Abstract
Background
An unprecedent increase in the number of cases and deaths reported from dengue virus (DENV) infection has occurred in the southwestern Indian ocean in recent years. From 2017 to mid-2021 more than 70,000 confirmed dengue cases were reported in Reunion Island, and 1967 cases were recorded in the Seychelles from 2015 to 2016. Both these outbreaks displayed similar trends, with the initial circulation of DENV-2 which was replaced by DENV-1. Here, we aim to determine the origin of the DENV-1 epidemic strains and to explore their genetic characteristics along the uninterrupted circulation, particularly in Reunion.
Methods
Nucleic acids were extracted from blood samples collected from dengue positive patients; DENV-1 was identified by RT-qPCR. Positive samples were used to infect VERO cells. Genome sequences were obtained from either blood samples or infected-cell supernatants through a combination of both Illumina or MinION technologies.
Results
Phylogenetic analyses of partial or whole genome sequences revealed that all DENV-1 sequences from Reunion formed a monophyletic cluster that belonged to genotype I and were closely related to one isolate from Sri Lanka (OL752439.1, 2020). Sequences from the Seychelles belonged to the same major phylogenetic branch of genotype V, but fell into two paraphyletic clusters, with greatest similarity for one cluster to 2016–2017 isolate from Bangladesh, Singapore and China, and for the other cluster to ancestral isolates from Singapore, dating back to 2012. Compared to publicly available DENV-1 genotype I sequences, fifteen non-synonymous mutations were identified in the Reunion strains, including one in the capsid and the others in nonstructural proteins (NS) (three in NS1, two in NS2B, one in NS3, one in NS4B, and seven in NS5).
Conclusion
In contrast to what was seen in previous outbreaks, recent DENV-1 outbreaks in Reunion and the Seychelles were caused by distinct genotypes, all likely originating from Asia where dengue is (hyper)endemic in many countries. Epidemic DENV-1 strains from Reunion harbored specific non-synonymous mutations whose biological significance needs to be further investigated.
Similar content being viewed by others
Background
Dengue virus (DENV) is the most widespread mosquito-borne flavivirus worldwide. More than 390 million people in over 129 countries are exposed to dengue virus infections with an estimated 20,000 deaths every year [1, 2]. DENV is transmitted through the bite of infected female mosquitoes belonging to the Aedes genus; the species Aedes aegypti being a primary vector while Aedes albopictus plays a secondary role [3]. The DENV genome consists of a positive-sense single-stranded linear RNA of approximately 11 kilobases. Four DENV serotypes (DENV-1 to DENV-4) are currently known. Molecular analyses have highlighted the diversity of DENV genotypes belonging to each of the four serotypes [4, 5].
The southwestern Indian ocean islands have a history of periodic outbreaks of DENV, but Reunion Island has been an exception in recent years. Since an outbreak of the virus in 2017, there has been continuous circulation of DENV on the island with over 70,000 confirmed cases between 2017 and 2022 (Table 1). This continuous presence of the virus on the island may suggest that dengue has become endemic [6].
At the beginning of the epidemic wave, DENV-2 was the first serotype detected on the island [9, 10]. At the end 2019, among the 25,000 confirmed cases and 20 deaths, DENV-2 still represents the major serotype, but a few cases of DENV-1 and DENV-3 were also reported [11]. During the course of the epidemic, DENV-1 was also observed to co-circulate with DENV-2, and rapidly became the dominant serotype. DENV-1 was the only identified serotype among the 29,577 confirmed cases by week 35 in 2021 [12]. A similar trend was also observed in the Seychelles where an outbreak of DENV-2 started in 2015, being replaced by DENV-1 in 2016 (MOH Seychelles). Interestingly, the DENV-2 strains that circulated at the beginning of these outbreaks are 99.8% similar to each other and 93% similar to a 2013 strain from Singapore, indicating a single introduction of DENV-2, presumably from Asia [10]. As DENV-1 subsequently succeeded DENV-2 in these two islands, it would be interesting to see whether the DENV-1 outbreaks were also initiated by a single introduction event.
