Abstract
Background
Immunodeficient patients, particularly HIV patients, are at risk of opportunistic infections. Nontuberculous mycobacteria can cause severe complications in immunodeficient patients.
Case Presentation
We describe a 57-year-old HIV patient, primarily presented with coughs and constitutional symptoms, with a unique Mycobacterium genavense abdominal, pulmonary, and central nervous system infection, accompanied by intracranial masses.
Conclusion
The diagnosis of NTM, including M. genavense, must always be considered by clinicians in immunodeficient patients, especially those with HIV, who have a compromised immune system.
Similar content being viewed by others
Background
Nontuberculous mycobacterial (NTM) infections are a major concern for HIV-infected patients. Their main habitats in the environment are water sources and dust [1] and they can infect multiple organs in the host [2]. Mycobacterium genavense (M. genavense) is reported to be responsible for more than 10% of disseminated NTM infections [3, 4]. Abdominal organs, including lymph nodes, liver, spleen and gastrointestinal tracts are the main targets for M. genavense infection [5]. Despite the improvements in survival by antiretroviral therapies (ART) in the recent years, the prognosis of this infection remains poor [5] due to the long treatment periods and high prevalence of side effects, plus non-specific diagnostic and treatment tools [6,7,8].
The disseminated presentation of the infection has been repeatedly reported across the world; however, involvement of the central nervous system (CNS) is rarely observed. In this paper, we will discuss a case admitted to our center and review the published literature to investigate the diagnostic means of this unique form of infection.
Case presentation
Our patient is a 57-year-old male, with a four-month history of HIV infection (CD4 count = 10/µL, HIV viral load > 3,000,000 copies/ml) and cytomegalovirus (CMV) retinitis, who complained of worsening fatigue and nausea for two months. He also complained of progressive unintentional weight loss (over 30 kg in this period) and a productive cough. No vision or sensory symptoms was reported. Additionally, there was no evidence of fever, loss of consciousness, or cognitive-behavioral demonstration; however, he had given a history of disrupted gait. Although, the neurological (i.e., finger-to-nose, heel-to-shin, and limb forces) and meningitis tests (i.e., neck stiffness, Brudzinski’s and Kernig’s signs) were normal. This could be related to the severe generalized weakness, as no neurological deficit was identified. He was on a drug regimen consisting of Truvada® (emtricitabine 200 mg– tenofovir disoproxil 300 mg) q24h, dolutegravir 50 mg q24h, and valganciclovir 450 mg q12h and was compliant with treatment. Laboratory results showed a pancytopenia (WBC = 1,500/µL, Hb = 6 g/dL, and platelets = 125,000/µL) and elevated CRP level of 92 mg/L. Lung CT-scan showed a 9 mm×6 mm nodule in the right middle lobe and a tree-in-bud pattern at the lower levels of the left lung (Fig. 1). Abdominal ultrasound investigation revealed an enlarged spleen (15.5 cm), multiple enlarged paraaortic lymph nodes, and a 7 mm lymph node in the porta hepatis.
A cerebrospinal fluid (CSF) analysis indicated a decreased glucose level (24 mg/dL) as well as a normal protein level of 28 mg/dL and a WBC count of 0–1/L. Acid fast bacilli (AFB) staining in both sputum and CSF was positive. Viral, namely CMV and varicella zoster virus, and fungal diagnostic tests on the CSF specimen for probable agents were negative. No pathological finding in brain MRI was observed at this stage. Considering Mycobacterium tuberculosis (MTB) infection, we started an anti-MTB empirical treatment, consisting of isoniazid, pyrazinamide, ethambutol, and rifampin (liver function tests were normal). Due to the pharmacokinetic interactions of rifampin and dolutegravir [9], we increased the dose of dolutegravir to 50 mg q12 h. No MTB growth was observed in the culture and GeneXpert® molecular tests of sputum and CSF were negative for MTB, raising the probability of NTM infection. Due to low blood cell counts, lymphadenopathy, and splenomegaly, we performed a simultaneous bone marrow biopsy and observed foamy histiocytes and NTM presence (AFB staining positive and GeneXpert® negative) (Fig. 2). Clarithromycin was added to the previous regimen, for NTM infection coverage. At this stage, the results of previously-requested NTM polymerase chain reaction (PCR) analysis from the CSF specimen detected M. genavense presence (complete match to hsp65 gene) (Fig. 3). Considering the NTM infection in the respiratory system and bone marrow, and M. genavense meningitis, we made a disseminated M. genavense infection diagnosis. The patient was discharged with NTM combinational drug treatment (five-drug) accompanied by ART and valganciclovir as his medical condition was stable.
