo PK, Cannon JL, Vinjé J, Ndip LM. Molecular epidemiology of noroviruses in children under 5 years of age with acute gastroenteritis in Yaoundé, Cameroon. J Med Virol. 2019;91(5):738–43.
Rönnelid Y, Bonkoungou IJO, Ouedraogo N, Barro N, Svensson L, Nordgren J. Norovirus and Rotavirus in children hospitalised with diarrhoea after rotavirus vaccine introduction in Burkina Faso. Epidemiol Infect. 2020;148:e245.
Winder N, Gohar S, Muthana M. Norovirus: an overview of Virology and Preventative measures. Viruses 2022, 14(12).
Esposito S, Principi N. Norovirus Vaccine: priorities for Future Research and Development. Front Immunol. 2020;11:1383.
Atmar RL, Opekun AR, Gilger MA, Estes MK, Crawford SE, Neill FH, Graham DY. Norwalk virus shedding after experimental human infection. Emerg Infect Dis. 2008;14(10):1553–7.
Seo H, Duan Q, Zhang W. Vaccines against gastroenteritis, current progress and challenges. Gut Microbes. 2020;11(6):1486–517.
Hungerford D, Jere KC, Bar-Zeev N, Harris JP, Cunliffe NA, Iturriza-Gómara M. Epidemiology and genotype diversity of norovirus infections among children aged < 5 years following rotavirus vaccine introduction in Blantyre, Malawi. J Clin Virol. 2020;123:104248.
Weekly Epidemiological Record (WER). 18 December 2009, vol. 84, nos. 51/52 (pp. 533–540) [https://reliefweb.int/report/world/weekly-epidemiological-record-wer-18-december-2009-vol-84-nos-5152-pp-533-540-enfr. Accessed: 10/01/2024.].
Burke RM, Tate JE, Kirkwood CD, Steele AD, Parashar UD. Current and new rotavirus vaccines. Curr Opin Infect Dis. 2019;32(5):435–44.
Uprety T, Wang D, Li F. Recent advances in rotavirus reverse genetics and its utilization in basic research and vaccine development. Arch Virol. 2021;166(9):2369–86.
Lartey BL, Quaye O, Damanka SA, Agbemabiese CA, Armachie J, Dennis FE, Enweronu-Laryea C, Armah GE. Understanding Pediatric Norovirus Epidemiology: a decade of study among Ghanaian Children. Viruses 2020, 12(11).
Chen SY, Tsai CN, Chen CL, Chao HC, Lee YS, Lai MW, Chen CC, Huang WL, Chiu CH. Severe viral gastroenteritis in children after suboptimal rotavirus immunization in Taiwan. Pediatr Infect Dis J. 2013;32(12):1335–9.
Lu MC, Lin SC, Hsu YH, Chen SY. Epidemiology, clinical features, and unusual complications of Norovirus infection in Taiwan: what we know after Rotavirus vaccines. Pathogens 2022, 11(4).
International Vaccine Access Center (IVAC). Johns Hopkins Bloomberg School of Public Health [https://www.view-hub.org Accessed: 10/01/2024.].
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9. w264.
Liu YB, Zhao L, Ding J, Zhu J, Xie CL, Wu ZK, Yang X, Li H. Association between maternal age at conception and risk of idiopathic clubfoot. Acta Orthop. 2016;87(3):291–5.
Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72(1):39.
Li M, Yan Y, Wang C, Tu H. Hospital mortality of blunt abdominal aortic injury (BAAI): a systematic review and meta-analysis. World J Emerg Surg. 2023;18(1):26.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88.
Elfving K, Andersson M, Msellem MI, Welinder-Olsson C, Petzold M, Björkman A, Trollfors B, Mårtensson A, Lindh M. Real-time PCR threshold cycle cutoffs help to identify agents causing acute childhood diarrhea in Zanzibar. J Clin Microbiol. 2014;52(3):916–23.
