Abstract
Background
The aim of this study was to evaluate the role of Xpert MTB/RIF assay in the detection of Mycobacterium tuberculosis for differentiating tuberculosis intrathoracic lymphadenopathy from sarcoidosis intrathoracic lymphadenopathy.
Methods
The patients who were suspected to having sarcoidosis or tuberculosis intrathoracic lymphadenopathy at the Shanghai Pulmonary Hospital between October 1, 2020 and June 30, 2021 were retrospectively evaluated in this study. All patients underwent endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) and Xpert analysis. Differences in clinical and radiological features were recorded. The diagnostic performances of EBUS-TBNA Xpert, acid-fast bacilli, culture, and peripheral blood QuantiFERON-TB Gold (QFT) for differentiating sarcoidosis from tuberculosis intrathoracic lymphadenopathy were analyzed.
Results
A total of 119 patients were included in this analysis. Of those, 83 patients were finally diagnosed with sarcoidosis (N = 50) and tuberculosis (N = 33) intrathoracic lymphadenopathy. Young individuals were more likely to have tuberculosis versus sarcoidosis intrathoracic lymphadenopathy (P = 0.006). Markers of inflammation, including fever, leukocytes, and serum ferritin levels, were significantly higher in tuberculosis versus sarcoidosis intrathoracic lymphadenopathy (P < 0.01). Bilateral lung involvement and symmetry intrathoracic lymphadenopathy were more common in sarcoidosis intrathoracic lymphadenopathy (P < 0.01). In addition, the longest diameter of intrathoracic lymphadenopathy (in cm) was significantly larger in sarcoidosis intrathoracic lymphadenopathy (P = 0.001). However, the largest diameter of lung lesions was significantly shorter (P = 0.005). The sensitivity and specificity values of Xpert and QFT for differentiating these two diseases were 69.70% and 100%, and 96.43% and 91.84%, respectively.
Conclusion
Xpert MTB/RIF is recommended for the diagnosis of tuberculosis intrathoracic lymphadenopathy using EBUS-TBNA samples. A negative QFT suggests the exclusion of the diagnosis of tuberculosis intrathoracic lymphadenopathy.
Similar content being viewed by others
Introduction
Tuberculosis (TB), is a major public health problem worldwide, particularly in China. This common chronic infectious disease is caused by the Mycobacterium tuberculosis (MTB) complex. TB typically invades the lungs and affects other organs of the human body (extrapulmonary TB). Lymph nodes are the most common site of extrapulmonary TB [1]; intrathoracic lymph nodes, including mediastinal and hilar lymph nodes, are particularly affected, resulting in TB intrathoracic lymphadenopathy (TBIL). In adults, TBIL may be an isolated finding or associated with lung infiltration.
Sarcoidosis (SA) is a multisystem autoimmune disease, which primarily affects the lungs. The most common extrapulmonary organs involved in this disease are the skin, lymph nodes (LN), eyes, and liver [2]. Sarcoidosis intrathoracic lymphadenopathy (SAIL), including Scadding stages I and II, is the most common form of sarcoidosis intrathoracic lymphadenopathy. The etiology of sarcoidosis is currently unknown. MTB was previously considered to be the cause of sarcoidosis. However, recent studies have suggested that mycobacterial antigens are not the pathogenic cause of sarcoidosis [3, 4]. Therefore, finding the evidence of TB infection is the key for distinguishing these two diseases. Owing to their similar clinical and radiological profiles, as well as the same granulomatous pathological features when there was no caseous necrosis, it is difficult to distinguish sarcoidosis from TB. Moreover, extrapulmonary TB samples carry a lower MTB load than respiratory samples. This difference affects the sensitivity of conventional methods in diagnosing TB, including smear microscopy, culture, or cytology.