Pathology sections of bone marrow biopsy specimen; Low magnification of bone marrow biopsy shows replacement of hematopoietic elements by numerous foamy macrophages (circled area) arranged in sheet (H&E section, x40 & x100). Higher magnification reveals histiocytes (circled area) containing abundant organisms (H&E section x400). Frequent positive acid-fast bacilli (white arrows) were present in foamy macrophages on Ziehl-Nielsen stain (x400)
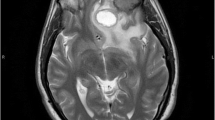
Four months later he presented with generalized fatigue and anorexia, while claiming he had not consumed the NTM prescription appropriately; however, he was compliant with ART and valganciclovir. There were no abnormal findings in the examinations. CD4 count was 13/µL and lung CT scan prevailed that the tree-in-bud pattern vanished but the nodule was present with no significant size change. According to his previous history, we requested a brain MRI, which showed a right hemispherical mass in the corpus callosum with mass effects on the ventricle, accompanied by edema and two lesions in both hemispheres of the cerebellum (Fig. 4). The patient did not consent for a cerebral biopsy to investigate potential diagnoses, such as toxoplasmosis and malignancies. However, considering the previously confirmed presence of M. genavense in CNS and poor Anti-NTM regimen compliance, the intracranial masses were most likely formed in the background of disseminated M. genavense infection. Anti-NTM drug combinations (ethambutol 1,200 mg q24h, rifampin 600 mg q24h, clarithromycin 500 mg q12h, levofloxacin 750 mg q24h, and amikacin 1,000 mg q24h) were initiated in conjunction with ART, valganciclovir, and prophylactic trimethoprim-sulfamethoxazole. We started dexamethasone eight mg q12h for perilesional edema. The next MRI within one week showed that the edema had regressed and reduced cerebral mass size, favoring the diagnosis. After the treatment, his clinical progression was desirable and he was discharged with the same prescriptions as of admission (except for amikacin, which was discontinued due to a rise in serum creatinine up to 1.8 mg/dL, and then improved before discharge to baseline by discontinuing the drug and hydrating). Dexamethasone was replaced by prednisolone, which was tapered gradually over the following weeks. Three months later, his symptoms were relieved, his drug compliance was complete, and he was clinically stable (Fig. 5).
Brain MRI reveals a right sided mass (white arrowheads) in the corpus callosum with perilesional edema (white arrows) and mass effects on the ventricle, and two lesions (white arrowheads) in the hemispheres of cerebellum accompanied by edema (white arrows). [Left to right: T1 with gadolinium contrast, T2, and FLAIR views]
Discussion and conclusions
M. genavense is one of the most common causes of mycobacterial infections in avians, especially parrots [10]. Colonization of M. genavense in the human body is common and almost always does not result in disease; however, a case of disseminated infection has recently been published in a previously healthy pet keeper, hypothesizing the zoonotic transmission probability [11]. As a widespread family, NTM could be isolated from various specimens due to colonization or specimen contamination [12, 13]. M. genavense has been detected more frequently in HIV patients [5, 12]. Despite this fact, multiple reports have discussed M. genavense infection in non-HIV immunodeficiencies, namely sarcoidosis, solid organ recipients, and primary immunodeficiencies [14,15,16,17]. Diagnostic and treatment challenges are the most significant challenges in disease management.
As well as constitutional symptoms, M. genavense commonly manifests symptoms involving the gastrointestinal and abdominal organs, including abdominal pain, hepatosplenomegaly, and lymphadenopathy [3, 5]. Thomsen et al. have hypothesized that more frequent abdominal manifestations might be a result of the presence of the microorganism in the GI tract of the infected [18]. Only a few cases with M. genavense CNS involvement have been reported worldwide. After a systematic literature review in PubMed, Embase, and Web of Science online databases with ‘Genavense’ AND ‘HIV’ keywords, we have identified seven cases with background immunodeficiency plus CNS M. genavense infection. Five cases were HIV infected [19,20,21,22,23] and the remaining two were primary immunodeficiency cases (including a case of hypogammaglobulinemia [24] and an Adenosine Deaminase deficiency patient with a history of gastrointestinal M. genavense infection [25]). Table 1 provides the main clinical and laboratory characteristics of HIV cases with M. genavense CNS involvement.
