Abstract
Objectives
The increasing prevalence of severe Mycoplasma pneumoniae pneumonia (SMPP) poses a significant threat to the health of children. This study aimed to characterise and assess the outcomes in children with SMPP.
Methods
We retrospectively analysed children hospitalised for M. pneumoniae pneumonia (MPP) between January and December 2022. Retrospectively, demographic, clinical, underlying diseases, laboratory and radiological findings, and treatment outcomes were collected and analysed. Disease severity was defined as severe or general according to the Guideline for diagnosis and treatment of community-acquired pneumonia in children (2019 version).
Results
Over a 12-month observation period, 417 children with MPP were enrolled, 50.6% (211/417) of whom had SMPP, with the peak incidence observed in winter. Of the 211 children with SMPP, 210 were treated and discharged with improvement, while one child with congenital heart disease died of cardioembolic stroke. A significantly higher proportion of patients with SMPP had underlying diseases, extrapulmonary complications (myocardial and digestive system involvement), and bacterial co-infection. A total of 25 (12%) children with SMPP received mechanical ventilation. The median duration of mechanical ventilation was 3 days. All children were treated with macrolide antibiotic. A significantly higher proportion of patients with SMPP received antibiotic other than macrolides, methylprednisolone sodium succinate, intravenous immunoglobulin and anticoagulation, compared with patients with general MPP (GMPP). Children with SMPP had significantly higher levels of white blood cells, neutrophil percentage, C-reactive protein, procalcitonin, interferon-γ, interleukin (IL)-2, IL-5, IL-6, IL-8, IL-10 and significantly lower percentages of lymphocytes, monocytes, and natural killer cells, compared with GMPP group.
Conclusion
Our findings suggest that severely ill children have more pronounced inflammatory reaction and extrapulmonary complications. For effective management of children with SMPP, hormonal, prophylactic, anticoagulant therapy, as well as the use of antibiotics other than macrolides for bacterial co-infections, could be incorporated into treatment regimens.
Similar content being viewed by others
Background
Severe pneumonia is the leading infectious disease that causes death in children under five years of age. It is estimated that 740,180 deaths occur annually worldwide due to severe pneumonia, accounting for 22% of deaths in children under 5 years of age [1]. Mycoplasma pneumoniae has long been considered an important aetiology of pneumonia and is commonly isolated from children [2]. Although most children with M. pneumoniae pneumonia (MPP) appreciably recover, some present with worsening clinical symptoms and imaging findings, defined as severe MPP (SMPP) [3]. In 2022, MPP was detected in most of children with pneumonia and caused a substantial increase in hospitalisations, severe illnesses, even deaths [4]. Research on active surveillance of hospitalised children suffering from SMPP in China is scarce [5, 6]. In order to improve treatment and to reduce morbidity and mortality, it is vital to recognize the factors contributing to the severity of MPP [7]. This study sought to characterise the demographic and clinical features of paediatric SMPP patients in 2022, which has been rarely described in the literature.
Methods and materials
Ethics approval
This study was carried out according to the protocol which was reviewed and approved by the Institutional Review Board of the Children’s Hospital of Hebei (CHH) (Approval No. 202101). The Ethics Committee of CHH approved this study protocol and waived the obligation for informed consent because of the retrospective nature of the study.
Study participants
Paediatric patients (aged <16 years) admitted to our hospital between January and December 2022 with a discharge diagnosis of MPP were enrolled in this study. Demographics, clinical data, laboratory findings, radiological and bronchoalveolar lavage findings were retrieved from the inpatient electronic records and analysed retrospectively.
MPP diagnoses and disease severity
MPP was confirmed according to Guideline for diagnosis and treatment of community-acquired pneumonia in children (2019 version) [8], including: 1) a presence of an infiltrate on chest radiography reported by two licensed radiologists, 2) fever, cough, or abnormal lung auscultation; and 3) the positive detection of M. pneumoniae DNA in lower respiratory tract specimens by real-time polymerase chain reaction (PCR) and the positive detection of specific MP antibody in sera using a micro-particle agglutination test. According to the Chinese guidelines on CAP, severe disease was defined as the presence of one or more of the following manifestations: (1) Radiography: infiltration of 2/3 of one lung, multilobar infiltration, pleural effusion, pneumothorax, atelectasis, lung necrosis or lung abscesses; (2) Hypoxemia: cyanosis; marked increase in respiratory rate; marked chest wall retractions, tracheal tugging or nasal flaring; O2 saturation less than 92%; (3) Extrapulmonary complications; (6) Persistent high fever for more than 5 days; or (7) reluctance or inability to feed. All the other subjects were considered to have GMPP [9].
