Abstract
Background
Nontuberculous mycobacteria (NTM) are ubiquitous, environmental bacteria that can cause chronic lung disease. Persons with cystic fibrosis (pwCF) are at high risk for NTM. Approximately 1 in 5 pwCF in the United States (U.S.) is affected by pathogenic NTM species, and incidence rates of NTM have been increasing among pwCF as well as in the general population. Prevalence of NTM pulmonary infections (PI) varies widely across the United States because of geographic variation in environmental exposures. This study will present updated region-level incidence of NTM infections in the cystic fibrosis (CF) population in the U.S.
Methods
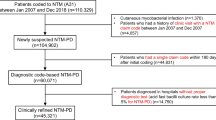
We used the Cystic Fibrosis Foundation Patient Registry (CFFPR) data for the period 2010 through 2019. Our study population comprised persons with CF ≥ 12 years of age who had been tested for NTM PI. We included only registry participants with NTM culture results. We defined incident cases as persons with one positive mycobacterial culture preceded by ≥ two negative mycobacterial cultures. We defined non-cases as persons with ≥ two negative mycobacterial cultures. We estimated average annual NTM PI incidence by region. Using quasi-Poisson models, we calculated annual percent change in incidence by region.
Results
We identified 3,771 incident NTM infections. Of these cases, 1,816 (48.2%) were Mycobacterium avium complex (MAC) infections and 960 (25.5%) were Mycobacterium abscessus infections. The average annual incidence of NTM PI among pwCF in the U.S. was 58.0 cases per 1,000 persons. The Northeast had the highest incidence of MAC (33.5/1,000 persons tested) and the South had the highest incidence of M. abscessus (20.3/1,000 persons tested). From 2010 to 2019, the annual incidence of total NTM PI increased significantly by 3.5% per year in the U.S.
Conclusions
NTM PI incidence is increasing among pwCF. Identifying high risk areas and increasing trends is important for allocating public health and clinical resources as well as evaluating interventions.
Similar content being viewed by others
Background
Nontuberculous mycobacteria (NTM) are ubiquitous environmental bacteria which are increasingly associated with pulmonary infections (PI) in both the general population and in high-risk groups [1, 2]. Persons with cystic fibrosis (pwCF) are at particularly high risk for NTM PI in the United States (U.S.). We previously found that the 5-year period prevalence (2010–2014) for NTM PI was 20% among pwCF, and that NTM PI prevalence was increasing [3]. NTM PI is associated with increased morbidity among pwCF [4, 5]: pwCF with chronic Mycobacterium abscessus PI experienced significantly greater lung decline rates than persons without NTM PI [4]. In 2010, the Cystic Fibrosis Foundation (CFF) started collecting data regarding NTM testing and culture results. The Cystic Fibrosis (CF) Foundation and the European Cystic Fibrosis Society (ECFS) have recommended that pwCF be screened for NTM, defined as performing annual cultures among persons spontaneously expectorating with a stable clinical course [6]. Although we are not able to differentiate screening versus clinically indicated testing in our dataset, the availability of NTM culture data from a high proportion of this population presents an opportunity to describe NTM lung infections in a relatively representative sample from this population. The objective of this study was to expand on our previous study by estimating the incidence of NTM PI among pwCF during a 10-year period. We also sought to evaluate temporal trends and geographic patterns of NTM incident PI among pwCF in the U.S.
Methods
We obtained patient data from the Cystic Fibrosis Foundation Patient Registry (CFFPR) [7] which represents 90% of pwCF in the U.S. Persons are enrolled in the CFFPR through CF Foundation-accredited care centers, which collect data from medical records and questionnaires [7]. We defined persons tested for mycobacteria as persons from whom a mycobacteria respiratory specimen was collected and cultured. Our study population comprised pwCF ≥ 12 years of age who were tested for mycobacteria from 2010 to 2019. This study was deemed not human subjects research because data were deidentified. Incident cases were defined as pwCF who had ≥ two negative mycobacterial cultures in two different calendar years, followed by one positive culture at any point. Subjects were defined as cases based on their first positive culture. Any subsequent cultures were excluded from our analysis. Non-cases were defined as those pwCF who had ≥ two negative cultures in two different years. A case could count as a non-case until the year of the incident infection. We geocoded patient zip codes using the United States Postal Service (USPS) zip code database [8]. Incidence was estimated as the proportion of pwCF identified as cases each year using the total tested population with culture results in that year as the denominator. We calculated average annual incidence of total NTM, Mycobacterium avium complex (MAC), and M. abscessus at national and state levels in R [9].
