Abstract
The Xpert MTB/RIF test (Xpert) can help in the accurate screening of tuberculosis, however, its widespread use is limited by its high cost and lack of accessibility. Pooling of sputum samples for testing is a strategy to cut expenses and enhance population coverage but may result in a decrease in detection sensitivity due to the dilution of Mycobacterium tuberculosis (Mtb) by sample mixing. We investigated how the mixing ratio affected the detection performance of Xpert. We used frozen sputum samples that had been kept after individual Xpert assays of the sputa from Mtb-confirmed TB patients and non-TB patients. Our results showed that the overall sensitivity of the Xpert pooling assay remained higher than 80% when the mixing ratio was between 1/2 and 1/8. When the mixing ratio was raised to 1/16, the positive detection rate fell to 69.0%. For patients with either a high sputum Mtb smear score ≥ 2+, a time-to-positive culture ≤ 10 days, or an Xpert test indicating a high or medium abundance of bacteria, the pooling assay positivity rates were 93.3%, 96.8%, and 100% respectively, even at a 1/16 mixing ratio. For participants with cavities and cough, the pooling assay positivity rates were 86.2% and 90.0% at a 1/8 ratio, higher than for those without these signs. Our results show that the Xpert pooled assay has a high overall sensitivity, especially for highly infectious patients. This pooling strategy with lower reagent and labor costs could support TB screening in communities with limited resources, thereby facilitating reductions in the community transmission and incidence of TB worldwide.
Similar content being viewed by others
Introduction
Tuberculosis (TB), a global epidemic caused by Mycobacterium tuberculosis (Mtb), is a leading cause of illness and death worldwide [1]. Although the World Health Organization (WHO) launched its ambitious “END TB” plan about a decade ago, progress has been sluggish in reducing TB-related mortality and morbidity. This is partly due to the fact that up to 30–40% of TB patients are undiagnosed [1, 2]. Individuals without typical clinical signs and symptoms or those without access to healthcare, especially in resource-limited communities, are more likely to experience underdiagnosis and might spread the disease among households and communities, preventing decreases in TB-related illness and deaths.
Actively screening and treating these concealed cases is an ideal strategy to eliminate TB. And yet, due to the lack of suitable screening techniques, screening for TB in large populations is highly challenging. Symptom screens and chest X-ray radiography (CXR) are the most used screening methods [3], but they have limited specificity in predicting TB, and do not directly reflect infectivity. Xpert MTB/RIF test (Xpert), a WHO-recommended rapid diagnostic test for TB, typically exhibits higher accuracy (sensitivity plus specificity) than symptom screens and chest X-rays [4]. To date, however, the value of Xpert for TB screening in large populations remains limited, primarily due to (1) the high cost of Xpert cartridges for a single test, (2) the low cost-effectiveness due to the fact that the majority of tests return negative results, and (3) the time- and labor-intensity due to insufficient lab technicians and instruments. These shortcomings are particularly evident in areas where resources are limited, but where extensive or continuous monitoring is required.
Testing of pooled sputum samples is a feasible cost-saving strategy to facilitate TB screening of large populations [5]. Usually, sputa from several individuals are mixed into a pool for testing, and a negative test result can rule out TB infection. If the result is positive, all samples are retested separately to determine which patients are TB-positive. By combining samples, this assay’s benefits include increased testing efficiency but, more importantly, reduced testing costs. The most noticeable disadvantage is the possibility of a decrease in detection sensitivity due to the dilution of Mtb by sample mixing. A mixing ratio that is too high may significantly impair Xpert’s sensitivity due to TB’s typical paucibacillary nature, thus limiting its value for TB screening in a large population.
To investigate the effect of pool size on the sensitivity of the Xpert assay, we mixed sputum samples from bacteriologically confirmed TB patients with sputum samples from non-TB patients in different ratios (from 1/2 to 1/16) for the Xpert assay. The effects of the dilution ratio on the overall rate of detection and the influence of estimated Mtb loads were assessed.
Methods
Sample collection
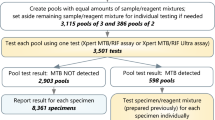
All participants included in this study were outpatients with presumptive TB who underwent Xpert testing between July and October 2022. We collected 3–5 mL of spot sputum from each recruited patient, added reagents equal to the volume of sputum, and tested with the Gene Xpert MTB/RIF kit according to the manufacturer’s instructions. Whether the Xpert test was positive or negative, the samples with a remaining volume of more than 2 mL were frozen at -80 °C and included in the study. Patients were allocated into an Xpert-positive group (TB group) and an Xpert-negative group (non-TB group) based on the Xpert results. Patients in the non-TB group were only included after a rigorous clinical evaluation that included CXR, sputum culture and smear, and responsiveness to antituberculosis chemotherapy. The samples from the non-TB group were thawed and mixed to a pool (non-TB samples pool, NSP). The demographics and diagnostic information of TB patients were collected. Shenzhen Third People’s Hospital’s Ethics Committee approved this study (No. 2022-008).