The exclusion criteria were as follows: 1) solid tumor or hematological malignancy 2) history of recent hospitalisation (<90 days), and 3) immune deficiency or received corticosteroids prior to admission. Cardiovascular complications included myocardial damage, pericardial effusion, and heart failure. Neurological involvement included febrile convulsions, epilepsy, intracranial haemorrhage, and encephalitis. Digestive complications included abnormal liver function, gastritis, and diarrhea.
Pathogen detection
A multiplex PCR-based platform (GenomeLab system) was utilized to detect M. pneumoniae and ten other pathogens including influenza virus, respiratory syncytial virus, adenovirus, parainfluenza virus, rhinovirus, metapneumovirus, bocavirus, coronavirus and Chlamydia pneumoniae. Multiplex-PCR was performed as previously described [10]. Bacterial and fungal cultures of respiratory secretions and blood samples were obtained according to the protocols developed in our diagnostic laboratory. Specimens used for sputum culture included bronchoalveolar lavage fluid (BALF), negative pressure aspiration by tracheal intubation and induced sputum (IS). Children who underwent bronchial lavage were provided with bronchial lavage fluid. Children who underwent tracheal intubation provided the deep sputum by negative pressure suctioning through tracheal intubation. Other children provided induced sputum. A trained nurse utilized a sterile negative pressure suction catheter to induce a cough to obtain an IS sample. Evidence of bacterial co-infection in our study was demonstrated using blood cultures from sterile sites or induced sputum from non-sterile sites. For positive respiratory specimens, it was considered to be a bacterial co-infection if the clinician judged it clinically relevant and provided appropriate antibiotic treatment.
Statistical analysis
Medians with interquartile ranges were used for continuous variables, and counts (%) were used for categorical variables. Continuous and categorical variables were analysed using the Mann–Whitney U test and Fisher’s exact test. Univariate analysis was performed to identify the differences between patients with SMPP and those with general MPP (GMPP). Logistic regression analysis was performed to select the variables associated with SMPP. Statistical significance was set at p < 0.05. 3. All data analyses were performed using SPSS (version 25.0; SPSS Inc., Chicago, IL, USA).
Treatment and outcomes
All patients were treated according to the expert consensus on the diagnosis and management of M. pneumoniae in children [8]. Outcomes recorded were recovery, discharge, transfer to a community hospital, and death.
Results
General patients’ information
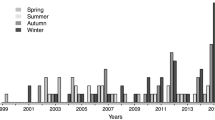
During the observation period, 417 children with MPP were enrolled. The characteristics of the study cohort are presented in Table 1. The median age was 5.5 years, ranging from 0.2–15 years, with a female-to-male ratio of 0.75. The severe case rate was 50.6% (211/417). Analysis of the onset time showed that the epidemic occurred between August and October (autumn in the northern hemisphere) (Fig. 1). The peak period of SMPP was observed in winter. No significant differences in age or sex were found between the SMPP and GMPP groups (p>0.05; Table 1).
Clinical presentation and outcome
The clinical features of the patients on admission are presented in Table 1. Compared to children with GMPP, those in the SMPP group had significantly longer fever and disease duration (both p<0.001) and hospital duration (p=0.007). A significantly greater proportion of patients in the SMPP group had underlying diseases (13.7% vs. 3.9%, p<0.001) and extrapulmonary complications, including myocardial (p=0.033) and gastrointestinal (p<0.001) involvement, than those in the GMPP group. A total of 25 RMPP cases warranted mechanical ventilation, with an median ventilation duration as 3 days (IQR 2, 5). The respiratory failure occurred in 15, with statistically significant differences compared to patients with GMPP (all p<.001, Table 1).