Kabue JP, Meader E, Hunter PR, Potgieter N. Norovirus prevalence and estimated viral load in symptomatic and asymptomatic children from rural communities of Vhembe district, South Africa. J Clin Virol. 2016;84:12–8.
Trainor E, Lopman B, Iturriza-Gomara M, Dove W, Ngwira B, Nakagomi O, Nakagomi T, Parashar U, Cunliffe N. Detection and molecular characterisation of noroviruses in hospitalised children in Malawi, 1997–2007. J Med Virol. 2013;85(7):1299–306.
Rossouw E, Brauer M, Meyer P, du Plessis NM, Avenant T, Mans J. Virus etiology, diversity and clinical characteristics in South African children hospitalised with gastroenteritis. Viruses 2021, 13(2).
Ouédraogo N, Kaplon J, Bonkoungou IJ, Traoré AS, Pothier P, Barro N, Ambert-Balay K. Prevalence and Genetic Diversity of Enteric Viruses in children with Diarrhea in Ouagadougou, Burkina Faso. PLoS ONE. 2016;11(4):e0153652.
Moyo S, Hanevik K, Blomberg B, Kommedal O, Vainio K, Maselle S, Langeland N. Genetic diversity of norovirus in hospitalised diarrhoeic children and asymptomatic controls in Dar Es Salaam, Tanzania. Infect Genet Evol. 2014;26:340–7.
Makhaola K, Moyo S, Lechiile K, Goldfarb DM, Kebaabetswe LP. Genetic and epidemiological analysis of norovirus from children with gastroenteritis in Botswana, 2013–2015. BMC Infect Dis. 2018;18(1):246.
Kebe O, Fernandez-Garcia MD, Zinsou BE, Diop A, Fall A, Ndiaye N, Vinjé J, Ndiaye K. Prevalence and genetic characterization of noroviruses in children with acute gastroenteritis in Senegal, 2007–2010. J Med Virol. 2022;94(6):2640–4.
Japhet MO, Famurewa O, Adesina OA, Opaleye OO, Wang B, Höhne M, Bock CT, Mas Marques A, Niendorf S. Viral gastroenteritis among children of 0–5 years in Nigeria: characterization of the first Nigerian aichivirus, recombinant noroviruses and detection of a zoonotic astrovirus. J Clin Virol. 2019;111:4–11.
Gelaw A, Pietsch C, Mann P, Liebert UG. Molecular detection and characterisation of sapoviruses and noroviruses in outpatient children with diarrhoea in Northwest Ethiopia. Epidemiol Infect. 2019;147:e218.
El Qazoui M, Oumzil H, Baassi L, El Omari N, Sadki K, Amzazi S, Benhafid M, El Aouad R. Rotavirus and norovirus infections among acute gastroenteritis children in Morocco. BMC Infect Dis. 2014;14:300.
Abugalia M, Cuevas L, Kirby A, Dove W, Nakagomi O, Nakagomi T, Kara M, Gweder R, Smeo M, Cunliffe N. Clinical features and molecular epidemiology of rotavirus and norovirus infections in Libyan children. J Med Virol. 2011;83(10):1849–56.
Bonkoungou IJO, Ouédraogo N, Tamini L, Teguera RK, Yaméogo P, Drabo MK, Medah I, Barro N, Sharma S, Svensson L, et al. Rotavirus and Norovirus in children with severe diarrhea in Burkina Faso before Rotavirus vaccine introduction. J Med Virol. 2018;90(9):1453–60.
Howard LM, Mwape I, Siwingwa M, Simuyandi M, Guffey MB, Stringer JS, Chi BH, Edwards KM, Chilengi R. Norovirus infections in young children in Lusaka Province, Zambia: clinical characteristics and molecular epidemiology. BMC Infect Dis. 2017;17(1):92.
Nordgren J, Nitiema LW, Ouermi D, Simpore J, Svensson L. Host genetic factors affect susceptibility to norovirus infections in Burkina Faso. PLoS ONE. 2013;8(7):e69557.
