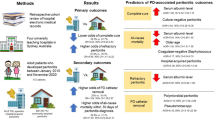
Abstract
The present study aimed to explore the pathogenic spectrum and risk factors of peritoneal dialysis-associated peritonitis (Peritoneal dialysis associated peritonitis, PDAP) in Yongzhou, Hunan, China. The clinical and epidemiological data on regular peritoneal dialysis (Peritoneal dialysis, PD) between January 2016 and December 2020 in Yongzhou were collected for retrospective analysis. The related factors of peritonitis were evaluated by single-factor analysis, while risk factors of refractory PDAP were evaluated by multivariate logistic regression analysis.172/331 172 (51.9%) patients developed peritonitis. The risk factors of PDAP in PD patients included high C-reactive protein (C-reactive protein, CRP), low albumin(Albumin, ALB), low hemoglobin (Hemoglobin, Hb), low educational level (junior high school or lower), preference of spicy food, irregular diet, low annual household income, unfavorable fluid exchange conditions, unstable employment (including working as a farmer), and unfavorable humidity conditions (P < 0.05). 63/172 (36.6%) PDAP patients were intractable cases with a pathogenic bacteria positive rate of 74.60% in the peritoneal dialysate cultures, and 109/172 patients were non-intractable cases with a pathogenic bacteria positive rate of 53.21%. Gram-positive bacteria (G+) were detected in most of the dialysate cultures, with Staphylococcus epidermidis (S. epidermidis) as the most common type, while Escherichia coli (E. coli) was the most common Gram-negative bacteria (G-). Gram-positive bacteria were sensitive to vancomycin and linezolid, while G- bacteria were sensitive to imipenem and amikacin. Lifestyle, educational level, and environmental factors are the major contributors to PDAP in PD patients. Fungal and multi-bacterial infections are the major causes of death; PD is stopped for such patients.
Similar content being viewed by others
Introduction
peritoneal dialysis is the most important alternative therapy for end-stage renal disease (ESRD) that protects remnant kidney function and can be easily administered at home [1, 2]. peritoneal dialysis-associated peritonitis can lead to peritoneal ultrafiltration and dialysis effects, thereby leading to complications that can result in PD failure and even death in patients [3,4,5].
peritoneal dialysis-associated peritonitis is defined according to Guidelines for the Prevention and Treatment of Peritoneal Dialysis Guidelines from International Association in 2022. After 5 days of appropriate antibiotic treatment, the PD fluid remains cloudy, or the white blood cell count in the permeated fluid continues to be > 0.1 × 109/L [6].
Several factors can induce PDAP, and its pathogenesis is complicated. Some studies showed have shown that hypoalbuminemia is common in the maintenance of PD patients [7, 8]. The incidence of peritonitis in PD patients with hypoalbuminemia is increased significantly [9,10,11,12]. Based on the results of multiple observational studies (Dialysis outcomes and practice patterns study, DOPPS) analysis, persistent low potassium is also a risk factor for peritonitis [13,14,15,16].
Due to the widespread use of antibiotics in clinical practice, pathogenic bacteria spectrum and drug resistance of PDAP in PD centers are constantly changing, the resistance of pathogens to antibiotics shows an increasing trend, and the proportion of refractory PDAP increases year by year. In 2010, the International Society for Peritoneal Dialysis (International society for peritoneal dialysis, ISPD) emphasized the adjustment of medication according to the pathogen profile and treatment experience of PDAP in each dialysis center [17]. Yonzhou, a city in the southern part of Hunan, China, consists mostly of mountainous areas with an underdeveloped economy and insufficient access to the transport network. For patients with uremia in Yongzhou, PD is the major alternative therapy because frequent visits to the hospital in a week would be difficult. Based on the specific geographical location, environment, economy, and living habits, the present study analyzed the epidemiological data, pathogenic spectrum, drug sensitivity, and prognosis of PDAP patients in Yongzhou and explored the risk factors of PDAP in PD patients to provide suggestions for the prevention and management of the disease and evidence for the formulation of regional prevention, treatment strategies, and guidelines.