Mycobacterial culture remains the gold standard for the diagnosis of active TB. However, this approach requires 2–4 weeks to yield results, and its sensitivity for lymphatic TB is poor [5]. In 2013, the World Health Organization recommended the use of Xpert MTB/resistance to rifampin (Xpert MTB/RIF, referred to as Xpert) for some types of extrapulmonary TB, such as TB lymphadenitis and TB meningitis [6]. The cost of this method is similar to that of MTB culture; however, it offers the advantages of a short cycle, faster process, automation. A meta-analysis analyzed 18 studies involving 4,461 samples; they found that, in lymph node tissues or aspirates, the pooled sensitivity of Xpert versus culture was 83.1% (95% confidence interval: 71.4–90.7%) [7]. Moreover, it can be used for differentiating tuberculosis from sarcoidosis [8].
In the present study, we aimed to evaluate the role of Xpert in the detection of MTB for differentiating TBIL from SAIL. To achieve our objective, we used fresh specimens obtained through endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA, referred to as EBUS). Moreover, we compared the clinical features of the two diseases to detect differences that could assist us in reaching a definitive diagnosis.
Methods
Study design
All patients (n = 119) suspected to having SAIL or TBIL during the initial inpatient period at Shanghai Pulmonary Hospital (Shanghai, China) between October 1, 2020 and June 30, 2021 were retrospectively evaluated and followed-up for > 6 months. The last follow-up was performed on February 1, 2022. All patients underwent EBUS-TBNA from the mediastinal, hilar, and interlobar LN to obtain tissue specimens for Xpert analysis.
Inclusion and exclusion criteria
The inclusion criteria were: (1) age 18–80 years; (2) suspected SAIL or TBIL with or without lung infiltration during the initial inpatient period diagnosed by two specialist physicians and two radiologists; (3) final diagnosis of sarcoidosis and TB during the follow-up period; (4) having underwent EBUS-TBNA and Xpert examinations; (5) negative sputum acid-fast bacilli (AFB) smear prior to EBUS; and 5) no treatment with anti-TB drugs, glucocorticoids, or immunosuppressive agents for > 1 month prior to EBUS.
The exclusion criteria were: (1) presence with any malignancy; (2) presence of fungal or other infections detected through histopathological or microbiological analysis; (3) lack of a correct definitive diagnosis during the follow-up period; and (4) lost to follow-up.
The medical records of all patients, including demographic data (sex, age, and race), medical history (symptoms, presence of comorbidities, diagnostic test, and treatment outcome), laboratory results, and the features of chest high-resolution computed tomography (HRCT) were collected.
Endobronchial ultrasound-guided transbronchial needle aspiration and ultrasonography procedure
EBUS-TBNA was performed using a dedicated bronchoscope with a linear ultrasound transducer (BF-UC260F-OL8; Olympus, Tokyo, Japan). The target LN were punctured with a 21-gauge needle (NA-201SX-4022; Olympus, Tokyo, Japan). All patients provided written informed consent before undergoing the bronchoscopy. Two or more punctures were performed on each target lymph node to obtain at least two tissue core specimens. One of those specimens was prepared for histological examination, while the other specimens were utilized for AFB, Xpert, and MGIT960 culture. Ultrasonography was conducted with model HDI 5000, 7–12 MHz (Philips Medical Systems, Bothell, WA, USA) to evaluate the sizes (in cm) of superficial LN by measuring the largest diameters. Nodal masses with size > 5 mm were identified as bulky lesions. The size of LN was assessed by measuring the largest and smallest diameters on the ultrasound screen, and the long axis/short axis (L/S) ratio was calculated.
Xpert MTB/RIF procedure
The rpoB gene is the target of Xpert. The Xpert assay was performed according to the instructions provided by the manufacturer (Cepheid, Sunnyvale, CA, USA). The sample reagent was added to ≥ 0.5 mL of decontaminated specimen at a 3:1 ratio. After shaking, the mixture was incubated for 15 min at room temperature. Thereafter, part of the mixture (2 mL) was transferred to the Xpert test cartridge. The semiquantitative results of the Xpert were obtained based on the cycle threshold in each sample using the Xpert software within 2 h.
Diagnosis of TBIL and SAIL
TBIL
The diagnosis of TBIL was based on the presence of MTB in all samples. Biopsies performed using pathological tissues showed granulomatous reaction with caseation necrosis or multinucleated giant cells associated with epithelioid histiocytes. Possible (based on clinical, imaging, and histological assessments in this study) TB according to the European Centre for Disease Prevention and Control [9] when the symptoms improved, the size of LN was decreased, and/or the lung infiltration resolved after the standard anti-TB treatment is also considered as TBIL.