In 2021, DENV-1 circulation in Reunion coincided with a significant increase of severe cases and hospitalizations when compared to 2018 and 2019 [12]. For instance, several severe ophthalmological cases related to DENV-1 infection were reported, notably in 2020 and 2021 [12]. Moreover, while severe forms and deaths were most frequently observed in older patients with comorbidities such as diabetes and hypertension, increasing numbers of DENV1-induced mortalities were observed in younger patients (including children) without comorbidities [12]. Although such an increase in severity was observed for the first time in Reunion, these symptoms were already described in Asia where dengue is endemic [13, 14]. To investigate whether the current epidemic DENV-1 strains from Reunion and the Seychelles were similar to each other and to those known in Asia, we performed whole-genome sequencing and phylogenic analyses to trace their origin and explore some genetic features.
Methods
Origin of samples
Biological samples from Reunion Island were originated from two different collections. The CARBO collection consisted of a prospective cohort of patients with arbovirus infections, registered on clinicaltrials.gov (NCT01099852). The CARBO was started by the University Hospital of La Martinique in 2010 and extended to include patients from Reunion in 2018. The other collection, DEMARE, was established as part of an epidemiological cross-sectional study conducted in 2019–2020. Both collections were approved by The French Committee for the Protection of Individuals. Written consent was obtained from all participants. Blood samples from the Seychelles were collected by the Ministry of health of the Seychelles during outbreaks between January 2015 and December 2016.
Bloods were collected from serologically confirmed patients presenting one or several dengue symptoms including fever, myalgia, headache, asthenia and thrombocytopenia and without recent travel history. To increase the probability of detecting the presence of viral RNA, the samples used in the study were from patients included in the cohort within the first seven days of the onset of symptoms. Collections were performed by accredited professionals in 10 mL EDTA tubes. Within the two hours after collection, samples were centrifuged at 2,000 g for 10 min at 20 °C. Supernatants were transferred into new tubes, homogenized by inversion, aliquoted into new 2 mL tubes, labeled, then frozen and stored at -80 °C until used.
Viral RNA extraction and amplification
To ascertain the presence and the amount of DENV-1 RNA in the selected samples, a reverse transcription quantitative real-time PCR (RT-qPCR) was performed. Briefly, total nucleic acids were extracted from the serum samples using the QIAamp® Mini Kit purification according to the manufacturer’s recommendation (QIAGEN). For RT-qPCR, we used the QIAGEN OneStep kit following the manufacturer’s instructions (QIAGEN). A mixed solution was prepared with RNA template (5 µl), a TaqMan probe (FAM-ACACCTCAAGCTAA-TAMRA) and primers (Forward 5’-GAACATGGRACAAYTGCAACYAT-3’; Reverse 5’-CCGTAGTCDGTCAGCTGTATTTC-3’) specific to DENV-1. The thermocycler program consisted of a retrotranscription step of 45 min at 45 °C, denaturation for 5 min at 95 °C followed by 40 cycles of amplification (72 °C for 5 s and 56 °C for 60 s). Viral RNA copy number was estimated against a standard curve following the methodology published by the HAS (Haute Autorité de Santé, France). Plasmids containing targeted DENV-1 were synthesized by GeneCust (France) and used as the standard curve at concentrations of 101 to 108 RNA copy per µl.
The E gene was amplified to confirm RT-qPCR-positive samples. cDNA was synthesized from extracted RNA using the ProtoScript® II Reverse Transcriptase Kit with random primers following the manufacturer’s instructions (New England BioLab, USA) and amplified using a nested PCR protocol, as previously described [9]. Briefly, we designed degenerated primers targeting a fragment (~ 700–800 bp) of the E gene encoding the envelope protein. The first round of amplification reaction was performed with primers DNV1-E-F1 (CAC TGG TGG AAG AAC AAG ACG C) and DNV1-E-R2 (CMA CDG AYG TGA ACA CYC CTC C) generating an approximately 1100-bp fragment. The second round used the primers DNV1-E-F2 (ACG GAG CTC TYA CAT TGG ACT G) DNV1-E-R2 (CMA CDG AYG TGA ACA CYC CTC C) and generated a 750-bp fragment. PCR amplification was performed on a PCR System 2700 Thermocycler (ABI Applied Bio-system ™). Amplification programs were as follows: 94 °C for 2 min; 3 cycles of 95 °C for 5 s, 60 °C for 30 s, 72 °C for 30 s; 3 cycles of 95 °C for 5 s, 55 °C for 30 s, 72 °C for 30 s; and 28 cycles of 95 °C for 5 s, 50 °C for 30 s, 72 °C for 1 min 30 s; 72 °C for 7 min. Amplified products were checked first on electrophoresis gel (Additional file) then samples showing amplicons of expected sizes were Sanger sequenced (Genoscreen, Lille, France).