We used a quasi-Poisson model to estimate the annual percent change (APC) in NTM incidence by region during the study period. These models controlled for annual testing rate by region and were weighted by population size. The annual testing rate was included as an independent variable, while the log of the total population of pwCF screened for NTM was used as our denominator (population offset). Models were run separately for each region of the US: the Northeast, South, Midwest, and West.
Results
The national average annual testing rate was 20.0%. The rate of NTM testing increased significantly by 1.1% annually, ranging from 19.1% to 2013 to 23.1% in 2019. We identified 3,771 incident NTM PI cases. Of these cases, 1,816 (48.2%) had MAC infections and 960 (25.5%) had M. abscessus infections (Table 1). The average annual incidence of total NTM PI was 58.0 per 1,000 persons tested. The cumulative incidence for the study period was 393.7 per 1,000 persons tested. The average annual incidence was 27.9 per 1,000 for MAC and 14.8 per 1,000 for M. abscessus. The cumulative incidence over the 7-year study period was 189.6 per 1,000 for MAC and 100.2 per 1,000 for M. abscessus.
Total NTM PI incidence was highest in the South (64.1/1,000 persons tested; Table 2). M. abscessus incidence was highest in the South (20.3/1,000 persons tested; Table 2) and MAC incidence was highest in the Northeast (33.5/1,000 persons tested; Table 2; Fig. 1a). MAC represented 60.7% of cases in the Northeast and 42.7% percent of cases in the South. The average annual incidence of M. abscessus was nearly twice as high (20.3/1,000 persons tested) in the South compared with the Northeast (10.6/1,000 persons tested) and the Midwest (9.7/1,000 persons tested) (Table 2; Fig. 1b).
Overall, annual incidence of NTM PI increased significantly in the U.S. (Table 3). By region, significant (p < 0.05) annual increases were observed in the Northeast (APC, 7.7%), South (APC, 4.1%), and Midwest (APC, 4.4%) (Table 3). In the West, total NTM increased by 1.8% annually but this change was not significant (Table 3). With respect to species-specific trends, overall MAC PI increased nationally by 4.4% per year and in the Northeast by 11.0% per year (Table 3; Fig. 2a). We did not observe significant changes in M. abscessus incident PI rates during the study period (Table 3; Fig. 2b).
Discussion
We found that NTM PI incidence was increasing among pwCF, with regional differences in species-specific incidence and trends. Overall, the South had the highest NTM PI incidence, and the highest proportion of M. abscessus PI. In the Northeast, MAC was predominant with an increasing PI incidence. These findings are consistent with prior work that has identified a high prevalence of NTM PI and disease in the South, as well as a high burden of M. abscessus [3]. A prior analysis for the period 2010–2014 [3] found an increasing prevalence of 5.3% per year, with overall increases in MAC annual prevalence as well as significant increases in Middle Atlantic states (NJ, NY, PA). The same study found that M. abscessus did not increase significantly during 2010–2014.
This project has some important limitations. In states with lower rates of NTM testing, those tested may be more likely to be symptomatic, and therefore more likely to be cultured and test positive, resulting in an overestimation of NTM positivity among pwCF. We did not have access to data that indicated cases which were clinically stable and screened for NTM and which cases had clinical indicators of NTM PI and were tested for NTM. In addition, the case definition used in this study may overestimate the rate of NTM PI across the U.S. The clinical significance of a single positive culture result for NTM in pwCF is uncertain and may represent transient infection rather than a clinically significant infection, as NTM can be isolated from the respiratory tract of patients who never develop progressive NTM pulmonary disease [10]. Although we are interested in temporal and spatial trends of NTM species other than MAC and M. abscessus, our dataset lacked sufficient sample sizes for other species groups, precluding analysis.