Esteves A, Nordgren J, Tavares C, Fortes F, Dimbu R, Saraiva N, Istrate C. Genetic diversity of norovirus in children under 5 years of age with acute gastroenteritis from Angola. Epidemiol Infect. 2018;146(5):551–7.
Mikounou Louya V, Vouvoungui C, Koukouikila-Koussounda F, Veas F, Kobawila SC, Ntoumi F. Molecular characterization of norovirus infection responsible for acute diarrhea in Congolese hospitalized children under five years old in Brazzaville, Republic of Congo. Int J Infect Dis. 2019;88:41–8.
Dove W, Cunliffe NA, Gondwe JS, Broadhead RL, Molyneux ME, Nakagomi O, Hart CA. Detection and characterization of human caliciviruses in hospitalized children with acute gastroenteritis in Blantyre, Malawi. J Med Virol. 2005;77(4):522–7.
Payne DC, Vinjé J, Szilagyi PG, Edwards KM, Staat MA, Weinberg GA, Hall CB, Chappell J, Bernstein DI, Curns AT, et al. Norovirus and medically attended gastroenteritis in U.S. children. N Engl J Med. 2013;368(12):1121–30.
Bányai K, Estes MK, Martella V, Parashar UD. Viral gastroenteritis. Lancet. 2018;392(10142):175–86.
Oluwatoyin Japhet M, Adeyemi Adesina O, Famurewa O, Svensson L, Nordgren J. Molecular epidemiology of rotavirus and norovirus in Ile-Ife, Nigeria: high prevalence of G12P[8] rotavirus strains and detection of a rare norovirus genotype. J Med Virol. 2012;84(9):1489–96.
O’Ryan M, Riera-Montes M, Lopman B. Norovirus in Latin America: systematic review and Meta-analysis. Pediatr Infect Dis J. 2017;36(2):127–34.
Siafakas N, Zerva L, Hatzaki D, Lebessi E, Chronopoulou G, Paraskakis I, Pournaras S. Molecular epidemiology of noroviruses in children in South Greece, 2013–2015. J Med Virol. 2018;90(11):1703–11.
Sarmento SK, de Andrade J, Miagostovich MP, Fumian TM. Virological and Epidemiological Features of Norovirus Infections in Brazil, 2017–2018. Viruses 2021, 13(9).
Chen C, Yan JB, Wang HL, Li P, Li KF, Wu B, Zhang H. Molecular epidemiology and spatiotemporal dynamics of norovirus associated with sporadic acute gastroenteritis during 2013–2017, Zhoushan Islands, China. PLoS ONE. 2018;13(7):e0200911.
Farahmand M, Moghoofei M, Dorost A, Shoja Z, Ghorbani S, Kiani SJ, Khales P, Esteghamati A, Sayyahfar S, Jafarzadeh M, et al. Global prevalence and genotype distribution of norovirus infection in children with gastroenteritis: a meta-analysis on 6 years of research from 2015 to 2020. Rev Med Virol. 2022;32(1):e2237.
Cannon JL, Bonifacio J, Bucardo F, Buesa J, Bruggink L, Chan MC, Fumian TM, Giri S, Gonzalez MD, Hewitt J, et al. Global trends in Norovirus genotype distribution among children with Acute Gastroenteritis. Emerg Infect Dis. 2021;27(5):1438–45.
Munjita SM. Current Status of Norovirus Infections in Children in Sub-Saharan Africa. J Trop Med 2015, 2015:309648.
Mans J, Armah GE, Steele AD, Taylor MB. Norovirus Epidemiology in Africa: a review. PLoS ONE. 2016;11(4):e0146280.
Zhou H, Wang S, von Seidlein L, Wang X. The epidemiology of norovirus gastroenteritis in China: disease burden and distribution of genotypes. Front Med. 2020;14(1):1–7.