Our case is the sixth reported HIV case with M. genavense CNS involvement. Previously, the microorganism was isolated from the CSF of two cases [20, 23], and one developed a cerebral mass without other organ involvement [23]. Other three cases suffered from intracranial masses, but their CSF were not examined [19, 21, 22]. One of these patients had simultaneous lung nodule and pleural involvement [22], while the authors of the remaining studies did not address further involvement [19, 21]. The present case is a composite abdominal, pulmonary, and CNS infection caused by M. genavense, accompanied by intracranial lesions, which is unique. Disseminated M. genavense diagnosis was highly plausible in this case due to the synchronicity of M. genavense isolation from CNS, and pulmonary and bone marrow NTM involvement. Also, due to the history of CNS involvement and poor drug compliance, the intracranial masses are likely to be related to the M. genavense background; However further measures would be mandatory in the case of clinical course reverse or probable drug resistance. Interestingly, no neurological abnormality was present in physical examination in the second admission, similar to three of the previous cases [21,22,23]. This may originate from the chronic and insidious and chronic clinical progression of the NTM [26, 27]. Imaging and pathology beside molecular tests play a key role in M. genavense infection confirmation. However, the diagnosis should be highly concerned when NTM infection is present (when culture and TB molecular tests are inconclusive) in an immune-compromised patient. Microbiology tests must rely on molecular assessments [28], as the time to identification in fortified growth media could be as long as 91 days or more [14, 20, 29].
Recent introduction of ART has improved overall survival significantly; however, M. genavense could be lethal [5]. Treatment of the disease remains controversial. A recent individual patient meta-analysis concluded that macrolides might be related to lower fatalities; and other agents, such as amikacin, have no significant association with survival [5]. It has been shown that azithromycin, ethambutol, and rifampicin combination may be effective for M. genavense lung disease [28].
CNS infection treatment knowledge is restricted to previous experiences. Kuczynski et al. and Berman et al. initiated corticosteroid and an anti-NTM regimen without mass resection and the patients were stable after nine [23] and twelve months [19]. The Belgian case died roughly two weeks after treatment (clarithromycin, ethambutol, rifabutin, moxifloxacin, and amikacin) due to unsteady hemodynamics and consciousness [20]. Toussi et al. performed lesion resection due to high pre- and intraoperative malignancy suspicion and no follow-up was provided [21]. Another case presented with pulmonary involvement of M. genavense with an incidental, asymptomatic intracranial mass. The patient underwent surgical lesion excision; nevertheless, his condition worsened and he died due to respiratory failure [22]. Our patient was primarily diagnosed with M. genavense meningitis with no pathologic findings on imaging. However, he developed multiple intracranial masses after months. Medication noncompliance might be a reason for this progression, and one reason is the high quantity of medications [30, 31]. The NTM infection has a poor prognosis, with a mortality rate estimated at 32–39.3% among HIV patients [5, 12], which illustrates the pathogen’s invasiveness and ineffective treatment methods. A recent systematic review of NTM CNS infections reported a 37.5% case fatality rate; although this review did not include any M. genavense cases [32].
Clinicians must always consider the diagnosis of NTM, including M. genavense, in immunodeficient patients, especially those with HIV. The outcomes remain unfavorable despite ART and antibiotic developments. Due to the low prevalence of the disease, no consensus management of CNS involvement is available. As we have reviewed, invasive CNS treatment must be decided according to medical status due to possible lack of effectiveness. Macrolides, ethambutol, and rifamycins might improve disseminated infection outcomes and should be considered first-line treatment. Further multicenter prospective studies might identify poor outcomes predictors.
Data availability
Not applicable.
Abbreviations
- NTM:
-
Nontuberculous Mycobacterium
- ART:
-
Antiretroviral Therapy
- CNS:
-
Central Nervous System
- CMV:
-
Cytomegalovirus
- AFB:
-
Acid Fast Bacilli
- MTB:
-
Mycobacterium tuberculosis
- CSF:
-
Cerebrospinal Fluid
- PCR:
-
Polymerase Chain Reaction
References
Honda JR, Virdi R, Chan ED. Global Environmental Nontuberculous Mycobacteria and Their Contemporaneous Man-Made and Natural Niches. Front Microbiol 2018, 9:2029.
Sharma SK, Upadhyay V. Epidemiology, diagnosis & treatment of non-tuberculous mycobacterial diseases. Indian J Med Res. 2020;152(3):185–226.