Methods
Study design and participants
The epidemiological and clinical data of 331 patients on regular PD in Yongzhou were collected between January 2016 and December 2020. All data were analyzed retrospectively. According to the International Society for Peritoneal Dialysis (ISPD) guidelines (2016) [18], PDAP can be diagnosed based on at least two of three criteria: (1) symptoms and signs, such as abdominal pain, cloudy dialysate, and/or fever; (2) white blood cell (White blood cell, WBC) count of ≥ 100 × 106/L and multinucleated cell rate of ≥ 50% in the dialysate; (3) positive dialysate smear or culture. Intractable PDAP was diagnosed based on the criteria for PDAP and any one of the following: (1) no remission after five days of antibiotics and cloudy dialysate; (2) high WBC count after treatment; (3) fungal peritonitis; (4) complications with the PD catheter exit-site and tunnel infection; (5) new or recurrent peritonitis.
The inclusion criteria were as follows: (1) patients with > 3 months of experience in maintenance PD for uremia; (2) patients between 18- and 80-years-old; (3) patients on regular PD for at least 3 months; The exclusion criteria were as follows: (1) patients without complete clinical and follow-up records or participating in other clinical studies; (2) patients with fully recovered kidney function after PD; (3) patients producing bloody PD fluid; (4) patients producing chylous PD fluid; (5) patients with mental disorders and are unable to cooperate during treatment; (6) patients with comorbid acute or chronic blood system diseases; (7) patients with cerebrovascular accidents, such as cerebral infarction and hemorrhage; (8) patients with comorbid severe communicable diseases. Accordingly, all patients were assigned to the PDAP (Each patient with two or more PDAP cases was counted as one case, and the relevant data of the last peritonitis were selected) and non-PDAP groups. The PDAP group was further subdivided into intractable and non-intractable PDAP groups. This study was approved by the Ethics Committee of Yongzhou Central Hospital, and all patients signed the informed consent (No. 2,022,071,301).
Sample collection
In sterile conditions, a volume of 10 mL dialysate (retained in the abdominal cavity for at least 4 h) was collected and placed in a blood culture bottle for testing using the Bact/ALERT 3D automated microbial detection system (Biomérieux, France). Then, the dialysate in the positive bottle was smeared and seeded on the Columbia blood agar and chocolate blood agar. The quality control bacteria included E. coli, (ATCC 25,922), Staphylococcus aureus (S. aureus, ATCC 25,923), and Pseudomonas aeruginosa (ATCC 27,853). The drug susceptibility test was conducted according to the method described by the Clinical and Laboratory Standards Institute (CLSI) (2016), using BACTEC™ 9000 (Becton Dickinson, USA), sterile paper discs (Oxiod, UK), and E-test plastic strips (Autobio, Zhengzhou, China).
Cure and withdrawal criteria
According to ISPD guidelines, the cure criteria were as follows: (1) complete remission of PDAP signs and symptoms; (2) dialysate WBC count of < 100 × 106/L and multinucleated cell rate < 50%, two consecutive negative cultures; (3) no recurrence after discontinuation of antibiotics. The withdrawal criteria [18, 19] were as follows: no alleviation within 2–3 weeks of antibacterial therapy, fungal peritonitis, intractable catheter exit-site and tunnel infection, multiple infections leading to PD catheter withdrawal, switching to hemodialysis, or death.
Statistical analysis
SPSS 23.0 (IBM Corp.) was used for the data analysis. The enumeration data were expressed in percentage and ratio and compared using chi-square test. The measurement data were expressed as mean ± standard deviation (SD). Normally distributed data were subjected to t-test or one-way analysis of variance (ANOVA). For non-normal distribution and uneven variance, the data were subjected to rank-sum test. Factors in the univariate analysis were incorporated into the logistic regression model. P < 0.05 was considered statistically significant. Whonet 5.6 was used for the drug susceptibility test.
Results
Pathogenic spectrum
Among the 172 patients with PDAP, 63 (36.6%) were intractable cases. The pathogenic bacteria-positive rate in the PD cultures was 74.60% (47 cases). Gram-positive bacteria were detected in 24 cases (38.10%), among which S. epidermidis accounted for the highest proportion (9 cases, 14.29%). Gram-negative bacteria were detected in 18 cases (28.57%), and E. coli accounted for the highest proportion (8 cases, 12.70%). Furthermore, 2 (3.17%) cases presented multiple infections, and 3 (4.76%) had fungal infections. Moreover, 109 (63.37%) patients developed non-intractable PDAP. The peritoneal dialysate cultures of these patients presented a pathogenic bacteria-positive rate of 53.21% (58 cases). Among these cases, 32 (29.36%) were positive for G+, with 8 (7.34%) cases positive for S. epidermidis, while 26 (23.85%) were positive for G- with 8 (7.34%) cases positive for E. coli (Table 1).