All children with GMPP either recovered or experienced an improvement, whereas one boy aged 7 years in the SMPP group died. He had underlying congenital heart disease. During admission, a cardioembolic stroke occurred, and a mucus plug was found under a fibreoptic bronchoscope.
Treatment
All 417 children were treated with macrolide. A significantly higher proportion of patients with SMPP received antibiotic other than macrolides, methylprednisolone sodium succinate, intravenous immunoglobulin, and anticoagulant treatment (all p<0.001), compared with patients with GMPP (Table 1).
Laboratory testing results
Laboratory tests showed that the SMPP group had a significantly greater percentage of white blood cells and neutrophils, and a significantly lower percentage of lymphocytes, monocytes, and natural killer (NK) cells (Table 2). In the SMPP group, the inflammatory indicators were remarkably greater including C-reactive protein (CRP), procalcitonin (PCT), as well as interferon (IFN)-γ, interleukin (IL)-2, IL-5, IL-6, IL-8, and IL-10 (Table 2). There were no significant differences in serum IL-4, IL-12p, IL-17A, IL-1β, IFN-α and tumor necrosis factor-α concentrations between the two groups (Table 2).
Co-infections
Co-infections occurred more frequently in the SMPP group (80/211, 37.9%) than in the GMPP group (35/206, 17.0%) (p<0.001; Table 3). Among these, bacterial co-infections occurred in 25 SMPP (25/211, 11.8%) and 10 GMPP cases (10/206, 4.8%). The SMPP group had a significantly higher percentage of bacterial co-infections (p=.010; Table 3). Among these, S. pneumoniae was the most common bacterium, with 10 severe cases and three common cases. In addition, viral co-infections were observed in 25 and 55 patients in the GMPP and SMPP groups, respectively (p<0.001), with rhinovirus being the most frequently identified (Table 3). It should be noted that among the culture-positive strains, one case of Staphylococcus epidermidis and one case of Staphylococcus capitis were not treated with the appropriate antibiotics and were excluded as positive infections.
Multiple logistic regression analysis for SMPP markers
To evaluate markers for differentiating SMPP and GMPP, 417 cases were subjected into a non-conditional multiple logistic regression analysis of. The level of IL-2 was a protective factor, and white blood cell count, CRP, IL-5, IL-6, fever duration, and digestive system complications had significantly higher predictive values as risk factors for SMPP, and the corresponding odds ratio values were 1.125, 1.047, 1.485, 1.016, 1.286, and 4.900, respectively (Table 4).
Discussion
Although M. pneumoniae often causes community-acquired pneumonia, the epidemiology of severe infections that pose a life-threatening risk in children is poorly characterized [7]. Here, we explored the severity of MPP and its related factors in children with MPP from January and December 2022 and present the following key findings: 1) half of the MPP cases were severe and occurred mainly in winter, 2) a greater proportion of children with SMPP received antibiotics other than macrolides, and 3) higher levels of pro-inflammatory ILs, gastrointestinal complications as well as duration of fever were risk factors for severe MPP.
MPP can cause severe pulmonary infections and extrapulmonary complications in adults and children [11]. During the 2010–2013 M. pneumoniae epidemic, the hospital mortality rate of adult patients with MPP admitted to intensive care was 29.4% [3]. A large study of paediatric patients with MPP from 2011–2019 in Taiwan revealed emerging cases requiring extracorporeal membrane oxygenation [12]. Carrim et al. treated 103 hospitalised patients with MPP and found that an age of <6 years was an independent risk factor of severe pneumonia [13]. In this study, we observed a severe rate of more than half of hospitalised children with MPP, and extrapulmonary complications included digestive, cardiovascular, and neurological system complications. A series of 332 paediatric patients hospitalised with MPP between 2007–2017 revealed that 25.6% (85/332) of the children experience complications related to the skin, digestion, nervous system, and cardiovascular system [14]. A study conducted in China found that 55.9% (33/59) of children with MPP between 2013-2014 had myocardial damage [15]. In our study, myocardial damage, pericardial effusion, and heart failure were observed especially in the SMPP group. Kammer et al. showed that 24.7% (22/89) of paediatric patients hospitalised in Germany between 2000-2013 had neurological symptoms and signs [16]. We observed febrile seizures, epilepsy, and encephalitis only in the SMPP group. Mia et al. reported that in 2010–2011, 33% (246/746) of children with MPP admitted to a paediatric hospital in Denmark had nausea or vomiting complications [17]. Othman et al. reported children with MPP exhibited coryza, diarrhoea and vomiting especially in children less than 5 years[18]. We observed that the gastrointestinal complication was a dependent risk factor for SMPP. These data suggest that extrapulmonary complications of MPP are common in children and are more frequent in severe cases. Therefore, the management of extrapulmonary complications should be a critical part of the therapeutic regimens for MPP.