SAIL
The diagnosis of mediastinal sarcoidosis was reached according to the official American thoracic society clinical practice guideline [10]. Histological specimens showed non-caseous necrotizing granulomas without other known causes of granulomatosis (evidence of TB, fungi, etc.). Stage I (mediastinal or hilar lymphadenopathy) and stage II (lymphadenopathy accompanied by pulmonary infiltrations) were included.
Statistical analysis
The mean ± standard deviation was used for measurement data, while data with skewed distribution were described using the interquartile range. For continuous variables, the t-test was used to determine mean differences between groups. Non-normally distributed data were analyzed by the Mann–Whitney U test. For categorical variables, differences were assessed using the chi-squared or Fisher’s exact test. Two-tailed P-values < 0.05 indicate statistically significant differences. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of various diagnostic methods were calculated. All statistical analyses were performed using IBM SPSS statistics version 23.0 (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
Of the 119 patients analyzed, 83 were eventually diagnosed with SAIL (N = 50) and TBIL (N = 33) (Fig. 1). All patients underwent EBUS-TBNA and Xpert analysis. We found that young individuals were more likely to have TBIL than SAIL (Table 1).
In addition, a lower proportion of women had TBIL compared with SAIL (N = 16, 48.48% vs. N = 38, 76%; P = 0.01). The body mass index was lower in patients with TBIL versus SAIL. Chest distress (P = 0.001) and dyspnea (P = 0.010) more often occurred in patients with SAIL, while fever (P = 0.002) was more frequently observed in those with TBIL. There were no differences detected in other symptoms (Supplementary Table 1). Markers of inflammation, including serum amyloid A, leukocytes, erythrocyte sedimentation rate, C-reactive protein levels, and serum ferritin concentration, were higher in TBIL versus SAIL, particularly leukocytes and serum ferritin (P < 0.01). Although the eosinophil ratio was significant higher in SAIL versus TBIL, only seven patients exhibited values above the normal. The patients with SAIL had higher serum angiotensin levels than those with TBIL. Extrathoracic involvement was more often observed in SAIL compared with TBIL (29 vs. 9, respectively; P = 0.006) (Supplementary Table 1). There were no significant differences in comorbidities (emphysema, coronary heart disease, hypertension, and diabetes) between SAIL and TBIL (P = 0.283) (Supplementary Table 1).
Imaging features
We found that most patients with intrathoracic lymphadenopathy had concurrent pulmonary infiltration. Notably, 92% (46/50) of SAIL patients and 87.88% (29/33) TBIL patients exhibited lung involvement (P = 1.000). Moreover, patients with SAIL were at a higher risk of infiltration into bilateral lungs versus those with TBIL (90.91% vs. 37.93%, respectively; P < 0.01). The largest diameter of lung lesions was smaller in patients with SAIL versus TBIL (P < 0.01) (Table 2).
A significantly larger number of stations of intrathoracic lymphadenopathy (e.g., 2R/L, 4R/L, 5, 6, 7, 8, 9, 10R/L, and 11R/L) involved per patient were detected in SAIL versus TBIL (P < 0.01). Similarly, the LN were more likely to show symmetry in patients with SAIL versus TBIL (P < 0.01). TBIL is mostly asymmetric, and the largest involved sites were the right paratracheal (2R [N = 6] and 4R [N = 7]) and subcarinal lymph (station 7 [N = 14]) (Fig. 2). Station 7 (N = 46) was the largest site involved in SAIL (P < 0.01). The longest diameter of intrathoracic lymphadenopathy (measured in cm) of SAIL was significantly larger than that of TBIL. Nonetheless, patients with TBIL had significantly higher lymph node calcification than those with SAIL. Stations 4R and 7 were the most common sites of lymph node puncture in these two diseases.
Eleven and six patients with SAIL and TBIL, respectively, underwent positron emission tomography–computed tomography (PETCT). Among patients with SAIL, seven (63.64%), two, and two were diagnosed with sarcoidosis, infection disease, and malignant tumor, respectively. Among patients with TBIL, two and four patients were diagnosed with TB and malignant tumor, respectively.