The drug resistance test results were similar for both intractable and non-intractable PDAP. Gram-positive bacteria detected from patients in both groups were sensitive to vancomycin and linezolid, but had a high resistance rate to penicillin G, oxacillin, clindamycin, cephazolin, and levofloxacin. Furthermore, G- were sensitive to imipenem and amikacin, but exhibited a high drug resistance rate for ceftazidime, gentamicin, ampicillin/sulbactam, piperacillin/tazobactam, and levofloxacin. Among the three fungal infection cases, one case was sensitive to all drugs, including flucytosine, fluconazole, voriconazole, amphotericin B, and itraconazole, while the other two cases were resistant to fluconazole, flucytosine, and voriconazole but were sensitive to the remaining drugs (Table 2).
Analysis of risk factors for PDAP
The univariate analysis revealed that dialysis time, blood Hb, blood CRP, blood albumin (ALB), lactic dehydrogenase (Lactic dehydrogenase, LDH) in dialysate, occupation, educational level, income, diet preference, diet regularity, sanitation of fluid exchange conditions, and humidity, had a statistically significant impact on the occurrence of PDAP (P < 0.05, Tables 3 and 4). Furthermore, multivariate analysis revealed that high blood CRP [odds ratio (OR) = 12.354, 95% confidence interval (CI): 1.351–42.873)], low blood ALB (OR = 0.937, 95% CI 0.850–0.984), low blood Hb (OR = 0.924, 95% CI: 0.819–0.973), low educational level (junior high school or lower) (OR = 5.181, 95% CI: 1.514–15.379), preference of spicy food (OR = 4.563, 95% CI: 1.473–12.819), irregular diet (OR = 5.018, 95% CI: 1.419–11.328), low annual household income (OR = 4.133, 95% CI: 1.378–9.572), unfavorable fluid exchange conditions (OR = 3.572, 95% CI: 1.311–7.458), unstable employment (including working as a farmer) (OR = 4.933, 95% CI: 1.152–8.583), and unfavorable humidity conditions as, too high (OR = 2.951, 95%CI 1.257 ∼ 6.782) or too low (OR = 3.970, 95% CI: 1.182–5.637) were risk factors for PDAP in PD patients (P < 0.05, Table 5).
Outcomes of patients
Among the 63 patients with intractable PDAP, 45 were cured, and 18 stopped the PD (17 patients were transferred to hemodialysis after catheter removal, and one patient died). Patients with fungal and multiple infections had a poor prognosis. Among the 109 patients with non-intractable PDAP, 99 were cured, and 10 stopped the PD (all patients transferred to hemodialysis after catheter removal). Among the 159 patients who did not develop peritonitis, 6 patients stopped the PD (5 patients were transferred to hemodialysis after catheter removal, one patient received a kidney transplant), and no death occurred (Supplementary Table 1).
Discussion
In the present study, PDAP occurred in 172/331 included patients, with an incidence (51.96%) significantly higher than that reported previously [20]. The findings indicated that PDAP should be under intensive focus and that other risk factors should also be explored to improve its prevention and treatment.
The study showed that the CRP levels in PDAP patients was significantly higher than the control group, which may serve as a predictive biomarker for the occurrence of peritonitis. C-reactive protein, a major indicator of inflammation, can activate the complement system to produce immune complexes, thereby damaging the endangium and indirectly affecting the pro-inflammatory factors. High CRP levels can be used to independently predict the severity of intractable peritonitis, which is consistent with previous findings [21,22,23]. Blood ALB is a critical parameter in evaluating nutritional status. Hypoalbuminemia is a common pathological condition of PD patients and is closely correlated to the host’s immune and inflammatory scenario. This can weaken a patient’s response and increase the risk of infection. Furthermore, hypoalbuminemia has been identified as a risk factor for the early occurrence of PDAP [24], which is consistent with the present findings, wherein low blood ALB was identified as a risk factor of PDAP and an independent risk factor for intractable PDAP. hemoglobin is also an indicator of nutrition in PD patients. In uremia patients, a decrease in hemopoietin can reduce hemopoiesis and cause renal anemia. Also, poor dietary intake would decrease the supply of nutrients for Hb synthesis, further aggravating anemia. Moreover, the pro-inflammatory factors are activated in the microinflammatory environment, thereby weakening host immunity and triggering inflammation. The present study revealed that a low Hb level is a risk factor for PDAP and an independent risk factor for intractable PDAP. Thus, the nutritional status should be monitored in PD patients to improve their condition and reduce the risk of PDAP [25,26,27].