Pooling assay
Samples were thawed on the day of the assay. The NSP was used to serially dilute the Xpert-positive samples. A 1/2 ratio mixed sample was obtained by adding 1 mL NSP to 1 mL Xpert-positive sputum, then two-fold dilutions of this to 1/4, 1/8, and 1/16 were made in NSP. The dilutions were stirred with a vortex mixer and then added to the Xpert assay cartridge (Fig. 1).
Statistical analysis
Following the manufacturer’s guidance, the Mtb load of the samples was defined as high, medium, low, and very low when the CT values of Xpert were < 18, 18–22, 22–28, and > 28, respectively. The positivity rate of the Xpert pooling assay was calculated variously after including all patients, patients with or without cavities and cough, with diverse Xpert and smear grades, and time-to-positive (TTP) of cultures. These data were visualized by GraphPad 8.0.2.
Results
Residual sputum samples were collected from 87 TB patients who had positive Xpert tests (Table 1) and 281 non-TB patients (Table S1). Of these, 74.7% were male, 55.2% were middle-aged people (between the ages of 30 and 60), and 98.9% were without HIV infection. The CXR of these individuals indicated that 74.7% had cavities in their lungs. In addition, 80.5% of participants had a significant cough. The proportions with high, medium, low, and very low Mtb loads defined by the CT values of Xpert were 19.5%, 31.0%, 30.0%, and 19.5%, respectively. All samples in the group with a high Mtb load had a smear grade of 2+ or higher, as compared to 26.9% and 23.6% in the low and very low groups, respectively. In the high and medium groups, mycobacterial cultures were 100% positive, while in the low and very low groups, they were 77.0% and 76.5% positive, respectively.
The overall positive rate of the pooling test was 82.8% when the mixing ratio was less than 1/8. When the mixing ratio was raised to 1/16, the positive detection rate fell to 69.0% (Fig. 2a). Positive rates of the pooling tests were higher in patients with cavities or cough than in those without, with positive rates of 86.2% (with cavities) and 90.0% (with cough) at a mixed 1/8 ratio, respectively (Fig. 2b and c). For patients with medium or high bacterial load indicated by Xpert, the pooling assays yielded 100% positivity even at a 1/16 mixing ratio (Fig. 2d). In sputum samples with a smear score of 2+ or higher, the pooling assays were 97.7% positive at 1/8 mixture and 93.3% positive at 1/16 mixture (Fig. 2e). When the TTP of culture was less than 10 days, the positive rate of the mixed test was greater than 96.8% at 1/16 mixture (Fig. 2f).
Positivity rates of Xpert pooling assays for the diagnosis of tuberculosis. (a) All patients. (b) Patients with (n = 18) or without cavitation (n = 65 ). (c) Patients with (n = 70) or without cough (n = 16). (d) Patients with very low (n = 17), low (n = 26), medium (n = 17), and high (27) Mtb load defined by the CT values from Xpert. (e) Patients with negative (n = 26), 1+ (n = 10), 2+ (n = 10), 3+ (n = 9), and 4+ (n = 26) grade of smear. (f) Patients with < 5 (n = 8), 5–10 (n = 24), 10–20 (n = 27), 20–42 (n = 15), > 42 (n = 7) time-to-positive culture (TTP).
Discussion
Our research shows that the overall sensitivity of the Xpert assay remained higher than 80% when the mixing ratio was between 1/2 and 1/8, which is comparable to the positive rates of several earlier studies of sputum sample pooling (89.1–91.8%) [5,6,7]. More notably, we observed that patients with high sputum Mtb load (smear ≥ 2+, TTP ≤ 10 days, and Xpert medium or high), cough, or cavities had a higher positive rate by the Xpert pooling assay, even at a 1/16 mix ratio. This implies that at certain mixing ratios, Xpert maintains a high level of sensitivity while effectively screens out almost all patients who are considered to be at high risk of transmission. When resources are limited, it may be more cost-effective to prioritize pooled screening to find and treat highly infectious cases in order to reduce the community transmission of tuberculosis; the public health value of Xpert pooling assay in a large population could be very substantial. The lower reagent and labor costs being incurred, particularly when positive results are infrequent may permit such screening to be repeated regularly for timely detection of TB in the community.
The Xpert assay has a low limit of detection (LOD) at 131 cfu/mL. This is significantly better than 10,000 cfu/mL for smears and very close to the LOD for solid culture [4]. Xpert, therefore, maintains a comparatively high sensitivity even after the sample has been diluted. However, the use of the Xpert pooling assay to detect patients who have low Mtb load (Xpert low or very low and smear less than 1+) remains a problem due to inadequate sensitivity in this population, as demonstrated here. None of the samples with a very low Mtb load tested positive when sputum samples were mixed at a ratio of 1/16. Even with a 1/4 mixing ratio, only 53.0% of samples were positive. This inadequate sensitivity at low loads is consistent with Lao’s finding that pooled tests identified 40% of TB patients (2/5) with very low Mtb loads at a 1/4 mixing ratio [6]. The next-generation version of Xpert, Xpert MTB/RIF Ultra (Xpert Ultra), has improved sensitivity (LOD 15.6 cfu/mL [8], close to that of liquid culture); in the pooling strategy it could give results 100% consistent with individual detection. The use of the Xpert Ultra may offset the reduction of sensitivity caused by pooling samples with very low Mtb loads, but the increased cost per sample may make the Xpert Ultra pooling test less attractive to countries with limited resources. The balance between sensitivity and cost of testing by pooling assay should be carefully assessed alongside the prevalent TB characteristics before the screening of large populations begins.