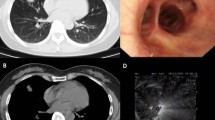
HRCT and PETCT features of SAIL and TBIL. (a and b) HRCT showing miliary nodules in both the bronchovascular bundle and interlobular septa before (a) and 6 months after (b) treatment of a male patient with prednisone. (c and d) HRCT showing that most miliary nodules were similar in terms of size, and their distribution bears no relation to the airways before (c) and 12 months after (d) treatment of a male patient with anti-TB therapy. (e and f) PETCT indicating bilateral and symmetrical of SAIL (e) and asymmetric unilateral TBIL (f) HRCT, high-resolution computed tomography; PETCT, positron emission tomography–computed tomography; SAIL, sarcoidosis intrathoracic lymphadenopathy; TB, tuberculosis; TBIL, tuberculosis intrathoracic lymphadenopathy
Involvement of other organs
The rate of extrathoracic organ involvement (particularly skin, eyes, spleen, and kidneys) was higher in SAIL versus TBIL (Supplementary Table 1). The supraclavicular LN were the mostly frequently involved site compared with cervical, axillary, and inguinal LN. Necrosis of superficial lymph node tissues was more commonly noted in TBIL versus SAIL (N = 6, 42.86% vs. N = 1, 5.26%, respectively; P = 0.026). There were no significant differences in the longest diameters and L/S ratios between SAIL and TBIL. Endobronchial nodules and bronchial pachymucosa/mucosal swelling (nodules [N = 4] and others [N = 3]) were major features of bronchial involvement in SAIL. Neoplasm with caseous necrosis, edematous-hyperemia, ulcer, and tracheobronchial stenosis were often observed in TBIL (neoplasm with caseous necrosis [N = 5] and others [N = 4]).
Diagnostic performances of Xpert, AFB, culture, and QuantiFERON-TB gold
Xpert did not detect MTB in all patients with SAIL. Among those with TBIL, positive results were obtained for 23 (69.70%) patients (P < 0.01). This approach yielded negative findings for other diseases and undiagnosed diseases (Table 3).
Xpert yielded negative results for 10 patients with TBIL. The diagnosis of these 10 patients was reached as follows: diagnosed by the histopathologic findings with caseous necrosis of cervical lymph nodes (N = 2); concurrent with endobronchial TB (N = 1); associated with lung TB diagnosed by positive bronchoalveolar lavage fluid Xpert (N = 1); diagnosed by positive sputum TB culture (N = 2); and 4 patients (two with positive AFB of bronchoscopy brush specimens) associated with lung involvement were confirmed to having TB by the alleviated clinical symptoms and radiological features after 6 months of anti-TB therapy. The rate of positive results from the culture of samples obtained through EBUS-TBNA were 0% and 24.24% in SAIL and TBIL, respectively (P < 0.01). There was no significant difference in EBUS AFB between the two diseases. QFT examination of peripheral blood yielded positive results for 27 (96.43%) and four (8.16%) patients with TBIL and SAIL, respectively. The results were inconclusive for two patients with TBIL and one patient with SAIL. Three patients with TBIL did not undergo QFT.
Discussion
TB lymphadenitis is more frequent in children and females, with the peak age of onset ranging 15–30 years in high TB burden countries [11]. In the present study, the age of patients with TBIL ranged 26.5–54.5 years and only 36.36% (12/33) were aged > 40 years, these patients were markedly younger than those with SAIL (80%, 40/50, p < 0.01). TB is an infectious disease caused by MTB. Although most patients with TB lymphadenitis did not exhibit special symptoms [12], those with TBIL were more likely to experience fever in our study. Moreover, indicators of infection (i.e., leukocytes, erythrocyte sedimentation rate, C-reactive protein, serum amyloid A, and serum ferritin) were higher in TBIL, particularly leukocytes and serum ferritin. These data suggested that, unlike SAIL, TBIL is an infectious disease. Importantly, 30–60% of patients with sarcoidosis are asymptomatic, and the disease is often discovered accidentally during a chest examination. In this study, patients with SAIL were more likely to experience chest distress and dyspnea versus those with TBIL, which may be related to the compression caused by mediastinal lymph node enlargement. Renston et al. found that 41% of patients with sarcoidosis had peripheral blood eosinophilia [13]. We found that the ratio of eosinophils in peripheral blood was significantly higher in SAIL versus TBIL, which may indicate different pathogenic processes. Nevertheless, further studies are required to determine how eosinophils participate in the pathogenesis of sarcoidosis. In addition, there were no differences in the nutritional and immune status of patients in the two groups in terms of albumin, globulin, IgG, IgM, IgA, IgE, C3, C4, CD4, and CD8 levels. However, patients with SAIL had a higher body mass index than those with TBIL (Table 1).