In addition, a low education level (junior high school or lower), preference for spicy food, irregular diet, low annual household income, unfavorable fluid exchange conditions, unstable employment (including working as a farmer), and unfavorable humidity conditions (too high or too low) were risk factors of PDAP. These factors may represent the geological, climatic, educational, and lifestyle conditions in Yongzhou. An epidemiological investigation in Yongzhou identified the following features. First, Yongzhou is located in the south of Hunan, China, which is surrounded by mountains on three sides and occupied by low hills. This region has a mid-subtropical continental and monsoonal climate, which causes high humidity indoors, favoring the proliferation of bacteria. Second, Yongzhou is an underdeveloped region with a population that mainly comprises rural farmers who lack health education and bacteria-controlling knowledge. Third, the population in Yongzhou likes spicy and pickled foods. The long-term intake of these foods can distort the gut microinflammatory environment, and their irregular diet can also disrupt gastrointestinal function and may trigger peritonitis. Fourth, due to low household income, patients are often lost to follow-up, which delays the treatment of PDAP. Fifth, most of the patients raise poultry in their courtyards, which might pollute the fluid exchange environment. Based on these five factors, it can be inferred that doctors, patient’s family members, and local governments need to cooperate and propose a plan for the prevention and treatment of PDAP. The strategy should encompass health education, hygiene training, follow-up, fluid-exchange standardization, healthy diet, insurance coverage, environmental protection, and individualized therapy.
In the present study, the positive rate for intractable-PDAP-related bacteria was 74.60%, while the positive rate for non-intractable-PDAP-related bacteria was 53.21%; however, both were lower than that reported previously (78% and 81.52%, respectively) [28, 29]. This phenomenon could be explained by repeated infection or previous use of antibiotics before culture [30]. Furthermore, G + were the dominant pathogenic bacteria, which is in agreement with previous findings [31, 32]. The G + bacteria were represented by S. epidermidis, a coagulase-negative Staphylococcus that habitats on the skin. Its infection can induce the recurrence of PDAP. In addition, the high infection rate in the present study was consistent with that reported by previous studies [33, 34], indicating that the incorrect operation of fluid exchange remains the main factor for PDAP. G- bacteria were represented by E. coli, suggesting that PDAP is correlated with intestinal infection. Also, its infection rate is lower than that of S. epidermidis [35,36,37], indicating that PDAP is closely correlated with dirty food, constipation, diarrhea, and chronic enteritis.
According to ISPD guidelines [10], PD centers should use antibiotics with an antibacterial spectrum that covers G + and G- bacteria. The selection of drugs should be based on bacterial distribution and resistance. In the present study, the G + bacteria in PDAP patients were sensitive to vancomycin and linezolid, but resistant to cefazolin, while the G- bacteria were sensitive to imipenem and amikacin, but resistant to ceftazidime and gentamicin. Therefore, for PDAP patients in Yongzhou, vancomycin (or linezolid) and imipenem (or amikacin) were recommended before reporting the chemosensitivity assay results or after the completion of the bacterial culture.
With the widespread use of antimicrobial drugs, there has been a gradual increase in the prevalence of resistant strains. The initial treatment regimen adopted by our institution during this period consisted of first-generation cephalosporins (cefazolin) combined with third-generation cephalosporins (cefotaxime), resulting in relatively high rates of resistance to these antibiotics. Recent studies [38,39,40] have shown an upward trend in resistance rates to commonly used antibiotics such as cefazolin, ampicillin, and cefotaxime. Additionally, research indicates that Gram-positive pathogens may develop resistance to almost all clinically available antimicrobial drugs, making them more likely to produce multidrug-resistant (MDR) strains compared to Gram-negative bacteria [41]. According to the 2022 ISPD guidelines [42], antibiotic selection should be based on the specific circumstances of the healthcare institution and should cover both Gram-positive and Gram-negative bacteria. For Gram-positive bacterial infections, first-generation cephalosporins or vancomycin are recommended, while for Gram-negative bacterial infections, third-generation cephalosporins or aminoglycosides are preferred. It is imperative for us to adhere to, but not blindly follow, the ISPD guidelines, and regularly assess the spectrum of hospital pathogens and initial treatment regimens to minimize the emergence of resistant strains.