Virus isolation
Viral isolation assays were conducted by inoculating the DENV-1 PCR-positive sera from viremic patients onto Vero E6 cell monolayers. Cultures were checked every day. When cytopathic effects were observed, supernatants (passage 1) were collected by centrifugation and stored at -80 °C. The presence of viral RNA was confirmed by RT-qPCR, as above, and by titration of viral infectious particles using plaque-forming unit assay [10].
Sequencing and genomic analysis
Genomic data were generated using either Oxford Nanopore Technologies (MinION) sequencing based on the amplicon tiling protocol, or Illumina shotgun sequencing, or by combining data from both sequencing platforms [15].
For Illumina sequencing, libraries were generated from 10 ng of cDNA using the Celero™ PCR Workflow with Enzymatic fragmentation (DNA-Seq) following the manufacturer’s instructions. Sequencing was performed on the MiSeq platform, with 1 * 170 bp single-end reads. Demultiplexed sequences were provided by the sequencing company (Biofidal, Lyon, France). Sequences were quality trimmed and adapters removed using Trimmomatic v0.39 [16]. Trimmed reads were mapped to reference sequence NC_001477.1 using bowtie2 [17]. Geneious v9.1.8 [18] was used to inspect and curate mapped sequence data.
For Oxford Nanopore Technology’s MinION sequencing, an amplicon tiling protocol was used in conjunction with DENV1 primers from the Oxford Centre for Arbovirus Discovery, Diagnostics, Genomics and Epidemiology (https://www.caddecentre.org/protocols/). Briefly, cDNA was amplified in two independent PCR reactions. PCR products were pooled, dA-tailed using the NEBNext® Ultra™ II End Repair/dA-Tailing Module then barcoded using the Nanopore Native Barcoding Expansion kit (EXP-NBD104). Barcoded amplicons were then purified using a 0.4x volume of AMPure-XP SPRI beads, washing the beads with an excess volume of Nanopore’s small fragment buffer (SFB) to ensure that un-ligated barcode molecules were removed. Purified amplicons were then pooled in equimolar proportions before adapter ligation and sequencing, following the manufacturer’s instructions. The sequencing run was left for 24 h and stopped when predicted coverage exceeded 1000-fold for each genome. Base-calling and demultiplexing were performed using guppy (v4.0.11). Base-calling used default parameters in accurate mode, and demultiplexing was performed using the “require_barcodes_both_ends” parameter to minimize sample crosstalk. Reads were then assembled using Medaka v1.0.3 (https://nanoporetech.github.io/medaka/) mimicking the ARTIC network bioinformatics standard operating procedure for SARS-CoV-2 sequencing (https://artic.network/ncov-2019/ncov2019-bioinformatics-sop.html), subsampling amplicon coverage to 400x and using genome accession NC_001477.1 for read mapping. Geneious v9.1.8 was used to inspect and curate mapped sequence data. Consensus base-calling required a minimum of 30-fold coverage.
For samples from the Seychelles, only partial genomic assemblies were obtained by this method, as not all primer pairs produced amplified products. The partial assembly data were thus combined with Sanger sequence data from the envelope region of the genome to produce contiguous consensus sequences that spanned a 4,838 bp region at the 5’ end of the DENV genome. This region corresponded to base positions 156–4,994 of the reference genome NC_001477.1 and contained genetic data from the capsid, membrane glycoprotein, envelope, NS1, NS2a and NS2b regions of the genome.
Phylogenetic analysis
Global phylogenetic comparisons were carried out using the E-gene, which is typically used for identifying dengue genotypes [5]. The E-gene region was extracted from all reference sequences in the NCBI database using custom scripts. Alignments were generated using MAFFT [19], and the alignment was curated by eye in Geneious v9.1.8. IQTree2 (v2.1.3) was used to identify optimal substitution model parameters and to generate a bootstrapped maximum-likelihood phylogeny with 1000 replicates. Phylogenetic tree representations were generated in R, using the “ggtree” package [20].