Recent advances in cystic fibrosis transmembrane conductance regulator (CFTR) modulator therapy, such as the development of Trikafta®, have resulted in reduced sputum production and reduced risk of NTM PI among pwCF [11]. A recent analysis has found a decline in the prevalence of NTM positive cultures among pwCF, consistent with increasing usage of highly effective modulator treatment [12]. Nevertheless, because pwCF represent a highly susceptible population who are at high risk of NTM infection and disease, burden and trends in this population are likely indicators of risk in the non-CF susceptible population and provide insights into regional differences and trends. The patterns found here are similar to those in the non-CF population, with overall increases in NTM pulmonary disease [2], and regional differences in NTM species-specific distribution, with a predominance of M. abscessus in the South and MAC in the Northeast [13]. Further work is needed to understand environmental exposures [14,15,16,17,18,19] or other factors driving these trends, for optimum allocation of clinical and public health resources.
Conclusions
The continued increase in NTM lung infections among pwCF represents a public health and clinical burden of NTM in the U.S. Although increased uptake of CFTR modulator therapies, such as Trikafta® appears to have resulted in a decreased risk of NTM lung infections among pwCF, continued surveillance for NTM lung infections is needed in both the CF and non-CF populations to assess the burden of these infections and evaluate trends.
Data Availability
The data that support the findings of this study are not publicly available because they were obtained from the Cystic Fibrosis Foundation through a Data Use Agreement. However, the dataset may be obtained upon request from the Cystic Fibrosis Foundation at the following contact address: datarequests@cff.org.
References
Dean SG, Ricotta EE, Fintzi J, Lai YL, Kadri SS, Olivier KN, et al. Mycobacterial testing Trends, United States, 2009–2015(1). Emerg Infect Dis. 2020;26(9):2243–6.
Winthrop KL, Marras TK, Adjemian J, Zhang H, Wang P, Zhang Q. Incidence and prevalence of Nontuberculous Mycobacterial Lung Disease in a large U.S. Managed Care Health Plan, 2008–2015. Ann Am Thorac Soc. 2020;17(2):178–85.
Adjemian J, Olivier KN, Prevots DR. Epidemiology of Pulmonary Nontuberculous Mycobacterial Sputum positivity in patients with cystic fibrosis in the United States, 2010–2014. Annals of the American Thoracic Society. 2018;15(7):817–26.
Esther CR Jr, Esserman DA, Gilligan P, Kerr A, Noone PG. Chronic Mycobacterium abscessus infection and lung function decline in cystic fibrosis. J Cyst Fibros. 2010;9(2):117–23.
Olivier KN, Weber DJ, Lee JH, Handler A, Tudor G, Molina PL, et al. Nontuberculous mycobacteria. II: nested-cohort study of impact on cystic fibrosis lung disease. Am J Respir Crit Care Med. 2003;167(6):835–40.
Floto RA, Kenneth NO, Lisa S, Charles LD, Jean-Louis H, Jerry AN, et al. US cystic Fibrosis Foundation and european cystic Fibrosis Society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis. Thorax. 2016;71(Suppl 1):i1.
Knapp EA, Fink AK, Goss CH, Sewall A, Ostrenga J, Dowd C, et al. The cystic Fibrosis Foundation Patient Registry. Design and methods of a National Observational Disease Registry. Ann Am Thorac Soc. 2016;13(7):1173–9.
United States. ZIP Code Database.
Team RC. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2021.
Daley CL, Iaccarino JM, Lange C, Cambau E, Wallace RJ, Andrejak C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur Respir J. 2020;56(1):2000535.
Ricotta EE, Prevots DR, Olivier KN. CFTR modulator use and risk of nontuberculous mycobacteria positivity in cystic fibrosis, 2011–2018. ERJ Open Res. 2022;8(2).