In the present study, two cases with multiple infection were tested for G-, which may have originated from enteral infection. The treatment had poor efficacy, and the infection recurred repeatedly. Finally, PD was replaced by hemodialysis. Three cases presented fungal infection, which included two cases of Torulopsis glabrata (T. glabrata) and one case of Candida Krusei. The case infected with T. glabrata was switched to antifungal therapy but died. This finding suggested that fungal infections or multiple infections are the main causes of death or withdrawal from PD. For these patients, studies recommended that the catheter should be withdrawn earlier [43, 44]. Moreover, the peritoneal function should be retained, and systemic infection should be prevented.
For refractory peritonitis, we need to identify relevant factors and administer targeted treatments for the underlying causes. Multidrug resistance poses a high risk for refractory and recurrent peritonitis. Refractory peritonitis is a major reason for peritoneal dialysis (PD) patients withdrawal, potentially leading to residual infections affecting PD re-initiation and reducing technique survival rates [45]. Recurrent peritonitis increases the risk of further recurrence and relapse. Given the high prevalence of multidrug-resistant infections in peritoneal dialysis-associated peritonitis (PDAP) patients, it is crucial to promptly identify and control their risk factors, minimize the occurrence of multidrug infections, and initiate appropriate antibiotic therapy based on pathogen distribution and resistance characteristics. This is essential for reducing adverse clinical outcomes such as refractory and recurrent peritonitis.
Conclusions
In summary, This study retrospectively analyzed the clinical and epidemiological data of peritoneal dialysis (PD) patients in Yongzhou City from January 2016 to December 2020.Ultimately, it was found that for PDAP patients, G + bacteria were sensitive to vancomycin and linezolid, while G- bacteria were sensitive to imipenem and amikacin. Lifestyle, educational level, and environmental factors are the major contributors to PDAP in PD patients. Fungal and multi-bacterial infections are the major causes of death; PD is stopped for such patients.
Study limitation
The present retrospective study was based on the data obtained from a single center in Yongzhou, thus lacking data from multiple centers. Presently, the investigators are establishing a PD database of patients from other areas. A multicenter and prospective study would be performed based on this database to provide evidence for PDAP prevention and treatment.
Data availability
All data generated or used during the study are available from the corresponding author by request.
References
National Kidney Foundation. NKF-DOQI clinical practice guidelines for peritoneal dialysis adequacy [J]. Am J Kidney Dis. 1997;30(3 Suppl 2):S67–136.
Andy Tang SO, Carolisna YI, Sakura D, et al. Demo-graphic characteristics and outcomes of continuous ambulatory peritoneal dialysis related peritonitis in Miri General Hospital. Malaysia [J] Med J Malaysia. 2019;74(4):270–4.
Lapic I, Padoan A, Bozzato D, et al. Erythrocyte sedimenta-tion rate and C -reactive protein in acute inflammation [J]. Am J Clin Pathol. 2020;153(1):14–29.
Wu H, Ye H, Huang R, et al. Incidence and risk factors of peritoneal dialysis-related peritonitis in elderly patients: a retro-spective clinical study[J]. Perit Dial Int. 2020;40(1):26–33.
Wang HH, Huang CH, Kuo MC et al. Microbiology of peritoneal dialysis -related infection and factors of intractable peritoneal di -alysis related peritonitis: a ten -year single-center study inTaiwan [J]J microbiol Immunol Infect,2019,52 (5):752–9.
Li PK, Chow KM, Cho Y, et al. ISPD peritonitis guideline recommendations: 2022 update on prevention and treatment[J]. Perit Dial Int. 2022;42(2):110–53.
Davenport A. Peritonitis remains the major clinical complication of peritoneal dialysis: the London, UK, peritonitis audit 2002 ⁃ 2003[J]. Perit Dial Int. 2009;29(3):297–302.
Zha Y, Qian Q. Protein nutrition and malnutrition in CKD and ESRD[J]. Nutrients. 2017;9(3):208.
Boudville N, Kemp A, Clayton P, et al. Recent peritonitis associates with mortality among patients treated with peritoneal dialysis[J]. J Am Soc Nephrol. 2012;23(8):1398–405.