Results
General characteristics
Among the 30 blood samples from Reunion and 14 from Seychelles collected within the first seven days of the onset of symptoms, only nine (PR1583, PR1615, PR1914, PR4443, PR4453, PR4463, PR4483, PR6594, P0409) of 2019 to 2021 outbreaks from Reunion and four (DS16177, DS16229, DS16232, DS16233) of 2016 outbreak from Seychelles tested positive for DENV-1 by RT-qPCR were further analyzed (Table 2). A higher viral titer was detected for the two samples PR1583 and PR6594 with approximately 107 RNA copies/µL. These two samples were used to infect VERO cells allowing the isolation of DENV-1 strains named RUN1-1583 and RUN1-6594, respectively, isolates which were also further analyzed (Table 2). Sequencing with Illumina generated complete genome sequences for 5 blood samples (PR4443, PR4453, PR4463, PR4483, PR6594) and the isolate RUN1-6594 (one passage) from Reunion. MinION technology allowed sequencing of almost complete genomes for 3 sera (PR1615, PR1914, P0409) and the isolate RUN1-1583 (one passage) from Reunion. The sequence of each isolated strain was identical to that obtained from the corresponding blood sample. Using MinION technology, only partial sequences were obtained from the four Seychelles samples. Upon sequence inspection, mismatches were identified in the DENV1_3, DENV1_11 and DENV1_12 primer pairs which may explain inefficient amplification of DENV1 genotype V. Further optimization of the amplicon tiling protocol would be required to allow efficient amplification and sequencing of all DENV-1 genotypes by this method.
Phylogeny
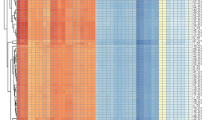
Phylogenetic analyses of the approximately 5000-bp genomic region of 3,891 publicly available sequences showed that all sequences from Reunion Island found in 2019, 2020 and 2021 belonged to genotype I of DENV-1 (Fig. 1a), and possessed nearly identical sequences, forming a monophyletic cluster in the phylogeny. These sequences showed greatest similarity (99.5% identity) to one isolate from Sri Lanka in 2020 (Accession number OL752439.1) and two sequences from China in 2016 (Accession numbers MN933661 and MN933663). Sequences from the Seychelles belonged to the same major phylogenetic branch of DENV-1 genotype V, but fell into two paraphyletic clusters (Fig. 1a). DS16177 showed greatest similarity (> 99% identity) to sequences from 2016 to 2017 isolated in Bangladesh, Singapore and China, whereas DS16229, DS16232, DS16233 and DS16243 showed greatest similarity to numerous ancestral isolates from Singapore, dating back to 2012. However, interpretations of isolate origins should consider the strong sampling bias that exists in available DENV sequence data (Fig. 1b).
Phylogenetic analyses of DENV. (a) Phylogenetic tree generated using a 4,838-bp region of the DENV genome (positions 156 to 4,994 of NC_001477.1). Inset are zoomed representations of the tree topology and bootstrap values for regions relevant to sequences generated as part of this study. (b) A graphical representation of the country of origin data for sequences included in the phylogenetic analysis
Further extending the phylogenetic comparison to include whole genomic data (Additional file) provided no strong evidence for the introduction of separate lineages in Reunion, as all whole genome sequences showed > 99.8% identity (between 0 and 19 SNPs).
Major non-synonymous mutations
Fifteen non-synonymous amino acid changes were specifically observed in all Reunion DENV-1 full genome sequences compared with other DENV-1 genotype I sequences publicly available (Fig. 2). Mutations consisted of N90S in the C gene, R989K, N1068S and K1116R in NS1, I1389M, V1451I and E1487K in NS2B, A2271T in NS4B. Seven mutations H2620Y, K2881R, V2906I, E3052D, S3059A, V3179I and I3322V were observed in NS5. As only partial sequences were generated from the Seychelles samples, reconstruction of full polyprotein was not possible, thus precluding the analysis of amino acid changes.
Schematic representation of conserved mutations identified within isolates from Reunion. Position numbers are expressed relative to the first conserved start methionine of all polyprotein sequences belonging to DENV1 as defined in [28]. Mutations are expressed relative to the majority amino acid sequence of DENV1 genotype I sequences from the same dataset. Positions are colored by mature protein: C, Capsid; prM, premembrane; E, envelope; NS, Nonstructrural. A: Alanine; D: Aspartic acid; E: Glutamic acid; H: Histidine; I: Isoleucine; K: Lysine; M: Methionine; N: Asparagine; R: Arginine; S: Serine; T: Threonine; V: Valine; Y: Tyrosine
Discussion
Since the recent dengue outbreak started in late 2017, uninterrupted transmission has occurred in Reunion Island. From 2017 to mid 2022, more than 70,000 confirmed cases were reported [6,7,8]. Circulation of DENV-2 in 2018 was followed by a major co-circulation of DENV-2 and DENV-1 at the end of 2019 and during 2020, with few cases of DENV-3. In 2021 and 2022, DENV-1 was the only serotype identified in Reunion Island [6]. The Seychelles were also confronted to dengue outbreaks years before (2015–2016) with a similar trend starting with DENV-2 which was replaced by DENV-1 [6].