Charles P, Lortholary O, Dechartres A, Doustdar F, Viard JP, Lecuit M, Gutierrez MC. Mycobacterium genavense infections: a retrospective multicenter study in France, 1996–2007. Med (Baltim). 2011;90(4):223–30.
Pechère M, Opravil M, Wald A, Chave JP, Bessesen M, Sievers A, Hein R, von Overbeck J, Clark RA, Tortoli E, et al. Clinical and epidemiologic features of infection with Mycobacterium genavense. Swiss HIV Cohort Study. Arch Intern Med. 1995;155(4):400–4.
Wetzstein N, Kessel J, Bingold TM, Carney J, Graf C, Koch BF, Meier F, Baumgarten J, Küpper-Tetzel CP, Khodamoradi Y, et al. High overall mortality of Mycobacterium genavense infections and impact of antimycobacterial therapy: systematic review and individual patient data meta-analysis. J Infect. 2022;84(1):8–16.
Varley CD, Streifel AC, Bair AM, Winthrop KL. Nontuberculous Mycobacterial Pulmonary Disease in the immunocompromised host. Clin Chest Med. 2023;44(4):829–38.
Joao I, Bujdáková H, Jordao L. Opportunist coinfections by Nontuberculous Mycobacteria and Fungi in Immunocompromised patients. Antibiot (Basel) 2020, 9(11).
Gopalaswamy R, Shanmugam S, Mondal R, Subbian S. Of tuberculosis and non-tuberculous mycobacterial infections - a comparative analysis of epidemiology, diagnosis and treatment. J Biomed Sci. 2020;27(1):74.
Dooley KE, Sayre P, Borland J, Purdy E, Chen S, Song I, Peppercorn A, Everts S, Piscitelli S, Flexner C. Safety, tolerability, and pharmacokinetics of the HIV integrase inhibitor dolutegravir given twice daily with rifampin or once daily with rifabutin: results of a phase 1 study among healthy subjects. J Acquir Immune Defic Syndr. 2013;62(1):21–7.
Palmieri C, Roy P, Dhillon AS, Shivaprasad HL. Avian mycobacteriosis in Psittacines: a retrospective study of 123 cases. J Comp Pathol. 2013;148(2–3):126–38.
Trauth J, Discher T, Fritzenwanker M, Imirzalioglu C, Arnold T, Steiner D, Richter E, Crisponi L, Grimbacher B, Herold S. Hodgkin Lymphoma after disseminated Mycobacterium genavense infection, Germany. Emerg Infect Dis. 2022;28(7):1506–9.
Ruas R, Abreu I, Nuak J, Ramos A, Carvalho T, Ribeiro M, Guimarães J, Sarmento A. Nontuberculous mycobacteria in a tertiary hospital in Portugal: a clinical review. Int J Mycobacteriology. 2017;6(4):344–8.
Schwenkenbecher P, Neyazi A, Donnerstag F, Ringshausen FC, Jacobs R, Stoll M, Kirschner P, Länger FP, Valizada E, Gingele S et al. Chronic granulomatous disease first diagnosed in adulthood presenting with spinal cord infection. Frontiers in Immunology 2018, 9(JUN).
Mahmood M, Ajmal S, Abu Saleh OM, Bryson A, Marcelin JR, Wilson JW. Mycobacterium genavense infections in non-HIV immunocompromised hosts: a systematic review. Infect Dis (Lond). 2018;50(5):329–39.
Denicolò S, Laydevant S, Fink J, Geiger C, Pizzini A, Sarcletti M, Zschocke J, Bellmann-Weiler R, Weiss G, Tancevski I. Sarcoid-like lesions obfuscating the diagnosis of disseminated Mycobacterium genavense infection in a patient with IL-12Rβ1-associated immunodeficiency. BMC Infect Dis. 2022;22(1):770.
Chen J, Nguyen M, Cheong E, Sean Riminton D, Reddel S. Refractory Mycobacterium genavense infection secondary to thymoma-associated endogenous IL-12 inhibitor. BMJ Neurol Open. 2022;4(1):e000285.
Baldolli A, Chocron R, Dargère S, Michon J, Daurel C, Thuillier-Lecouf A, Verdon R. Mycobacterium genavense infections in immunocompromised patients without HIV: Case Series of Solid Organ Transplant patients and Literature Review. Open Forum Infect Dis. 2022;9(10):ofac498.
Thomsen VO, Dragsted UB, Bauer J, Fuursted K, Lundgren J. Disseminated infection with Mycobacterium genavense: a challenge to physicians and mycobacteriologists. J Clin Microbiol. 1999;37(12):3901–5.