In intrathoracic sarcoidosis, the rate of bilateral hilar lymphadenopathy is 20–65%, while that of bilateral hilar lymphadenopathy associated with pulmonary infiltration is 20–40% [14]. Lymph nodes, especially thoracic LN, are among the most common sites of extrapulmonary TB [15]. However, the presence of isolated TBIL without a parenchymal lung lesion in adults is unusual, with an incidence rate of 0.25–5.8% [16]. In this study, solely mediastinal lymph node enlargement was not common (12% and 12.12% for SAIL and TBIL, respectively). Nodules were the main pulmonary features of the two diseases, including single, discrete, and miliary patterns. The formation of sarcoidosis granulomas occurs nearly exclusively along the lymphatic tracks. Hence, the typical feature of pulmonary sarcoidosis was nodular involvement in both the bronchovascular bundle and interlobular septa. This feature was typically symmetrical and demonstrated upper and middle lung zone predominance (Fig. 2). Miliary TB usually results from the acute hematogenous dissemination of TB bacilli in lungs. These nodules are uniform in size and their distribution bears no relation to the airways [17] (Fig. 2). There were no cavities in the lungs of patients with these two diseases. On computed tomography, LN in sarcoidosis are usually discrete, bilateral, and symmetrical, and rarely show a central hypodensity. In contrast, central necrosis and asymmetric conglomerate LN are frequently observed in TB [18]. Consistently, in our research, patients with TBIL mainly had asymmetric unilateral LN, especially in the drainage area of pulmonary lesions. Calcifications of the mediastinal or hilar LN were frequently observed in TBIL. In this study, extrathoracic organ involvement was more easily noted in SAIL versus TBIL.
It is critical to find evidence of MTB infection for differentiating sarcoidosis and TB. A positive culture for MTB using different specimens is currently the gold standard for the diagnosis of TB. However, the rate of positive culture was low for cases of lymphatic TB (2.1%) [19] due to difficulty in sampling from the LN and the paucibacillary nature of the specimens. Therefore, a rapid laboratory test is necessary for the diagnosis of TB. Xpert is an automated diagnostic test for the detection of MTB. It is a DNA-based test that detects the MTB rpoB gene [20]. The extraction, amplification, and detection by Xpert take place within a single-use multichambered cartridge, thus minimizing the risk of sample contamination. This method can provide results within 2 h. In this study, we used Xpert to find evidence of TBIL using specimens obtained through EBUS-TBNA. The rates of sensitivity, specificity, PPV, NPV, and accuracy of Xpert for differentiating SAIL from TBIL were 69.70%, 100%, 100%, 83.33%, and 87.95% respectively; these rates were higher than those of EBUS AFB and EBUS culture (Table 3). EBUS Xpert exhibited a higher PPV, suggesting that positive results in EBUS Xpert were associated with a higher likelihood for diagnosis of TB.