Using phylogenetic analyses, we showed that the DENV-1 sequences from Reunion were closely related to a 2020 strain circulating in Sri Lanka, whereas those from the Seychelles showed a greatest similarity for isolates from Bangladesh, Singapore and China. This result suggested that the DENV-1 epidemic strains in Reunion and Seychelles were probably imported from Asian countries. We identified a single DENV-1 lineage belonging to genotype I that circulated in Reunion since its emergences at the end of 2019 and subsequently in 2020 and 2021. On the contrary, two lineages of DENV-1 of genotype V occurred in the Seychelles in 2016. It should be noted that these are likely partial descriptions of the total genetic diversity of DENV on the two islands due to the limited sample availability for our study. Even so, our results were distinct to previous observations in the region where similar viral epidemic strains usually occurred in contemporary outbreaks. For instance, between 2003 and 2004 an outbreak caused by DENV-1 serotype started in the Seychelles [21] and then spread in Reunion [22]. The same DENV-1 serotype was involved in an outbreak in Toamasina, eastern Madagascar, two years later in 2006 [23], but a regional link could not be established as no sequence was found. More recently, dengue outbreaks due to DENV-2 occurred in 2016 in the Seychelles and then in 2018 in Reunion, and it was shown to involve the same cosmopolitan sub-lineage I [9, 10]. Moreover, the link of dengue outbreaks between these two islands has been demonstrated by the presence of dengue cases in Reunion imported from the Seychelles whether in 2016 [7] or in 2017 [8].
The presence of two distinct sub-lineages of DENV-1 in the Seychelles suggests at least two introduction events. Conversely, the full-genome phylogenetic analysis (Additional file) strongly suggests that sequenced DENV-1 genotype I strains in Reunion originated from a single introduction event. In these two countries, DENV-1 displaced the previously circulating DENV-2. The displacement phenomenon was also reported among genotypes of the same dengue serotype. For instance, during the DENV-2 outbreaks in several South American countries, the American genotype was replaced by the Asian genotype [24]. In some cases, lineage replacements were associated with an increased clinical severity [24, 25]. In Reunion, the introduction and subsequent dominance of DENV-1 was accompanied by severe cases with peculiar symptoms, notably the maculopathy. Ophthalmic complications associated with dengue, with certain dependence to serotype, have been increasingly described in recent times [13, 26]. The possible link between maculopathy observed in Reunion dengue patients and the emerging lineage DENV-1 genotype I needs to be further investigated.
Moreover, the probable single introduction of the DENV-1 genotype I in Reunion may have been be accompanied by a discrete micro-evolution event since fifteen exclusive non-synonymous mutations were found in different proteins, including capsid, NS1, NS2B, NS3, NS4B, and NS5. Such local micro-evolution events have been observed in Asia, as shown for instance in the Hunan province where several mutations were seen in DENV-2 outbreak (2018) after importation from neighboring areas having higher incidence of dengue [27]. The micro-evolution event in either structural or non-structural proteins can be neutral or under positive or negative selection with an impact in disease epidemiology [27, 28]. For instance, mutations in NS1 have been shown to influence production and secretion with impact on NS1 ELISA-based dengue detection in clinical samples [29]. In addition, it was shown that mutations involving changes from basic to acidic residues or vice versa tend to affect NS1 surface expression and secretion patterns of flavivirus with impact in host immunity [29, 30]. Here, mutations were observed also in both NS2B and NS5, two proteins that play a pivotal role in the activity of NS3 protease, the latter being also a therapeutic target against flaviviruses [31]. Strikingly, when studying mutations in NS2B of the flavivirus Zika, researchers found one particular mutation able to enhance transmission potential and to confer escape from pre-existing DENV immunity [32]. When analyzing several natural dengue variants from dengue severe patients, from mild to fatal cases, a link was established between mutations in NS5 and the virulent DENV phenotypes [33]. Whether the mutations found in DENV-1 epidemic strain played a role in dengue severe cases observed in Reunion needs to be further investigated. Particular attention should be paid in the three mutations (R989K, N1068S and K1116R) located in NS1, since expression of this nonstructural glycoprotein has been linked to dengue disease severity [14, 34, 35]. In addition, as NS1 antigen is a marker for routine diagnosis in rapid detection of dengue virus infection [36], the impact of these mutations on the efficiency of the tests may require further control.