Ozturk S, Soyluk O, Karakaya D, et al. Is decline in serum albumin an ominous sign for subsequent peritonitis in peritoneal dialysis patients?[J]. Adv Perit Dial. 2009;25:172–7.
Tian Y, Xie X, Xiang S, et al. Risk factors and outcomes of early ⁃ onset peritonitis in Chinese peritoneal dialysis patients[J]. Kidney Blood Press Res. 2017;42(6):1266–76.
Tian Y, Xie X, Xiang S et al. Risk factors and outcomes of high peritonitis rate in continuous ambulatory peritoneal dialysis patients: a retrospective study[J]. Med (Baltimore) 2016, 95(49): e5569.
Davies SJ, Zhao J, Morgenstern H, et al. Low serum potassium levels and clinical outcomes in peritoneal dialysis⁃International results from PDOPPS[J]. Kidney Int Rep. 2021;6(2):313–24.
Chuang YW, Shu KH, Yu TM, et al. Hypokalaemia: an independent risk factor of Enterobacteriaceae peritonitis in CAPD patients[J]. Nephrol Dial Transpl. 2009;24(5):1603–8.
Ribeiro SC, Figueiredo AE, Barretti P, et al. Low serum potassium levels increase the infectious ⁃ caused mortality in peritoneal dialysis patients: a propensity matched score study[J]. PLoS ONE. 2015;10(6):e0127453.
Liu D, Lin Y, Gong N, et al. Degree and duration of hypokalemia associated with peritonitis in patients undergoing peritoneal dialysis[J]. Int J Clin Pract. 2021;75(8):e14188.
Li PK, Szeto CC, Piraino B et al. Peritoneal dialysis related infections recommendations:2010 update[J]Perit Dial Int,2010,30(4):393–423.
Li PK, Szeto CC, Piraino B, et al. ISPD peritonitis recommendations:2016 update on prevention and treatment. Perit Dial Int. 2016;36(5):481–508.
Hibi,Arata et al. Kasugai. Peritoneal dialysis-associated catheter infection caused by Mycobacterium abscessus in an elderly patient who was successfully treated with catheter removal.[J].CEN Case Rep,2017.
Yılmaz. Fatih,Bora Peritoneal Dialysis Related Peritonitis by Sphingomonas Paucimobilis.[J].Ther Apher Dial,2017.
Amanathan K, Padmanabhan G. V i jayaraghavan B.Evaluation of continuous ambulatory peritoneal dialysis fluid C-reactire protein in patients with peritonitis[J]. Saudi J Kidney Dis Transpl 2016, 27(3) : 467–72.
Zhu YQ, Chen J, Ying LJ, et al. Pathogenic bacteria,antibiotic resistance and prognosis of peritonitis in peritoneal dialysis patients[J]. J Clin Nephrol. 2018;32(5):198–203.
Xiao-Jing L, Ling-Ju Y, Yong-Quan L et al. Clinical analysis of peritoneal dialysis-related peritonitis in a single center. 2017, 47(3):354–8.
Wu H, Huang R, Yi C, et al. Risk factors for early-onset Peritonitis in Southern Chinese Peritoneal Dialysis Patients[J]. Perit Dial Int. 2016;36(6):640–6.
Sukackiene,Diana et al. Rimsevicius. A case of successfully treated relapsing peritoneal dialysis-associated peritonitis caused by Gordonia bronchialis in a farmer.[J].Nephrol Ther,2017.
Ma,Ying. Wang Predictors of Short-term Outcomes of Patients with Peritoneal Dialysis-associated Peritonitis.[J].Zhongguo Yi Xue Ke Xue Yuan Xue Bao,2018,40(1):13–20.
Sarihan. Irem,Demir Serratia marcescens, Morganella morganii, Klebsiella oxytoca related peritonitis attacks in a patient on automated peritoneal dialysis: a case report.[J].Nefrologia,2017.
Shen SJ, Guan JC, Wang SM. Analysis of microbial spectrum and antibiotic resistance in 129 cases patients of peritoneal dialysis related peritonitis[J]. Chin J Gen Pract. 2018;35(5):389–95.
Chen CM, Nephrology DO. Analysis and research of type and tolerance of 34 cases with peritoneal Dialysis-related Peritonitis pathogenic Bacteria[J]. Volume 29. China & Foreign Medical Treatment; 2017. pp. 673–9. 4.