At present, the available tests are unable to accurately predict the progression of latent TB infection to active TB. In a systematic review, it was found that the interferon-gamma (IFN-γ) release assays (IGRAs) have a higher PPV than tuberculin skin tests. These findings suggested that individuals with positive IGRA results are more likely to progress to active TB than those with negative results [21]. QFT is a type of IGRA. Piotrowski proposed the use of QFT as a cost-effective diagnostic test, which can provide additional diagnostic information when a false-positive MTB culture result in a patient with sarcoidosis is highly suspected [22]. In the present study, the rates of sensitivity, specificity, PPV, NPV, and accuracy of QFT for differentiating SAIL from TBIL were 96.43%, 91.84%, 87.10%, 97.83%, and 93.51% respectively. These rates were higher than those of EBUS Xpert, except for specificity and NPV because there were also positive results in other and undiagnosed diseases. QFT with a highest NPV suggested that individuals with a negative QFT were more likely to exclude the diagnosis of TB and the lowest PPV indicated that if the patient had a positive QFT was not so strongly recommended to have the diagnosis of TB. Therefore, although QFT cannot provide a definitive diagnosis of active TB, it can assist us in excluding TB.
Limitations
First, owing to the insufficient number of specimens, some patients had false-negative results on EBUS Xpert, thereby decreasing the sensitivity of the method. Second, the QFT test was not performed in all patients, which may reduce the credibility of the results. Finally, this was a single-center retrospective study; hence, a multi-center prospective study is warranted to confirm the present findings.
Conclusions
Patients with TBIL were more likely to have an infection than those with SAIL; however, patients with SAIL were at a higher risk of extrathoracic involvement. Intrathoracic lymph nodes in SAIL were typically bilateral, symmetrical, and multifocal. Furthermore, the pulmonary radiological features of SAIL were bilateral nodes in both the bronchovascular bundle and interlobular septa. The use of EBUS Xpert is recommended for the diagnosis of TBIL. A negative QFT result can be utilized to exclude the diagnosis of TBIL.
Data Availability
All data generated or analyzed during this study are included in this published article and supplementary materials.
References
Peto HM, Pratt RH, Harrington TA, LoBue PA, Armstrong LR. Epidemiology of extrapulmonary Tuberculosis in the United States, 1993–2006. Clin Infect Dis. 2009;49(9):1350–7. https://doi.org/10.1086/605559.
Rossides M, Darlington P, Kullberg S, Arkema EV. Sarcoidosis: Epidemiology and clinical insights. J Intern Med. 2023;293(6):668–80. https://doi.org/10.1111/joim.13629.
Zhao MM, Du SS, Li QH, et al. High throughput 16SrRNA gene sequencing reveals the correlation between Propionibacterium acnes and sarcoidosis. Respir Res. 2017;18(1):28. https://doi.org/10.1186/s12931-017-0515-z.
Li QH, Zhang Y, Zhao MM, et al. Simultaneous amplification and testing method for Mycobacterium tuberculosis rRNA to differentiate sputum-negative Tuberculosis from sarcoidosis. Am J Physiol Lung Cell Mol Physiol. 2019;316(3):L519–24. https://doi.org/10.1152/ajplung.00172.2018.
Baykan AH, Sayiner HS, Aydin E, Koc M, Inan I, Erturk SM. Extrapulmonary tuberculosıs: an old but resurgent problem. Insights Imaging. 2022;13(1):39. https://doi.org/10.1186/s13244-022-01172-0.
World Health Organization. Xpert MTB/RIF Assay for the diagnosis of pulmonary and extrapulmonary TB in adults and children: policy update. Geneva: WHO; 2013.
Denkinger CM, Schumacher SG, Boehme CC, et al. Xpert MTB/RIF assay for the diagnosis of extrapulmonary Tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2014;44(2):435–46. https://doi.org/10.1183/09031936.00007814.
Dhooria S, Gupta N, Bal A, Sehgal IS, Aggarwal AN, Sethi S, Behera D, Agarwal R. Role of Xpert MTB/RIF in differentiating Tuberculosis from sarcoidosis in patients with mediastinal lymphadenopathy undergoing EBUS-TBNA: a study of 147 patients. Sarcoidosis Vasc Diffuse Lung Dis. 2016;33(7):258–66. PMID: 27758992.
European Centre for Disease Prevention and Control (ECDC). EU Case Definitions, Commission Decision 2012/506/EU. [cited 2019 Apr 12]. Available from: 2020.
Crouser ED, Maier LA, Wilson KC, et al. Diagnosis and detection of Sarcoidosis. An official American thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med. 2020;15(8):e26–e51. https://doi.org/10.1164/rccm.202002-0251ST.