Martiniano SL, Nick JA, Daley CL. Nontuberculous mycobacterial infections in cystic fibrosis. Clin Chest Med. 2022;43(4):697–716.
Spaulding AB, Lai YL, Zelazny AM, Olivier KN, Kadri SS, Prevots DR, et al. Geographic distribution of Nontuberculous Mycobacterial Species identified among clinical isolates in the United States, 2009–2013. Ann Am Thorac Soc. 2017;14(11):1655–61.
Foote SL, Lipner EM, Prevots DR, Ricotta EE. Environmental predictors of pulmonary nontuberculous mycobacteria (NTM) sputum positivity among persons with cystic fibrosis in the state of Florida. PLoS ONE. 2021;16(12):e0259964.
Lipner EM, Crooks JL, French J, Strong M, Nick JA, Prevots DR. Nontuberculous mycobacterial infection and environmental molybdenum in persons with cystic fibrosis: a case-control study in Colorado. J Expo Sci Environ Epidemiol. 2022;32(2):289–94.
Lipner EM, French JP, FalkinhamIII JO, Crooks JL, Mercaldo RA, Henkle E, et al. Nontuberculous mycobacteria infection risk and Trace Metals in Surface Water: a Population-based ecologic epidemiologic study in Oregon. Annals of the American Thoracic Society. 2022;19(4):543–50.
DeFlorio-Barker S, Egorov A, Smith GS, Murphy MS, Stout JE, Ghio AJ, et al. Environmental risk factors associated with pulmonary isolation of nontuberculous mycobacteria, a population-based study in the southeastern United States. Sci Total Environ. 2021;763:144552.
Lipner EM, French JP, Nelson S, Falkinham Iii JO, Mercaldo RA, Blakney RA, et al. Vanadium in groundwater aquifers increases the risk of MAC pulmonary infection in O’ahu, Hawai’i. Environ Epidemiol. 2022;6(5):e220.
Adjemian J, Olivier KN, Seitz AE, Falkinham JO 3rd, Holland SM, Prevots DR. Spatial clusters of nontuberculous mycobacterial lung disease in the United States. Am J Respir Crit Care Med. 2012;186(6):553–8.
Acknowledgements
This work was supported in part by the Divisions of Intramural Research, NIAID, NIH. The authors would like to thank the Cystic Fibrosis Foundation for the use of CF Foundation Patient Registry data to conduct this study. Additionally, we would like to thank the patients, care providers, and clinic coordinators at CF Centers throughout the United States for their contributions to the CF Foundation Patient Registry.
Funding
No outside funding was obtained for this study. This work was supported in part by the Divisions of Intramural Research of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Open Access funding provided by the National Institutes of Health (NIH)
Author information
Authors and Affiliations
Contributions
Study Conception: DRP. Data acquisition: DRP, EML, JEM. Study design: DRP, RAM, EML, JEM. Data curation and cleaning: JEM. Methodology: RAM, EML, DRP. Formal analysis: JEM. Visualization: JEM. Writing-original draft: JEM. Writing-review & editing: DRP, EML, RAM.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All methods were carried out in accordance with relevant guidelines and regulations by the National Institutes of Health’s (NIH) Office of human subjects Research Protection (OHSRP) and the cystic Fibrosis Foundation. The Institutional Review Board (IRB) of the NIH follows all international ethics rules, regulations, and standards. This research protocol was reviewed by NIH OHSRP, which determined that analyses of CFFPR data was “not human subjects research”, under the 2018 HHS Common Rule (45 CFR 46.102(e)[1](ii), 45 CFR 46.116 (b)[9](i)), because of the use of deidentified data and, accordingly, exempt from IRB review. For inclusion in the CFFPR, written informed consent was obtained from all subjects and their legal guardian(s) by accredited CF care centers.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Marshall, J.E., Mercaldo, R.A., Lipner, E.M. et al. Incidence of nontuberculous mycobacteria infections among persons with cystic fibrosis in the United States (2010–2019). BMC Infect Dis 23, 489 (2023). https://doi.org/10.1186/s12879-023-08468-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-023-08468-6