In this report, elevated leukocyte levels with a predominance of neutrophils were observed in children with severe pneumonia. Shimizu et al. reported that after M. pneumoniae infection, the M. pneumoniae lipid-associated membrane proteins can activate the toll-like receptor, leading to an increase in neutrophils [19]. CRP and PCT are sensitive indicators of the acute phase of inflammation. They are useful in identifying severe disease caused by MPP [20]. CRP levels are correlated with infection severity [21]. In the present study, patients with SMPP had a lower percentage of NK cell abnormalities than those with GMPP. Genome pathway analysis revealed that the NK cell-mediated cytotoxicity pathway was significantly upregulated in children with MPP [22]. Another study found that the number of NK cells and the expression of CD158b on their surface were altered in patients with severe acute respiratory syndrome and correlated with disease severity [23]. The study found that patients with severe disease had a significantly lower percentage of NK cells compared to those with GMPP. This suggests that NK cells may serve as a marker of disease severity; however, further mechanistic studies are required.
Cytokines are widely used as markers in children with infectious diseases [24]. In the present study, we observed the elevated serum concentrations of INF-γ, IL-2, IL-5, IL-6, IL-8, and IL-10 in patients with SMPP. ILs are a class of cytokines produced by a various of cells that play important regulatory roles in the immune system and have been implicated in the development and progression of MPP [25,26,27]. In severe pneumonia, immune cells in the lungs (e.g., macrophages and neutrophils) release interleukins, leading to an enhanced inflammatory response. M. pneumoniae infection increases IL-6 gene expression and its protein secretion [25]. Serum concentration of IL-6 could potentially indicate the severity and outcomes of MPP [26]. Our results were similar to those of Zhang et al., with the levels of IL-2, IL-10 and IFN-γ being higher in the SMPP group than in the GMPP group (p<.05) [27]. IFN-γ is one of the major cytokines involved in the inflammatory response after M. pneumoniae infection and can increase macrophage lysosomal activity and stimulate macrophage secretion of pro-inflammatory factors to exacerbate the inflammatory response [28]. Esposito et al. discovered that IL-5 was the only cytokine that presented substantially differences in the serum of children with acute M. pneumoniae infection and wheezing [29]. Narita et al. found that in patients with pleural effusion and a persistent fibrotic change in the lungs caused by M. pneumoniae infection, IL-8 was also detected. This indicates the crucial role for IL-8 in the pathogenesis of MPP [30]. It is important to note that the relationship between ILs and severe pneumonia is complex, and that IL levels and actions may be influenced by individual differences, infectious agents, and host immune status. Current strategies for the treatment of severe pneumonia focus on anti-infective therapy and a combination of supportive therapeutic measures [31]. To regulate the inflammatory response, several anti-inflammatory and immunomodulatory drugs need to be investigated for clinical application.
Previous studies have described bacterial co-infection in severely ill children with MPP [32]. In a study aimed at evaluating the frequency and impact of bacterial co-infection in children hospitalised with MPP, Song et al. showed that 2% (173/8612) of children with MPP had bacterial co-infection between 2006–2014, half of which were with S. pneumoniae [33]. In another study, 15.3% (9/59) of hospitalized children with MPP in Korea were found to have bacterial co-infection. In children under the age of 5 years, co-infection with S. pneumoniae was more likely to result in a longer duration of fever and hospital stay, compared to those infected with M. pneumoniae alone [34]. In addition, reports suggest that viruses, such as rhinovirus, may be independent causative agents of pneumonia, including severe pneumonia [35]. Human rhinovirus has emerged as an important cause of pneumonia due to its severity and poor prognosis in adults and children [32, 36]. We observed a substantially increase in the rate of viral and bacterial co-infections in children with SMPP. The results imply that early identification and swift, efficient antibiotic or antiviral treatment in SMPP children is crucial for preventing disease progression. Further investigation is warranted to determine the pathogenic role of this virus and identify specific bacteria that may be the culprit for the severe clinical presentations.