There are several limitations to this study. First, this is merely a lab simulation of a population-based pooling test in which negative and positive sputum samples are simply mixed in various ratios. Our study’s findings, however, may help to guide the selection of mixing ratios based on estimates of the proportion of infectious cases in the community. Second, we only focused on how different mixing ratios affected the precision of the mixed test in our study. The time and money to be saved by mixed testing also depend on the level of TB prevailing at the screening location, with smaller mixing ratios at higher prevalence rates being more cost- and time-effective than larger mixing ratios [9].
In conclusion, our results show that the Xpert pooled assay has high overall sensitivity, especially for highly infectious patients. This pooling strategy with lower cost and labor consumption could support TB screening in communities with limited resources, thereby reducing the community transmission and incidence of TB worldwide.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- Mtb :
-
Mycobacterium tuberculosis
- TB:
-
Tuberculosis
- WHO:
-
The World Health Organization
- CXR:
-
Chest X-ray radiography
- Xpert:
-
Xpert MTB/RIF test
- NSP:
-
Non-TB samples pool
- TTP:
-
Time-to-positive
- LOD:
-
Low limit of detection
- Xpert Ultra:
-
Xpert MTB/RIF Ultra
References
WHO. Global tuberculosis report 2022. Geneva: World Health Organization. In.; 2022.
WHO. Global tuberculosis report 2021. Geneva: World Health Organization. In.; 2021.
WHO Guidelines Approved by the Guidelines Review Committee. In: WHO consolidated guidelines on tuberculosis: Module 2: screening– systematic screening for tuberculosis disease. edn. Geneva: World Health Organization© World Health Organization 2021.; 2021.
Lawn SD, Mwaba P, Bates M, Piatek A, Alexander H, Marais BJ, Cuevas LE, McHugh TD, Zijenah L, Kapata N, et al. Advances in Tuberculosis diagnostics: the Xpert MTB/RIF assay and future prospects for a point-of-care test. Lancet Infect Dis. 2013;13(4):349–61.
Abdurrahman ST, Mbanaso O, Lawson L, Oladimeji O, Blakiston M, Obasanya J, Dacombe R, Adams ER, Emenyonu N, Sahu S, et al. Testing pooled sputum with Xpert MTB/RIF for diagnosis of Pulmonary Tuberculosis to increase affordability in low-income countries. J Clin Microbiol. 2015;53(8):2502–8.
Iem V, Chittamany P, Suthepmany S, Siphanthong S, Siphanthong P, Somphavong S, Kontogianni K, Dodd J, Khan JA, Dominguez J et al. Pooled testing of sputum with Xpert MTB/RIF and Xpert Ultra during tuberculosis active case finding campaigns in Lao People’s Democratic Republic. BMJ Glob Health 2022, 7(2).
Zishiri V, Chihota V, McCarthy K, Charalambous S, Churchyard GJ, Hoffmann CJ. Pooling sputum from multiple individuals for Xpert® MTB/RIF testing: a strategy for screening high-risk populations. Int J Tuberc Lung Dis. 2015;19(1):87–90.
Opota O, Mazza-Stalder J, Greub G, Jaton K. The rapid molecular test Xpert MTB/RIF ultra: towards improved tuberculosis diagnosis and rifampicin resistance detection. Clin Microbiol Infect. 2019;25(11):1370–6.
Dos Santos PCP, da Silva Santos A, de Oliveira RD, da Silva BO, Soares TR, Martinez L, Verma R, Andrews JR, Croda J. Pooling Sputum samples for efficient Mass tuberculosis screening in prisons. Clin Infect Dis. 2022;74(12):2115–21.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the Special Fund for Science and Technology Innovation of Guangdong (No. 2020B1111170014), the Shenzhen Fund for Guangdong Provincial High-level Clinical Key Specialties (No. SZGSP010), Shenzhen Natural Science Foundation (NO. JCYJ20220530163212027), the Shenzhen Clinical Research Center for Tuberculosis (No. 20210617141509001), and the Special Fund Of Shenzhen Central-leading-local Scientific and Technological Foundation (No. LCYX20220620105200001).
Author information
Authors and Affiliations
Contributions
JFZ, XHL, HML, and SHL conceived and designed the experiments. HH wrote the paper. JFZ, HH, ZH, WJL, and HML acquired, analyzed and interpreted the data. XHL and SHL gave overall supervision, and critical comments, and reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by Shenzhen Third People’s Hospital’s Ethics Committee (No. 2022-008). Informed consent was obtained from all patients or their legal guardians which were involved in this study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zeng, J., Huang, H., Liu, X. et al. Pooling sputum samples for the Xpert MTB/RIF assay: a practical screening strategy for highly infectious tuberculosis cases. BMC Infect Dis 24, 122 (2024). https://doi.org/10.1186/s12879-024-09020-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-024-09020-w