Assefa W, Eshete T, Solomon Y, Kassaye B. Clinicoepidemiologic considerations in the diagnosis of tuberculous lymphadenitis: evidence from a high burden country. Int J Infect Dis. 2022;124:152–6. https://doi.org/10.1016/j.ijid.2022.09.030.
Popescu MR, Călin G, Strâmbu I, et al. Lymph node Tuberculosis - an attempt of clinico-morphological study and review of the literature. Rom J Morphol Embryol. 2014;55(2 Suppl):553–67.
Renston JP, Goldman ES, Hsu RM, Tomashefski JF Jr. Peripheral blood eosinophilia in association with sarcoidosis. Mayo Clin Proc. 2000;75(6):586–90. https://doi.org/10.4065/75.6.586.
Book P, Sarcoidosis. A Guide for the Practicing Clinician, Marc A. Judson in Respiratory Medicine. 2014.
Ganchua SKC, White AG, Klein EC, Flynn JL. Lymph Nodes-the neglected battlefield in Tuberculosis. PLoS Pathog. 2020;16(8):e1008632. https://doi.org/10.1371/journal.ppat.1008632.
Ren X, Li K, Li L, Zhao G. Mediastinal tuberculous lymphadenitis presenting with insidious back pain in a male adult: a case report and review of the literature. J Int Med Res. 2021;49(1):300060520987102. https://doi.org/10.1177/0300060520987102.
Van Dyck P, Vanhoenacker FM, Van den Brande P, De Schepper AM. Imaging of pulmonary Tuberculosis. Eur Radiol. 2003;13(8):1771–85. https://doi.org/10.1007/s00330-002-1612-y.
Gupta D, Agarwal R, Aggarwal AN, Jindal SK. Sarcoidosis and Tuberculosis: the same Disease with different manifestations or similar manifestations of different disorders. Curr Opin Pulm Med. 2012;18(5):506–16. https://doi.org/10.1097/MCP.0b013e3283560809.
Pang Y, An J, Shu W, et al. Epidemiology of Extrapulmonary Tuberculosis among inpatients, China, 2008–2017. Emerg Infect Dis. 2019;25(3):457–64. https://doi.org/10.3201/eid2503.180572.
Kohli M, Schiller I, Dendukuri N, Dheda K, Denkinger CM, Schumacher SG, Steingart KR. Xpert® MTB/RIF assay for extrapulmonary Tuberculosis and rifampicin resistance. Cochrane Database Syst Rev. 2018;8(8):CD012768. https://doi.org/10.1002/14651858.CD012768.
Zhou G, Luo Q, Luo S, et al. Interferon-γ release assays or tuberculin skin test for detection and management of latent Tuberculosis Infection: a systematic review and meta-analysis. Lancet Infect Dis. 2020;20(12):1457–69. https://doi.org/10.1016/S1473-3099(20)30276-0.
Piotrowski WJ, Adam B, Gwadera Ł, et al. QuantiFERON-TB-GOLD In-Tube in patients with sarcoidosis. Adv Respir Med. 2018;86(5):234–9. https://doi.org/10.5603/ARM.2018.0037.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the National Natural Science Foundation of China (grant numbers 82000068, 81200046 and 81903977); Natural Science Foundation of Shanghai (grant numbers 20ZR1446800, 18ZR1431400 and 21ZR1447700); Science and Technology Innovation Research Project of Shanghai Science and Technology Commission, China (grant number 20Y11902700).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Xian He, Yuan Zhang, Ying Zhou, Li Li and Qiuhong Li. The first draft of the manuscript was written by Xian He and Qiuhong Li. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of Shanghai Pulmonary Hospital (approval number:K22-258) and all participants signed informed consent. And all methods were carried out in accordance with relevant guidelines and regulations or Declaration of Helsinki.
Consent for publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
He, X., Zhang, Y., Zhou, Y. et al. Xpert MTB/RIF assay for the differential diagnosis between sarcoidosis and tuberculosis intrathoracic lymphadenopathy. BMC Infect Dis 23, 725 (2023). https://doi.org/10.1186/s12879-023-08734-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-023-08734-7