Conclusion
The southwestern Indian ocean region is usually subject to dengue outbreaks with co-occurrence of a given dengue serotype alternating intense circulation and inter-epidemic periods. In contrast to what was observed previously, uninterrupted dengue circulation is occurring and we showed that recent DENV-1 outbreaks in Reunion and the Seychelles were caused by distinct genotypes, all probably originating from Asia. Strikingly, the unique DENV-1 genotype I lineage circulating during three consecutive years in Reunion harbored non-synonymous mutations whose biological significance need to be further investigated.
Availability of data and material
The datasets generated and analysed during the current study are available in the Genbank repository under the accession numbers ON631270, ON631271, ON631272, ON631273, ON631274, ON631275, ON631276, ON631277, ON631278, ON631279, ON631280, ON631281, ON631282, ON631283. Data from the CARBO study are not publicly available to protect patient privacy. They can be requested at the Department of research and innovation, Martinique University Hospital 97261 Fort-de-France.
Abbreviations
- DENV:
-
Dengue virus
- DENV-1:
-
Dengue virus serotype 1
- DENV-2:
-
Dengue virus serotype 2
- DENV-3:
-
Dengue virus serotype 3
- NS:
-
Nonstructural protein
- CARBO:
-
Cohorte arboviruses
- DEMARE:
-
Observational cross-sectional epidemiological study conducted in Madagascar and La Réunion
References
World Health Organization. Dengue and severe dengue. World Health Organ. https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed July 8, 2021).
Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496:504–7. https://doi.org/10.1038/nature12060.
Kramer MUG, Reiner RC Jr, Brady OJ, Messina JP, Gilbert M, et al. Past and future spread of the arbovirus vector Aedes aegypti and Aedes albopictus. Nat Microbiol. 2019;4:854–63. https://doi.org/10.1038/s41564-019-0376-y.
Vasilakis N, Weaver SC. Chapter 1 The History and Evolution of Human Dengue Emergence. In: Maramorosch K, Shatkin AJ, Murphy FA, editors. Adv. Virus Res., vol. 72, Elsevier; 2008, p. 1–76. Doi:https://doi.org/10.1016/S0065-3527(08)00401-6
Weaver SC, Vasilakis N. Molecular evolution of dengue viruses: contributions of phylogenetics to understanding the history and epidemiology of the preeminent arboviral disease. Infect Genet Evol. 2009;9:523–40. https://doi.org/10.1016/j.meegid.2009.02.003.
Hafsia S, Haramboure M, Wilkinson DA, Baldet T, Yemadje-Menudier L, Vincent M, et al. Overview of dengue outbreaks in the southern Indian Ocean and analysis of factors involved in the shift toward endemicity in Reunion Island: a systematic review. PLoS Neg Trop Dis. 2022;16:e0010547. https://doi.org/10.1371/1/journal.pndt.0010547.
Santé Publique France. Points Epidémiologiques - Dengue - Océan Indien https://www.santepubliquefrance.fr/regions/ocean-indien/documents/bulletin-regional/2016/surveillance-de-la-dengue-a-la-reunion.-point-au-08-avril-2016).
Santé Publique France. Points Epidémiologiques - Dengue - Océan Indien https://www.santepubliquefrance.fr/regions/ocean-indien/documents/bulletin-regional/2017/surveillance-de-la-dengue-a-la-reunion.-point-au-05-fevrier-2017).
Pascalis H, Turpin J, Roche M, Krejbich P, Gadea G, Atyame Nten C, et al. The epidemic of dengue virus type-2 cosmopolitan genotype on Reunion Island relates to its active circulation in the Southwestern Indian Ocean neighboring islands. Helyon. 2019;5:e01455. https://doi.org/10.1016/j.heliyon.2019.e01455.