Berman SM, Kim RC, Haghighat D, Mulligan ME, Fierer J, Wyle FC. Mycobacterium genavense infection presenting as a solitary brain mass in a patient with AIDS: case report and review. Clin Infect Dis. 1994;19(6):1152–4.
Kyrilli A, Payen MC, Antoine-Moussiaux T, Dewit S, Clumeck N. Meningitis and splenic infarction due to disseminated Mycobacterium genavense infection in an HIV patient. Case report and review of the literature. Acta Clin Belg. 2013;68(3):220–2.
Toussi A, Goodarzi A, Kulubya E, Lee DJ, Waldau B. Mycobacterium Genavense Granuloma mimicking a brain tumor: a Case Report. Cureus. 2017;9(8):e1547.
Vazquez E, Nicita D, Masini D, Matteo M, Costa N, Franze O, Trione N, Corti M. Mycobacterium genavense: a rare cause of cerebral mass lesion. Neurologia Argentina 2022.
Kuczynski AM, Krajden S, Spears J, Kus JV, Munoz DG, Ostrowski M, Chen Y, Supala-Berger A. Mycobacterium genavense Central Nervous System infection in a patient with AIDS. Can J Neurol Sci. 2023;50(2):305–7.
Uchino H, Terasaka S, Yamaguchi S, Kobayashi H, Kawai K, Kubota K, Ooe S, Houkin K. [Multiple infectious intracranial lesions of Mycobacterium genavense in an immunocompromised patient]. Brain Nerve. 2011;63(1):79–83.
Grunebaum E, Reid B, Naqvi A, Hershfield MS, Kim VH, Muller MP, Hicks LK, Lee E, Betschel S, Roifman CM. Morbidity in an adenosine deaminase-deficient patient during 27 years of enzyme replacement therapy. Clin Immunol. 2020;211:108321.
Winthrop KL, Chang E, Yamashita S, Iademarco MF, LoBue PA. Nontuberculous mycobacteria infections and anti-tumor necrosis factor-alpha therapy. Emerg Infect Dis. 2009;15(10):1556–61.
Ratnatunga CN, Lutzky VP, Kupz A, Doolan DL, Reid DW, Field M, Bell SC, Thomson RM, Miles JJ. The rise of Non-tuberculosis Mycobacterial Lung Disease. Front Immunol. 2020;11:303.
Lange C, Böttger EC, Cambau E, Griffith DE, Guglielmetti L, van Ingen J, Knight SL, Marras TK, Olivier KN, Santin M, et al. Consensus management recommendations for less common non-tuberculous mycobacterial pulmonary diseases. Lancet Infect Dis. 2022;22(7):e178–90.
Wilson ML, Stone BL, Hildred MV, Reves RR. Prolonged incubation of blood and bone marrow cultures in 12B bottles processed on the BACTEC 460 TB system does not increase microbial recovery. Diagn Microbiol Infect Dis. 1996;25(3):113–5.
Alsayed SSR, Gunosewoyo H. Tuberculosis: Pathogenesis, current treatment regimens and new drug targets. Int J Mol Sci 2023, 24(6).
Chiang CY, Centis R, Migliori GB. Drug-resistant tuberculosis: past, present, future. Respirology. 2010;15(3):413–32.
Meena DS, Kumar D, Meena V, Bohra GK, Tak V, Garg MK. Epidemiology, clinical presentation, and predictors of outcome in nontuberculous mycobacterial central nervous system infection: a systematic review. Trop Med Health. 2023;51(1):54.
Acknowledgements
Not applicable.
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
AH and MA drafted the primary manuscript. AH, MH, LA, SG, FA, and MA revised the manuscript. MH, LA, and SG were involved in the clinical management and follow-up of the patient. FA re-examined histopathology and reported the findings.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Informed consent was obtained from the patient. Imam Khomeini Hospital Complex Ethical Committee has approved this report under the code IR.TUMS.IKHC.REC.1402.276.
Consent for publication
Written informed consent was obtained from the patient for publication of case report and accompanying images.
Competing interests
The authors declare that they have no competing interests" in this section.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hassanzadeh, A., Hasannezhad, M., Abbasian, L. et al. Disseminated mycobacterium genavense infection with central nervous system involvement in an HIV patient: a case report and literature review. BMC Infect Dis 24, 437 (2024). https://doi.org/10.1186/s12879-024-09316-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09316-x