Our study has several limitations. The first one is that it was a retrospective study conducted in a single centre. Second, only inpatients were included, which restricts the generalizability of the results to other patients, particularly those with mild MPP who did not require hospitalization.
Conclusions
Conclusively, a much higher proportion of SMPP children are prone to extrapulmonary complications and viral or bacterial co-infections. Children with severe illness present with elevated inflammation and decreased adaptive immunity. Consequently, hormonal, prophylactic and anticoagulant therapies, as well as the use of antibiotics other than macrolides for bacterial co-infections, could be incorporated into the treatment regimens for the effective management of children with SMPP.
Availability of data and materials
Data is provided within the Figshare repository (https://doi.org/https://doi.org/10.6084/m9.figshare.25339039.v1).
Abbreviations
- MP:
-
Mycoplasma pneumoniae
- MPP:
-
Mycoplasma pneumoniae pneumonia
- SMPP:
-
Severe Mycoplasma pneumoniae pneumonia
- GMPP:
-
General Mycoplasma pneumoniae pneumonia
- CHH:
-
Children’s Hospital of Hebei
- BALF:
-
Bronchoalveolar lavage fluid
- IS:
-
Induced sputum
- CRP:
-
C-reactive protein
- PCT:
-
Procalcitonin
- IL:
-
Interleukin
- NK:
-
Natural killer
- RSV:
-
Respiratory syncytial virus
- PIV:
-
Human parainfluenza virus
- HCoV:
-
Human coronavirus
- ADV:
-
Adenovirus
- HBoV:
-
Human bocavirus
- HMPV:
-
Human metapneumovirus
- H. influenzae :
-
Haemophilus influenza
- K. pneumonia :
-
Klebsiella pneumoniae
- M. catarrhalis :
-
Moraxella catarrhalis
- MTB :
-
Mycobacterium tuberculosis
- S. pneumoniae :
-
Streptococcus pneumoniae
- S. aureus :
-
Staphylococcus aureus
- S. capitis :
-
Staphylococcus capitis
- A. pittii :
-
Acinetobacter pittii
References
Walker CLF, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, O’Brien KL, Campbell H, Black RE. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013;381(9875):1405–16.
Waites KB, Xiao L, Liu Y, Balish MF, Atkinson TP. Mycoplasma pneumoniae from the Respiratory Tract and Beyond. Clin Microbiol Rev. 2017;30(3):747–809.
Khoury T, Sviri S, Rmeileh AA, Nubani A, Abutbul A, Hoss S, van Heerden PV, Bayya AE, Hidalgo-Grass C, Moses AE, et al. Increased rates of intensive care unit admission in patients with Mycoplasma pneumoniae: a retrospective study. Clin Microbiol Infect. 2016;22(8):711–4.
Meyer Sauteur PM, Beeton ML, European Society of Clinical M, Infectious Diseases Study Group for M, Chlamydia I, the EMpSsg. Mycoplasma pneumoniae: delayed re-emergence after COVID-19 pandemic restrictions. Lancet Microbe. 2024;5(2):e100-e101.
Moynihan KM, Barlow A, Nourse C, Heney C, Schlebusch S, Schlapbach LJ. Severe Mycoplasma Pneumoniae Infection in Children Admitted to Pediatric Intensive Care. Pediatr Infect Dis J. 2018;37(12):e336–8.
Yan C, Xue GH, Zhao HQ, Feng YL, Cui JH, Yuan J. Current status of Mycoplasma pneumoniae infection in China. World J Pediatr. 2024;20(1):1–4.
Bajantri B, Venkatram S, Diaz-Fuentes G. Mycoplasma pneumoniae: A Potentially Severe Infection. J Clin Med Res. 2018;10(7):535–44.
Association RGotPBotCM, Clinics EBotCJoPP. expert consensus on the diagnosis and management of Mycoplasma pneumoniae pneumonia in children. Chinese Journal of Practical Paediatrics. 2015;30(17):1304-8.