Pascalis H, Biscornet L, Toty C, Hafsia S, Roche M, Desprès P, et al. Complete genome sequences of Dengue Virus type 2 epidemic strains from Reunion Island and the Seychelles. Microbiol Resour Announc. 2020;9. https://doi.org/10.1128/MRA.01443-19.
Santé Publique France. Points Epidémiologiques - Dengue - Océan Indien https://www.santepubliquefrance.fr/regions/ocean-indien/documents/bulletin-regional/2019/surveillance-de-la-dengue-a-la-reunion.-point-au-24-decembre-2019).
Santé Publique France. Points Epidémiologiques - Dengue - Océan Indien https://www.santepubliquefrance.fr/regions/ocean-indien/documents/bulletin-regional/2021/surveillance-de-la-dengue-a-la-reunion.-point-au-7-decembre-2021 (accessed May 8, 2022).
Hernandez-Delgado MA, Salvador B, Valdez-Garcia JE. Dengue fever: ophthalmological perspective. Int J Trop Dis. 2021;4:047.
Bhatt P, Sabeena SP, Varma M, Arunkumar G. Current understanding of the pathogenesis of dengue virus infection. Curr Microbiol. 2021;78:17–32. https://doi.org/10.1007/s00284-020-02284-w.
Quick J, Grubaugh ND, Pullan ST, Claro IM, Smith AD, Gangavarapu K, et al. Multiplex PCR method for MinION and Illumina sequencing of Zika and other virus genomes directly from clinical samples. Nat Protoc. 2017;12:1261–76. https://doi.org/10.1038/nprot.2017.066.
Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–20.
Langmead B, Salzberg S. Fast gapped-real alignment with Bowtie 2. Nat Methods. 2012;9:357–9. https://doi.org/10.1038/nmeth.1923.
Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, et al. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–9. https://doi.org/10.1093/bioinformatics/bts199.
Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30:772–80. https://doi.org/10.1093/molbev/mst010.
Yu G, Smith DK, Zhu H, Guan Y, Lam TT. ggtree: an r package for visualization and annotation of phylogenetic trees with their covariates and other associated data. Mol Biol Evol 2017;30:28–36. https://doi.org/10.1093/molbev/mst010.
D’Ortenzio E, Balleydier E, Baville M, Filleul L, Renault P. Dengue à la Réunion et dans les îles du sud-ouest de l’océan Indien. Médecine Mal Infect. 2011;41:475–9. https://doi.org/10.1016/j.medmal.2010.11.021.
Pierre V, Thiria J, Rachou E, Sissoko D, Lassale C, Renault P. Epidémie de dengue-1 à la Réunion en 2004, Journées INVS. https://www.invs.sante.fr/publications/2005/jvs_2005/poster_13.pdf
Ratsitorahina M, Harisoa J, Ratovonjato J, Biacabe S, Reynes J-M, Zeller H, et al. Outbreak of Dengue and Chikungunya Fevers, Toamasina, Madagascar, 2006. Emerg Infect Dis. 2008;14:1135–7. https://doi.org/10.3201/eid1407.071521.
Rico-Hesse R, Harrison LM, Salas RA, Tovar D, Nisalak A, Ramos C, et al. Origins of dengue type 2 viruses associated with increased pathogenicity in the Americas. Virology. 1997;230:244–51. https://doi.org/10.1006/viro.1997.8504.
Ramos-Cantañeda J, Bareto dos Santos F, Martínez-Vega R, Galvão de Araujo JM, Joint G, Sarti E. Dengue in Latin America: systematic review of molecular epidemiological trends. PLoS Neg Trop Dis. 2017;11:e00005224. https://doi.org/10.1371/journal.pntd.0005224.
Chee E, Sims JL, Jap A, Hock Tan B, Oh H, Che S-P. Comparison of prevalence of dengue maculopathy during two epidemics with different predominant serotypes. Am J Ophthamol. 2009;140:910–3.
Guan J, He Z, Qin M, Deng X, Chen J, Duan S, et al. Molecular characterization of the viral structural protein genes in the first outbreak of dengue virus type 2 in Hunan province, inland China in 2018. BMC Infect Dis. 2021;21:166. https://doi.org/10.1186/s12879-021-05823-3.
Stica CJ, Barrero RA, Muttay RZ, Devine GJ, Philippes MJ, Frentieu FD. Global evolutionary history and dynamics of dengue viruses inferred from whole genome sequences. Viruses. 2022;14:703. https://doi.org/10.3390/v14040703.