,Chang Jae Hyun,Oh Jieun, Park Sue K et al. Frequent patient retraining at home reduces the risks of peritoneal dialysis-related infections:a randomised study.[J].Scientific reports,2018,8(1).
BOUDVILLE N, JOHNSON D W, ZHAO J, et al. Regional Varia-Tion in the treatment and prevention of peritoneal dialysis-related infections in the peritoneal dialysis outcomes and practice patterns study[J]. Nephrol Dial Transpl. 2019;34(12):2118–26.
Yang J, Zhang WW, Wan C, et al. Analysis of antibiotic resistance in pathogenic bacteria from 91 episodes of peritoneal dialysis related peritonitis and its correlation to the prognosis of peritonitis[J]. Chin J Blood Purif. 2013;42(04):1202–6.
Li T, Ping S, Hu Q, et al. Analysis of pathogens and prognosis in the elderly patients with peritoneal dialysis-related peritonitis[J]. Chin J Kidney Disease Investigation(Electronic Edition). 2018;45(4):377–80.
Auguste BL, Girsberger M, Kennedy C, et al. Are adverse events in newly trained home dialysis patients related to learning styles? A single-centre retrospective study from Toronto, Canada]〕]. BMJ Open. 2020;10(1):e033315.
Hu S, Tong R, Bo Y, et al. Fungal peritonitis in peritoneal dialy- sis: 5-year review from a North China center [ J]. Infection. 2018;47(1):35–43.
Morrisette T, Canada RB, Padgeitt D, et al. Factors associated with increased hospital length of stay in peritoneal dialysis patients with peritonitis : a need for antimicrobial stewardship? [J]. Hosp Pharm. 2020;55(1):50–7.
Whitty. Rachel,Bargman Residual kidney function and Peritoneal Dialysis-Associated Peritonitis Treatment outcomes.[J].Clin J Am Soc Nephrol,2017.
Kitterer D, Latus J, Pöhlmann C et al. Microbiological Surveillance of peritoneal dialysis associated peritonitis: susceptibility profiles of a referral center in Germany over 32 years[J].PLoS One,2015,10(9): e0135969.
Zeng Y, Jiang L, Lu Y, et al. Peritoneal dialysis-related Peritonitis caused by gram-negative organisms:ten-years experience in a single center[J]. Ren Fail. 2021;43(1):993–1003.
Cho Y, Struijk DG. Peritoneal dialysis–related peritonitis: atypical and resistant organisms[J]. Semin Nephrol. 2017;37(1):66–76.
Karaman R, Jubeh B, Breijyeh Z. Resistance of gram-positive bacteria to current antibacterial agents and overcoming approaches [J]. Molecules. 2020;25(12):2888.
Li P, K,CHOW K M,CHO, Y. al.ISPD peritonitis guideline recommendations: 2022 update on prevention and treatment[J]. Perit Dial Int. 2022;42(2):110–53.
Li et al. Qing,Zheng. A pathogenetic role for M1 macrophages in peritoneal dialysis-associated fibrosis.[J].Mol Immunol,2018,94:131–139.
Yang L, Gong N, Jiang J, et al. Pathogenic bacteria and prognostic risk factors of intractable peritoneal dialysis related peritonitis[J]. J Practical Med. 2019;40(4):620–6.
Wang HH, Huang CH, Kuo MC, et al. Microbiology of peritoneal dialysis-related infection and factors of refractory peritoneal dialysis related peritonitis: a ten-year single-center study in Taiwan[J]. J Microbiol Immunol Infect. 2019;52(5):752–9.
Acknowledgements
We appreciate those who participated in the study for their cooperation and help.
Funding
The study was not supported by funding.
Author information
Authors and Affiliations
Contributions
LS Y and JW W wrote the main manuscript text and prepared all tables.BG Z and F Z collected data.LS Y, JW W and BG Z completed statistical analysis.All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Central Hospital of Yongzhou of China (Approval No. 2022071301) and all patients signed the informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
You, L., Zhang, B., Zhang, F. et al. Pathogenic spectrum and risk factors of peritoneal dialysis-associated peritonitis: a single-center retrospective study. BMC Infect Dis 24, 440 (2024). https://doi.org/10.1186/s12879-024-09334-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09334-9