Parra GI, Squires RB, Karangwa CK, Johnson JA, Lepore CJ, Sosnovtsev SV, Green KY. Static and evolving norovirus genotypes: implications for epidemiology and immunity. PLoS Pathog. 2017;13(1):e1006136.
Verhoef L, Hewitt J, Barclay L, Ahmed SM, Lake R, Hall AJ, Lopman B, Kroneman A, Vennema H, Vinjé J, et al. Norovirus genotype profiles associated with foodborne transmission, 1999–2012. Emerg Infect Dis. 2015;21(4):592–9.
Costa S, Fumian TM, Lima ICG, Siqueira JAM, Silva LDD, Hernández JDM, Lucena MSS, Reymão TKA, Soares LDS, Mascarenhas JDP, et al. High prevalence of norovirus in children with sporadic acute gastroenteritis in Manaus, Amazon Region, northern Brazil. Mem Inst Oswaldo Cruz. 2017;112(6):391–5.
Li HY, Zhang YG, Lei X, Song J, Duan ZJ. Prevalence of noroviruses in children hospitalized for acute gastroenteritis in Hohhot, China, 2012–2017. BMC Infect Dis. 2019;19(1):595.
Parra GI. Emergence of norovirus strains: a tale of two genes. Virus Evol. 2019;5(2):vez048.
Malm M, Tamminen K, Lappalainen S, Uusi-Kerttula H, Vesikari T, Blazevic V. Genotype considerations for virus-like particle-based bivalent norovirus vaccine composition. Clin Vaccine Immunol. 2015;22(6):656–63.
White PA. Evolution of norovirus. Clin Microbiol Infect. 2014;20(8):741–5.
Ogunsakin RE, Ebenezer O, Ginindza TG. A bibliometric analysis of the literature on Norovirus Disease from 1991–2021. Int J Environ Res Public Health 2022, 19(5).
Kwok K, Niendorf S, Lee N, Hung TN, Chan LY, Jacobsen S, Nelson EAS, Leung TF, Lai RWM, Chan PKS, et al. Increased detection of Emergent recombinant norovirus GII.P16-GII.2 strains in young adults, Hong Kong, China, 2016–2017. Emerg Infect Dis. 2017;23(11):1852–5.
Niendorf S, Jacobsen S, Faber M, Eis-Hübinger AM, Hofmann J, Zimmermann O, Höhne M, Bock CT. Steep rise in norovirus cases and emergence of a new recombinant strain GII.P16-GII.2, Germany, winter 2016. Euro Surveill 2017, 22(4).
Nagasawa K, Matsushima Y, Motoya T, Mizukoshi F, Ueki Y, Sakon N, Murakami K, Shimizu T, Okabe N, Nagata N, et al. Phylogeny and immunoreactivity of Norovirus GII.P16-GII.2, Japan, Winter 2016-17. Emerg Infect Dis. 2018;24(1):144–8.
De Andrade Jda S, Rocha MS, Carvalho-Costa FA, Fioretti JM, Xavier Mda P, Nunes ZM, Cardoso J, Fialho AM, Leite JP, Miagostovich MP. Noroviruses associated with outbreaks of acute gastroenteritis in the state of Rio Grande do sul, Brazil, 2004–2011. J Clin Virol. 2014;61(3):345–52.
Funding
This research did not receive any external funding.
Author information
Authors and Affiliations
Contributions
Study design: DO, DD and AKO. Search strategy and data extraction: DD, DO, AKO, AS and JS. Statistical analysis: DD, DO, AKO and AS. Supervision: DD, DO, AKO and AS. Manuscript editing: TRC, MAET, GZ, AAZ, LT, TMZ, ATY, DI, FWD, DO and JS. All authors have read and agreed to the publication of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dakouo, D., Ouermi, D., Ouattara, A.K. et al. Rotavirus vaccines in Africa and Norovirus genetic diversity in children aged 0 to 5 years old: a systematic review and meta-analysis. BMC Infect Dis 24, 547 (2024). https://doi.org/10.1186/s12879-024-09434-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09434-6