Ghosh A, Sukla S, Nath H, Saha R, De A, Biswas S. Non-structural protein 1 (NS1) variants from dengue virus clinical samples revealed mutations that influence NS1 production and secretion. Eur J Clin Microbiol Infect Dis. 2022;41:803–14. https://doi.org/10.1007/s10096-022-04441-4.
Youn S, Cho H, Fremont DH, Diamond MS. A short N-terminal peptide motif on flavivirus nonstructural protein NS1 modulates cellular targeting and immune recognition. J Virol. 2010;84:9516–32. https://doi.org/10.1128/JVI.00775-10.
Teramoto T, Choi KH, Padmanabhan R. Flavivirus proteases: The viral Achilles heel to prevent future pandemics. Antiviral Research 2023; 210:105516 2023
Regla-Nava JA, Wang Y-T, Fontes-Garfias CR, Liu Y, Syed T, Susantono M, et al. A zika virus mutation enhances transmission potential and confers escape from protective dengue virus immunity. Cell Rep. 2022;39:110655. https://doi.org/10.1016/j.celrep.2022.110655.
Cheng D, Huang S-W, Chin W-X, Hung S-J, Tsai H-P, Chu JJH, et al. Impac of intrahost NS5 nucleotide variations on dengue virus replication. Front Microbiol. 2022;13:894200. https://doi.org/10.3389/fmicb.2022.894200.
Paranavitane SA, Gomes L, Kamaladasa A, Adikari TN, Wickramasinghe N, Jeewandara C, et al. Dengue NS1 antigen as a marker of severe clinical disease. BMC Infect Dis. 2014;14:570. https://doi.org/10.1186/s12879-014-0570-8.
Puerta-Guardo H, Glasner DR, Harris E. Dengue virus NS1 disrupts the endothelial glycocalyx, leading to hyperpermeability. PLOS Pathog. 2016;12:e100. https://doi.org/10.1371/journal.ppat.1005738.
Costa VG, Marques-Silva AC, Moreli ML. A meta-analysis of the diagnostic accuracy of two commercial NS1 antigen ELISA tests for early dengue virus detection. PLoS ONE. 2014;9:e94655. https://doi.org/10.1371/journal.pone.0094655.
Acknowledgements
All physicians who participated in CARBO. The INSERM Research and Action Targeting Emerging Infectious Disease (REACTing) network who prepared the CARBO protocol. The CHU of Martinique and CHU of La Reunion for this collaboration.
Funding
This work was supported in part by the European Regional Development Fund (ERDF) through the RUNDENG project, number RE0022937, and the INTERREG though the VECTOBIOMES project, number RE0009962. The CARBO Cohort was supported by a grant from the French Ministry of Health (PHRC, 2009, n° 29 − 01), the French network for Research and Action targeting emerging infectious diseases (REACTING). The DEMARE collection was funded by the Swiss National Foundation for Scientific Research (SNFSR), project number 17953. SH and TB were supported by a PhD degree scholarship from Reunion University (Ecole doctorale STS) funded by DIRED/20181182 from the Conseil Régional de La Réunion.
Author information
Authors and Affiliations
Contributions
S.H and T.B: molecular analyses, investigation, writing-review and editing; D.A.W: molecular analyses, investigation, methodology, visualization, writing-original draft; C.A: supervision, writing-review and editing; J.B, M.L and J.G: supervision; L.B: molecular analyses, sample acquisition, investigation; O.D.S: sample acquisition, investigation; A.F and A.C: funding acquisition; A.B: conceptualization, investigation, writing-original draft, funding acquisition; P.M: conceptualization, formal analysis, investigation, writing-original draft, funding acquisition, writing-review and editing. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the French National Agency for the Safety of Medicines and Health Products (ANSM) (n°IDRCB 2010-A00282-37) and by the committee for the protection of individuals. All procedures were performed in accordance with relevant guidelines and regulations and written and signed informed consent was obtained from all participants included. Blood samples from the Seychelles were collected by the Ministry of Health of the Seychelles during outbreaks between January 2015 and December 2016.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.

Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hafsia, S., Barbar, T., Wilkinson, D.A. et al. Genetic characterization of dengue virus serotype 1 circulating in Reunion Island, 2019–2021, and the Seychelles, 2015–2016. BMC Infect Dis 23, 294 (2023). https://doi.org/10.1186/s12879-023-08125-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-023-08125-y