China NHCotPsRo, Medicine SAoC. diagnosis and treatment standard of community acquired pneumonia in Children (2019 Edition). Chin J Clin Infect Dis. 2019;12(1):6-13.
Wang L, Yang S, Yan X, Liu T, Feng Z, Li G. Comparing the yield of oropharyngeal swabs and sputum for detection of 11 common pathogens in hospitalized children with lower respiratory tract infection. Virol J. 2019;16(1):84.
Foy HM. Infections caused by Mycoplasma pneumoniae and possible carrier state in different populations of patients. Clin Infect Dis. 1993;17(Suppl 1):S37-46.
Charlotte Hsiung JC, Ma HY, Lu CY, Yen TY, Chi H, Liau YJ, Lai MJ, Chang LY, Huang LM. Children with Mycoplasma pneumoniae infection in Taiwan: Changes in molecular characteristics and clinical outcomes. J Formos Med Assoc. 2022;121(11):2273–80.
Carrim M, Wolter N, Benitez AJ, Tempia S, du Plessis M, Walaza S, Moosa F, Diaz MH, Wolff BJ, Treurnicht FK, et al. Epidemiology and Molecular Identification and Characterization of Mycoplasma pneumoniae, South Africa, 2012–2015. Emerg Infect Dis. 2018;24(3):506–13.
Gordon O, Oster Y, Michael-Gayego A, Marans RS, Averbuch D, Engelhard D, Moses AE, Nir-Paz R. The Clinical Presentation of Pediatric Mycoplasma pneumoniae Infections-A Single Center Cohort. Pediatr Infect Dis J. 2019;38(7):698–705.
Fan Q, Meng J, Li P, Liu Z, Sun Y, Yan P. Pathogenesis and association of Mycoplasma pneumoniae infection with cardiac and hepatic damage. Microbiol Immunol. 2015;59(7):375–80.
Kammer J, Ziesing S, Davila LA, Bultmann E, Illsinger S, Das AM, Haffner D, Hartmann H. Neurological Manifestations of Mycoplasma pneumoniae Infection in Hospitalized Children and Their Long-Term Follow-Up. Neuropediatrics. 2016;47(5):308–17.
Sondergaard MJ, Friis MB, Hansen DS, Jorgensen IM. Clinical manifestations in infants and children with Mycoplasma pneumoniae infection. PLoS One. 2018;13(4):e0195288.
Othman N, Isaacs D, Kesson A. Mycoplasma pneumoniae infections in Australian children. J Paediatr Child Health. 2005;41(12):671–6.
Shimizu T, Kida Y, Kuwano K. Lipid-associated membrane proteins of Mycoplasma fermentans and M. penetrans activate human immunodeficiency virus long-terminal repeats through Toll-like receptors. Immunology. 2004;113(1):121–9.
Huang JJ, Yang XQ, Zhuo ZQ, Yuan L. Clinical characteristics of plastic bronchitis in children: a retrospective analysis of 43 cases. Respir Res. 2022;23(1):51.
Jiang Y, Wang W, Zhang Z, Ma X, Sang Y, Wang J, Xu G, Feng Q, Zhao S. Serum amyloid a, C-reactive protein, and procalcitonin levels in children with Mycoplasma pneumoniae infection. J Clin Lab Anal. 2022;36(3):e24265.
Gao M, Wang K, Yang M, Meng F, Lu R, Zhuang H, Cheng G, Wang X. Transcriptome Analysis of Bronchoalveolar Lavage Fluid From Children With Mycoplasma pneumoniae Pneumonia Reveals Natural Killer and T Cell-Proliferation Responses. Front Immunol. 2018;9:1403.
National Research Project for Sars BG. The involvement of natural killer cells in the pathogenesis of severe acute respiratory syndrome. Am J Clin Pathol. 2004;121(4):507–11.
Kany S, Vollrath JT, Relja B. Cytokines in Inflammatory Disease. Int J Mol Sci. 2019;20(23):6008.
Hardy RD, Jafri HS, Olsen K, Wordemann M, Hatfield J, Rogers BB, Patel P, Duffy L, Cassell G, McCracken GH, et al. Elevated cytokine and chemokine levels and prolonged pulmonary airflow resistance in a murine Mycoplasma pneumoniae pneumonia model: a microbiologic, histologic, immunologic, and respiratory plethysmographic profile. Infect Immun. 2001;69(6):3869–76.
Lieberman D, Livnat S, Schlaeffer F, Porath A, Horowitz S, Levy R. IL-1beta and IL-6 in community-acquired pneumonia: bacteremic pneumococcal pneumonia versus Mycoplasma pneumoniae pneumonia. Infection. 1997;25(2):90–4.
Zhang Z, Dou H, Tu P, Shi D, Wei R, Wan R, Jia C, Ning L, Wang D, Li J, et al. Serum cytokine profiling reveals different immune response patterns during general and severe Mycoplasma pneumoniae pneumonia. Front Immunol. 2022;13:1088725.
Zhang Z, Wang H, Xie X, Chen R, Li J, Ni B, Yu P, Liu Z, Shao G, Xiong Q, et al. Long-Residence Pneumonia Vaccine Developed Using PEG-Grafted Hybrid Nanovesicles from Cell Membrane Fusion of Mycoplasma and IFN-gamma-Primed Macrophages. Small. 2021;17(34):e2101183.
Esposito S, Droghetti R, Bosis S, Claut L, Marchisio P, Principi N. Cytokine secretion in children with acute Mycoplasma pneumoniae infection and wheeze. Pediatr Pulmonol. 2002;34(2):122–7.
Narita M, Tanaka H, Yamada S, Abe S, Ariga T, Sakiyama Y. Significant role of interleukin-8 in pathogenesis of pulmonary disease due to Mycoplasma pneumoniae infection. Clin Diagn Lab Immunol. 2001;8(5):1028–30.
Cilloniz C, Torres A, Niederman MS. Management of pneumonia in critically ill patients. BMJ. 2021;375:e065871.
Wang K, Xi W, Yang D, Zheng Y, Zhang Y, Chen Y, Yan C, Tian G, An S, Li X, et al. Rhinovirus is associated with severe adult community-acquired pneumonia in China. J Thorac Dis. 2017;9(11):4502–11.
Song Q, Xu BP, Shen KL. Bacterial Co-infection in Hospitalized Children with Mycoplasma pneumoniae Pneumonia. Indian Pediatr. 2016;53(10):879–82.
Chiu CY, Chen CJ, Wong KS, Tsai MH, Chiu CH, Huang YC. Impact of bacterial and viral coinfection on mycoplasmal pneumonia in childhood community-acquired pneumonia. J Microbiol Immunol Infect. 2015;48(1):51–6.
Chidekel AS, Bazzy AR, Rosen CL. Rhinovirus infection associated with severe lower respiratory tract illness and worsening lung disease in infants with bronchopulmonary dysplasia. Pediatr Pulmonol. 1994;18(4):261–3.
Hartiala M, Lahti E, Forsstrom V, Vuorinen T, Ruuskanen O, Peltola V. Characteristics of Hospitalized Rhinovirus-Associated Community-Acquired Pneumonia in Children, Finland, 2003–2014. Front Med (Lausanne). 2019;6:235.
Acknowledgments
The study would not have been possible without the excellent support from clinical staff from the No.2 Respiratory Department at our hospital.
Funding
This study was supported by the Medical science research key project of Hebei province (20211225).
Author information
Authors and Affiliations
Contributions
L.W. and S.Y. wrote the manuscript drafts and performed formal analysis. S.K.L. helped interpret data. Y.H.G. aided analytical design. J.H.L. provide interpretation. W.J.L performed the acquisition of data. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This retrospective study received ethical approval from the institutional review board of ethics committee of the Children’s hospital of Hebei (CHH) (Approval No. 202101, official data March 4, 2021). The data access was also provided by CHH. The committee waived informed consent because the study was retrospective, there was no risk of harm to subjects, and all patients were anonymous.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yang, S., Lu, S., Guo, Y. et al. A comparative study of general and severe mycoplasma pneumoniae pneumonia in children. BMC Infect Dis 24, 449 (2024). https://doi.org/10.1186/s12879-024-09340-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09340-x